Abstract
PURPOSE
We aimed to evaluate the combination of the modified Response Evaluation Criteria In Solid Tumors (mRECIST) and contrast-enhanced ultrasonography (CEUS) as a tool for the assessment of hepatocellular carcinoma treated with transarterial chemoembolization.
MATERIALS AND METHODS
Forty-seven hepatocellular carcinoma patients (80 target tumors suitable for mRECIST measurements) were studied. They were treated with scheduled transarterial chemoembolization with doxorubicin-eluting microspheres every 5–7 weeks. Imaging follow-up (performed one month after each transarterial chemoembolization) included a standard, contrast-enhanced modality (computed tomography [CT] in 12 patients or magnetic resonance imaging [MRI] in 35 patients) and CEUS. The study focused on response evaluation after the third transarterial chemoembolization. CEUS required a bolus injection of an echo-enhancer and imaging with a dedicated, low mechanical index technique. The longest diameters of the enhancing target tumors were measured on the CEUS or CT/MRI, and mRECIST criteria were applied. Radiologic responses were correlated with overall survival and time to progression.
RESULTS
The measurements of longest diameters of the enhancing target tumors were easily performed in all patients. According to mRECIST-CEUS and mRECIST-CT/MRI, complete response was recorded in five and six patients, partial response in 22 and 21 patients, stable disease in 16 and 14 patients, and progressive disease in four and six patients, respectively. There was a high degree of concordance between CEUS and CT/MRI (kappa coefficient=0.84, P < 0.001). Responders (complete+partial response) according to mRECIST-CEUS had a significantly longer mean overall survival and time to progression compared to nonresponders (37.1 vs. 11.0 months, P < 0.001 and 24.6 vs. 10.9 months, P = 0.007, respectively).
CONCLUSION
The mRECIST-CEUS combination is feasible and has prognostic value in the assessment of hepatocellular carcinoma following transarterial chemoembolization.
Introduced in 2008 as an amendment of Response Evaluation Criteria In Solid Tumors (RECIST), the modified RECIST (mRECIST) focuses on the viable (enhancing on dynamic studies) tumoral components to assess the response of hepatocellular carcinoma (HCC) after locoregional or antiangiogenic treatment (1). mRECIST, which is based on the unidimensional measurement of the enhancing portions of target tumors, is simple and easy to apply in clinical practice. A high degree of concordance has been observed between mRECIST and another system of enhancement-based response criteria (the European Association for the Study of the Liver) that has been in use since 2001 (2–4). Moreover, there is growing evidence that mRECIST is a valuable prognostic tool that is able to predict overall survival in HCC patients treated with transarterial chemoembolization (TACE) (3, 4) or sorafenib (5). The mRECIST system was intended to be applicable to dynamic studies of computed tomography (CT) or magnetic resonance imaging (MRI), which are the standard modalities for an imaging assessment of tumor response (1). Contrast-enhanced ultrasonography (CEUS) plays a complementary role in the assessment of the efficacy of locoregional treatment, and at present, the mRECIST system has not been combined with CEUS. We believe that such a combination would be worth studying for several reasons. First, CEUS is capable of accurate, real-time, multiplanar imaging of tumoral enhancements prior to and after locoregional treatments (6–9). Thus, the basic calculation of mRECIST (namely, the measurement of the longest diameter of the enhancing tumor) can be easily performed on the appropriate CEUS images. Second, although the diagnostic accuracy of postinterventional CEUS has been adequately evaluated (6–8), little is known regarding the prognostic value of this modality. It would be, therefore, meaningful to categorize post-treatment CEUS findings with an established system of response criteria and to correlate the results with standard clinical endpoints. Finally, mRECIST could serve as a standardized framework for a comprehensive comparison between CEUS and the standard modalities for evaluating a tumor response.
To assess the feasibility and prognostic value of the combination of mRECIST and post-treatment CEUS, we performed a retrospective study in a series of HCC patients who underwent treatment with TACE and were monitored with CEUS and standard imaging modalities.
Materials and methods
Patients and treatment
A search of the digital database of the Radiology Department of Tzanio Hospital was performed to identify patients with newly diagnosed HCC according to The American Association for the Study of Liver Diseases (AASLD) guidelines (10) who were ineligible for curative treatment and underwent TACE between March 2008 and April 2012. Therapeutic decisions for the aforementioned patients were undertaken by a multidisciplinary tumor board consisting of a hepatologist, liver surgeon, medical oncologist, interventional radiologist, and pathologist. According to our TACE protocol, three scheduled sessions were performed every 5–7 weeks; patients were subsequently reevaluated by the tumor board before further treatment decisions were made. In general, subsequent treatment planning was similar to that described in a previously published cohort of HCC patients treated with TACE (11). Further treatment with TACE was undertaken in cases of disease progression (i.e., embolization on demand). Local thermal ablation (with radiofrequency or microwaves) was applied when the newly discovered lesions were smaller than 3 cm and at a suitable location. If disease progression was accompanied by development of a contraindication to locoregional treatment, oral antiangiogenic therapy (sorafenib) was started.
Patients provided written informed consent for TACE treatment. Institutional review board approval was not required for the retrospective collection and analysis of the study material.
Chemoembolization
The selection criteria and basic procedure for TACE were in line with those of previous work (11). Chemoembolization was performed with drug-eluting beads. During the first nine months of the study, we utilized 100–300 μm DC-Beads (Biocompatibles Ltd., Surrey, UK) loaded with doxorubicin (Adriblastina, Pfizer Italia S.r.L., Milano, Italy) at a dose of 75 mg drug/vial. For the rest of the study period, we utilized Hepasphere microspheres (Biosphere Medical, Paris, France) with dry state diameters of 50– 100 μm loaded with the same dose of doxorubicin as DC-Beads. Segmental or subsegmental TACE was routinely performed. Lobar TACE was performed only in cases of extensive (>75%) involvement of a liver lobe.
For superselective catheterization, a 2.7 F microcatheter (Progreat, Terumo Europe N.V, Leuven, Belgium or Renegade Hi-Flo Fathom, Boston Scientific, Natick, Massachusetts, USA) was utilized. Intraprocedural CEUS with the intra-arterial injection of echo-enhancer (12) was occasionally used to facilitate the selection of the appropriate tumor feeders, if the latter were not definitely detectable by angiography. The tumor-bearing liver segment and neighboring segments were scanned in CEUS mode during the injection of the echo-enhancer through the microcatheter. If the selected artery was actually tumor-feeding, the injection of the echo-enhancer was immediately detected by CEUS as a rapid, intense enhancement of the tumoral area. In contrast, if a nontumoral artery was selected, the intra-arterial injection of the echo-enhancer appeared on CEUS as an enhancement of nontumoral liver parenchyma, usually with a segmental or subsegmental distribution. Intraprocedural-intra-arterial CEUS findings were used only for guidance and not for diagnostic purposes.
Standard imaging protocol
These procedures were performed at several institutions, either with CT (4-or 64-detector scanners) or with MRI (field strength, 1.5 or 3 Tesla). A baseline study was performed 2–14 days prior to the first session of TACE, and follow-up was performed approximately one month after each session. For the purposes of this work, the technical requirements for both CT and MRI included the following: contiguous slices with a thickness equal to or less than 5 mm; absence of significant artifacts; and unenhanced and at least two enhanced (dynamic) acquisitions (at the arterial and portal venous phase) with clear depictions of the arterial uptake from viable tumor components and clear differentiation from the unenhanced (necrotic) parts. Dynamic MRI was performed after the intravenous injection of gadolinium. No liver-specific agents were used. The latter have been shown to increase the diagnostic accuracy of MRI in the post-TACE assessment of HCC (13); however, in our country, liver-specific contrast agents are not routinely used for postinterventional imaging.
CT and MRI studies were stored in the Digital Imaging and Communications in Medicine (DICOM) format in a personal computer, and retrospective evaluations were performed with dedicated software. The evaluations included the following: identification of the target, nontarget and new lesions; measurements of the targets and of their enhancing components on the axial sections; visual assessment of the tumor burden of the liver (greater or smaller than 50%); and assessment for macrovascular invasion and extra-hepatic tumor spread. The radiologist (M.G.P.), who reviewed the CT/MRI studies had 19 years of experience in cross-sectional abdominal imaging and was blinded to the findings of the CEUS. This work included only patients with tumors that could be considered “target lesions,” according to mRECIST (1).
Ultrasonographic imaging protocol
The schedule of pre- and postinterventional ultrasonography was similar to that of standard imaging. A baseline CEUS study was performed 1–7 days prior to the initiation of treatment; post-treatment CEUS was performed approximately one month (range, 27– 34 days; mean, 32.2 days) after each session of TACE. Each CEUS study was performed after the corresponding standard imaging investigation over an interval ranging from one to eight days (mean, 5.1 days).
We utilized three available ultrasonographic units (Esaote Megas GPX [Esaote, Genoa, Italy], Philips HD11 XE [Philips Ultrasound, Andover, Massachusetts, USA], and General Electric Logiq E9 [GE Healthcare, Milwaukee, Wisconsin, USA]) with CEUS capability and multifrequency (1–5 MHz or 2–5 MHz) curved array transducers.
Unenhanced B-mode and color Doppler ultrasonography was initially performed to identify the target tumors, to measure their maximum diameters and to collect other clinically relevant information, such as vascular invasion, presence of ascites, or newly appearing lesions.
For CEUS, a second-generation ultrasonography contrast agent (suspension of microbubbles of sulfur hexafluoride [SonoVue, Bracco Imaging S.p.A., Milano, Italy]) was injected as a bolus in a forearm vein, followed by a flush of normal saline. A dedicated, contrast-specific, continuous scanning, low mechanical index technique was utilized (mechanical index, 0.08–0.12). Our previous experience with the equipment indicated that, with the Megas and E9 machines, strong liver parenchymal and tumoral enhancement could be achieved after the administration of 2.4 mL (half of the full dose) of the echo-enhancer. On the other hand, a full dose (4.8 mL) of the echo-enhancer was required to observe adequate enhancement with the particular model of the HD11 at our disposal. Thus, in this study, we used the aforementioned doses for pre- and postinterventional CEUS, depending on the type of the equipment we utilized each time.
A technically adequate CEUS incorporated the following steps. First, a complete scan of the target tumors during the arterial phase was performed to clearly depict the viable (enhancing) and necrotic (non-enhancing) tumoral components and to identify the optimal plane for the measurement of the longest viable tumor diameter. Second, a scan of the rest of the liver was taken to determine the status of nontarget lesions (enhancement versus non-enhancement) and to assess potentially newly appearing lesions that were undetectable on unenhanced ultrasound. To keep the cost and duration of CEUS examination within reasonable limits, we included only technically adequate CEUS studies that could be accomplished with a maximum of two injections of the echo-enhancer. In this case, one injection was applied for each of the aforementioned steps at 10 min intervals. Images and video acquisitions of all of the CEUS studies were stored on the hard disk of the ultra-sonographic units and were reviewed by the same radiologist (H.M., with 14 and six years of experience in abdominal US and peri-interventional CEUS, respectively), who was unaware of the findings of standard imaging follow-up. This radiologist examined the stored video-clips frame-by-frame, isolated the appropriate images and performed measurements with electronic calipers on the monitor of each unit. A difference between CEUS and CT/MRI should be highlighted, as follows: regarding ultrasonography (B-mode or CEUS), obtaining true axial images is often difficult due to unsuitable anatomy or poor acoustic windows. Moreover, many HCCs (or their enhancing parts) are irregularly shaped and obliquely oriented. For these reasons, the ultrasonographic sections, which depicted the largest diameter of the viable tumor to the best advantage, were very often at an atypical, operator-defined imaging plane and not at a standard (e.g., axial) imaging plane.
Evaluation of response
mRECIST criteria were used to evaluate tumor responses on the basis of CT/ MRI and CEUS findings one month after the third session of TACE. If a complete response was achieved earlier, the respective session of TACE was taken into account. mRECIST system grades target lesion responses as follows: complete response (CR, disappearance of any intratumoral arterial enhancement in all target lesions), partial response (PR, at least a 30% decrease in the sum of diameters of viable target lesions, taking as reference the baseline sum of the diameters of target lesions), progressive disease (PD, an increase of at least 20% in the sum of the diameters of viable target lesions, taking as reference the smallest sum of the diameters of viable [enhancing] target lesions recorded since treatment started), and stable disease (SD, all other variations) (1). The “diameter of the viable target tumor” is the longest diameter of the enhancing target tumor (LDETT), as demonstrated on the axial section of a dynamic CT/ MRI (1). Also, in our study, the “diameter of the viable target tumor” was arbitrarily considered to be the LDETT on the appropriate CEUS section. To quantify the degree of necrosis (i.e., the decrease in enhancing tumor tissue) caused by the first three sessions of TACE and to apply mRECIST, the following formula was utilized:
For patients with more than two HCC foci, the two largest tumors at baseline were considered as the target tumors. This appears to be an acceptable simplification (14).
Although not included in the main purpose of this study, we also applied conventional RECIST criteria (version 1.1) (15) combined with the ultrasonographic findings following the third TACE, thereby seeking to investigate the differences between the size- and enhancement-oriented approaches for the evaluation of tumor response.
Patients with CR or PR were considered responders, while patients with SD or PD were nonresponders. Radiologic responses were correlated with the following two clinical end-points: 1) overall survival (OS), defined as the time between the date of the first TACE session and the date of the patient’s death, or the date at which the patient was last known to be alive; and 2) time to progression (TTP), defined as the time elapsed between TACE initiation and radiologic (CT/MRI) detection of the disease progression, according to mRECIST. To monitor the course of disease after the initial period of three sessions, standard imaging modalities were performed every 2–3 months or one month after each additional session of TACE.
Statistical analysis
Changes in quantitative values before and after treatment were evaluated with Wilcoxon signed-rank test. Survival data were analyzed by the Kaplan-Meier method and the log-rank test. Parameters that found to be significant in the univariate analysis underwent multivariate Cox regression analysis. The kappa coefficient was used to evaluate the degree of agreement between CEUS and CT/MRI in terms of response evaluation, according to mRECIST. The same coefficient was used to assess the concordance between RECIST and mRECIST systems. Statistical significance was defined as a P value of < 0.05. Statistical analyses were performed using a computer software (Statistical Package for Social Sciences, Version 19.0, SPSS Inc., Chicago, Illinois, USA).
Results
Forty-seven patients (37 males and 10 females; age range, 51–84 years, mean age, 67.5±8.5 years) with 80 target tumors were studied. At baseline, the longest diameter of the targets ranged from 23 to 163 mm (mean, 73±40 mm). Most of the patients presented with a cirrhotic background (usually with underlying hepatitis B viral infection), were of Child-Pugh class A and of Barcelona Clinic Liver Cancer (BCLC) stage B, and had three or fewer tumors. Extensive tumor burden (more than 50% of the entire liver) and macrovascular invasion (partial or peripheral portal vein thrombosis) were observed in a minority of subjects at baseline. The demographic and clinical data of the study population are provided in Table 1. The standard imaging modality was CT for 12 patients, while the rest received MRI. Intraprocedural-intra-arterial CEUS guidance was applied for 11 patients (16 sessions of TACE).
Table 1.
Baseline demographic and clinical data of study patients
| Mean±SD or n | ||
|---|---|---|
| Age (years) | 67.5±8.5 | |
| Gender (male/female) | 37/10 | |
| Cirrhotic background | 45/47 | |
| HBV | 34 | |
| HCV | 5 | |
| HBV+HCV | 1 | |
| HBV+HDV | 1 | |
| Alcohol usage | 2 | |
| Unknown | 2 | |
| Child-Pugh classification | ||
| A | 34 | |
| B | 13 | |
| BCLC stagea | ||
| A | 3 | |
| B | 36 | |
| C | 8 | |
| Tumor number | ||
| Solitary | 14 | |
| Two | 12 | |
| Three | 10 | |
| Four or more | 11 | |
| Maximum diameter of targets (mm) | ||
| Minimum | 23 | |
| Maximum | 163 | |
| Mean | 73 | |
| Median | 61 | |
| Tumor burden | ||
| >50% | 6 | |
| <50% | 41 | |
| Macrovascular invasion | ||
| Yes | 9 | |
| No | 38 | |
BCLC, Barcelona Clinic Liver Cancer stage. BCLC-A, patients of this study were unsuitable for curative treatment; BCLC-C, patients of this study had partial or peripheral portal vein thrombosis or hilar or perihepatic lymph node involvement.
HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis delta virus; SD, standard deviation.
During the period of this study, all of the 40 additional patients with newly diagnosed HCC who underwent TACE in our institution were excluded. In 18 patients, the CEUS was technically inadequate due to its poor visualization of the target lesions or of other liver areas. Seven patients received additional treatments. Six patients did not comply with the treatment schedule. Five patients had diffusely growing or atypically enhancing hepatomas; three patients had suboptimal standard imaging evaluations; and one patient died shortly after the first TACE (procedure-related complication; liver abscess).
Measurements of the longest diameters of the entire target tumors and of their enhancing components were easily accomplished in all 47 patients (Fig. 1). Moreover, calculations of the degree of necrosis post-TACE showed no significant differences between CEUS and CT/MRI (Table 2). One month after the third TACE, the evaluation of the response with mRECIST-CEUS identified five patients with CR, 22 patients with PR, 16 patients with SD, and four patients with PD. The corresponding results for the standard (CT/MRI) evaluation were CR in six, PR in 21, SD in 14, and PD in six patients. There was a high degree of concordance between CEUS and CT/MRI, both for the differentiation of responders from nonresponders (kappa=0.913, P < 0.001) and for the further classification of the response (kappa=0.84, P < 0.001).
Figure 1. a–f.
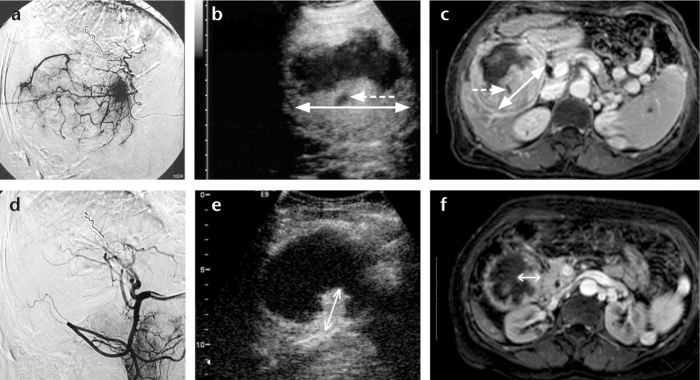
Imaging of a large hepatocellular carcinoma prior to treatment (upper row images) and following the third transarterial chemoembolization (TACE) (lower row images). Angiographic images at the beginning of the first session of TACE, prior to embolization (a) and immediately after completion of the third session of TACE (d) show the almost complete elimination of tumor blush as a result of TACE. The contrast-enhanced ultrasonography (CEUS) images of the same tumor at baseline (b) and at follow-up, one month after the third session of TACE (e), show a significant decrease in the viable (enhancing) components of the tumor. Axial contrast-enhanced MR images of the tumor at baseline (c) and one month after the third session of TACE (f) confirm the decrease of tumoral enhancement. Double-headed arrows indicate the longest diameters of the enhancing parts of the tumors. In accordance with mRECIST guidelines, small necrotic areas (dotted arrows) within the main bulk of enhancing tumor were not included in the measurements. This patient also had two smaller lesions (not shown) with complete lack of enhancement one month after the third TACE (both on CEUS and MRI) and was diagnosed as a partial response.
Table 2.
Measurements of the enhancing target tumors by CEUS and CT/MRI
| CEUS | CT/MRI | P | |
|---|---|---|---|
| Sum of LDETT (mm) | |||
| Baseline | 86.8±47.8 (41–271) | 84.1±47.0 (35–271) | 0.034 |
| One month after the third TACE | 57.0±53.4 (0–263) | 57.8±56.7 (0–268) | 0.679 |
| Degree of necrosis (%) | 40.9±36.0 (−21.1a–100) | 38.4±36.9 (−20a–100) | 0.4 |
Negative values indicate an increase rather than a decrease in the enhancing tumor after TACE.
CEUS, contrast-enhanced ultrasonography; CT/MRI, computed tomography/magnetic resonance imaging; LDETT, longest diameter of the enhancing target tumor; TACE, transarterial chemoembolization.
Discrepancies between CEUS and the standard techniques were observed in the following cases. In one patient, CEUS failed to confirm the complete disappearance of the enhancement of the target tumor and diagnosed PR instead of CR. CEUS failed to detect newly appearing lesions in two patients and misclassified them as SD, while they were correctly diagnosed as PD by means of MRI. Finally, in one patient, CEUS diagnosed a less than 30% decrease in an enhancing tumor (i.e., SD), although the CT measurements indicated a PR with a decrease exceeding 30%; in another patient, the opposite form of disagreement occurred.
Responders, according to mRECIST-CEUS, had a significantly longer mean OS compared to nonresponders (37.1 vs. 11.0 months, P < 0.001; Table 3, Fig. 2a). Of note, the differences in OS maintained their significance (P = 0.014), even when three other significant confounders (macrovascular invasion, tumor burden [<50% or >50%] and Child-Pugh score) were taken into account. mRECIST-CEUS was also predictive of the TTP (mean TTP for responders vs. nonresponders, 24.6 vs. 10.9 months, P = 0.007; Table 3, Fig. 2b).
Table 3.
Clinical end-points stratified by patient response according to mRECIST-CEUS
| Response according to mRECIST/CEUS | Overall survival (months) Estimate±SE (95%CI) | Time to progression (months) Estimate±SE (95%CI) | ||
|---|---|---|---|---|
|
|
|
|||
| Mean | Median | Mean | Median | |
| No Response | 11.02±1.33 | 8.00±3.44 | 10.97±1.48 | 10.00±1.53 |
| (8.42–13.62) | (1.26–14.74) | (8.06–13.87) | (7.01–12.99) | |
| Response | 37.19±3.34 | 43.00±7.55 | 24.67±3.11 | 30.00±6.50 |
| (30.65–43.74) | (28.20–57.80) | (18.57–30.77) | (17.27–42.73) | |
| Overall | 28.54±3.10 | 33.00±10.58 | 21.69±2.66 | 24.00±8.44 |
| (22.46–34.61) | (12.27–53.74) | (16.47–26.90) | (7.45–40.55) | |
CEUS, contrast-enhanced ultrasonography; CI, confidence interval; mRECIST, modified Response Evaluation Criteria In Solid Tumors; SE, standard error.
Figure 2, a, b.
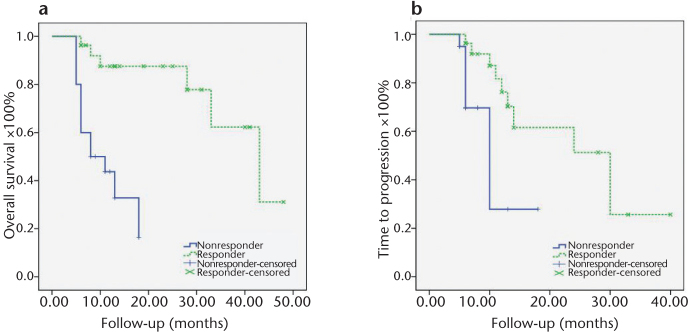
Kaplan-Meier curves illustrating the differences in overall survival (a) and in time to progression (b) between responders and nonresponders, according to mRECIST-CEUS classifications.
Conventional RECIST identified one patient with CR, six patients with PR, 35 patients with SD, and five patients with PD. Responses detected by RECIST were associated with a longer OS, but differences were of marginal statistical significance (mean OS for responders vs. nonresponders, 41.3 vs. 24.8 months, P = 0.050). The agreement between RECIST and mRECIST was poor (kappa=0.244, P = 0.001 for classification of response; kappa=0.230, P = 0.014 for diagnosing response vs. nonresponse). Of the 40 nonresponders according to RECIST, 20 were diagnosed as responders by mRECIST and had a significantly longer mean OS compared to the remaining 20 patients, who were diagnosed as nonresponders by both systems (34.1 vs. 11.0 months, P = 0.001).
Regarding the standard imaging evaluation of the 80 target tumors after the third TACE, 12 were characterized as completely necrotic, while 68 were found to have a variable extent of residual enhancing tissue. Corresponding CEUS examinations failed to detect residual enhancement in one of 68 target tumors. Moreover, as noted earlier, CEUS failed to diagnose the complete elimination of enhancement in one of 12 completely necrotic tumors. Thus, the sensitivity, specificity and diagnostic accuracy of CEUS in the detection of residual tumors post-TACE was 98.5%, 92.3%, and 97.5%, respectively.
Nontarget lesions and/or new lesions were present in 22 of 47 patients. Incorrect CEUS evaluation of those occurred in six of 22 cases (27.2%) and caused a misclassification of the response in two cases, as noted earlier. In the remaining four cases, CEUS misdiagnosis of the enhancement status of nontarget lesions was less significant; for example, the failure of CEUS to diagnose the complete necrosis of a small satellite would not affect response evaluation in a multifocal-HCC patient with two partially responding target tumors.
In total, 28 of the 47 patients were alive at the end of this study. Follow-up periods ranged from five to 48 months (mean, 15.3 months; median, 12 months).
Discussion
Our initial experience shows that, in the context of the post-TACE evaluation of the HCC, simple, linear measurements of the enhancing tumor can be readily performed on appropriate CEUS images; these results compare favorably with those of standard (CT/ MRI) imaging. Moreover, the combination of mRECIST-CEUS appears to be of prognostic value; this is supported by the significant differences in OS and TTP between responders and nonresponders. In line with other studies based on CT or MRI (16, 17), our mRECIST-CEUS protocol proved more suitable than the conventional RECIST system for the short-term assessment of tumor response. The former captured many more responders than the latter (27 vs. 7), and the ability of mRECIST to predict OS was associated with superior statistical power compared to RECIST. In a previous work (18), we applied CEUS and mRECIST system to evaluate HCC treated with sorafenib, and we reached similar conclusions regarding the feasibility of mRECIST-CEUS and the superiority of mRECIST over RECIST. A correlation between mRECIST-CEUS response and OS was also observed, although statistical significance was established only in the univariate analysis.
There were a few cases of discordance between CEUS and CT/MRI that merit further discussion. Differences in the measurements of the longest enhancing tumor diameter accounted for different classifications of response (SD instead of PR and vice versa) in two cases. For all measurements in CT or MRI studies, we utilized the axial plane. In contrast, as noted earlier, CEUS measurements were very often performed in a different, arbitrary, operator-defined imaging plane that captured the largest viable component of the target tumor during real-time CEUS imaging. This could explain variations between CEUS and CT/MRI measurements, particularly in large tumors with multiple, irregular islets of enhancing tissue. In two other patients, CEUS failed to detect newly appearing foci of HCC, which were declared a sign of disease progression. CEUS provides a smaller field of view compared to CT/MRI, and thus, scanning of the entire liver during the arterial phase (when most HCCs stand out clearly) may be challenging (19). Moreover, these two patients had numerous nontarget tumors at baseline and severe macronodular cirrhosis, which further compromised the ultrasonographic detection of new lesions.
On the other hand, CEUS appears to be highly efficient, if post-treatment evaluation is focused on one or two index lesions. In our series of 80 target tumors, CEUS could detect residual enhancement following treatment with a very high sensitivity and specificity. In several other studies comparing post-TACE CEUS with various reference standards (CT, biopsy, angiography), a sensitivity of 87%–100% and a specificity of 81%–100% were observed (6–8, 20, 21).
Several limitations of the present work should be acknowledged. First, the retrospective nature of the study is associated with various methodological weaknesses. Most importantly, we could not scan all patients with the same standard imaging modality (either CT or MRI) or with the same, consistently applied, acquisition protocol. Demographic and socio-economic factors, as well as the work load of the hospital’s imaging department, accounted for this limitation. Another source of inconsistency is the aforementioned inherent limitations of CEUS in depicting the targets in exactly the same planes as CT/MRI. We recognize that these drawbacks reduce the reproducibility of our observations and the strength of our conclusions. Second, all CEUS measurements were performed by one radiologist, and therefore, we were unable to assess the interobserver variability. Finally, the size of our sample did not allow us to incorporate other potentially significant prognostic factors or to perform a more thorough survival analysis.
In selected patients from this study, we utilized CEUS with intra-arterial injection of the echo-enhancer as a tool for the intraprocedural guidance of TACE. We recognize that this is not an established application for CEUS. However, recent studies have shown that intra-arterial CEUS during TACE is feasible, safe and capable of facilitating the selection of appropriate tumor feeders in difficult cases, if more sophisticated equipment (C-arm CT) is not available (12, 22, 23). We did not incorporate intraarterial CEUS findings into our data analysis, either as baseline information or at follow up; we considered this to be beyond the scope of this paper.
In conclusion, CEUS is a valuable method for depicting tumoral necrosis caused by TACE, and the resultant CEUS findings can be categorized by means of mRECIST. This approach appears to be practical and to correlate favorably with standard imaging and clinical end-points. CEUS should not be considered a first-line modality for the evaluation of tumor response after TACE; however, it is a useful complementary technique that appears to have not only diagnostic but also prognostic value.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 3.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–1316. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Kim BK, Kim KA, Park JY, et al. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer. 2013;49:826–834. doi: 10.1016/j.ejca.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Edeline J, Boucher E, Rolland Y, et al. Comparison of Tumor Response by Response Evaluation Criteria in Solid Tumors (RECIST) and Modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 6.Youk JH, Lee JM, Kim CS. Therapeutic response evaluation of malignant hepatic masses treated by interventional procedures with contrast-enhanced agent detection imaging. J Ultrasound Med. 2003;22:911–920. doi: 10.7863/jum.2003.22.9.911. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto M, Shirato K, Sugimori K, et al. Contrast-enhanced harmonic gray-scale sonographic-histologic correlation of the therapeutic effects of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:65–69. doi: 10.2214/ajr.181.1.1810065. [DOI] [PubMed] [Google Scholar]
- 8.Pompili M, Riccardi L, Covino M, et al. Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25:954–961. doi: 10.1111/j.1478-3231.2005.01135.x. [DOI] [PubMed] [Google Scholar]
- 9.Moschouris H, Malagari K, Papadaki MG, Kornezos I, Matsaidonis D. Contrast-enhanced ultrasonography of hepatocellular carcinoma after chemoembolisation using drug-eluting beads: a pilot study focused on sustained tumor necrosis. Cardiovasc Intervent Radiol. 2010;33:1022–1027. doi: 10.1007/s00270-010-9800-7. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 12.Moschouris H, Malagari K, Kalokairinou M, Stamatiou K, Marinis A, Papadaki MG. Contrast-enhanced ultrasonography with intraarterial administration of SonoVue for guidance of transarterial chemoembolization: an initial experience. Med Ultrason. 2011;13:296–301. [PubMed] [Google Scholar]
- 13.Bolog N, Pfammatter T, Müllhaupt B, Andreisek G, Weishaupt D. Double-contrast magnetic resonance imaging of hepatocellular carcinoma after transarterial chemoembolization. Abdom Imaging. 2008;33:313–323. doi: 10.1007/s00261-007-9244-y. [DOI] [PubMed] [Google Scholar]
- 14.Shim JH, Lee HC, Won HJ, et al. Maximum number of target lesions required to measure responses to transarterial chemoembolization using the enhancement criteria in patients with intrahepatic hepatocellular carcinoma. J Hepatol. 2012;56:406–411. doi: 10.1016/j.jhep.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Watanabe H, Sone M, et al. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16–22. doi: 10.3109/03009734.2012.729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 18.Moschouris H, Malagari K, Gkoutzios P, et al. Intermediate and advanced hepatocellular carcinoma treated with the antiangiogenic agent sorafenib. Evaluation with unenhanced and contrast-enhanced ultrasonography. Med Ultrason. 2012;14:87–94. [PubMed] [Google Scholar]
- 19.Moschouris H, Malagari K, Papadaki MG, et al. Short-term evaluation of liver tumors after transarterial chemoembolization: limitations and feasibility of contrast-enhanced ultrasonography. Abdom Imaging. 2011;36:718–728. doi: 10.1007/s00261-011-9690-4. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Kudo M, Minami Y, et al. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of sonazoid-enhanced harmonic sonography. Oncology. 2008;75(Suppl 1):99–105. doi: 10.1159/000173430. [DOI] [PubMed] [Google Scholar]
- 21.Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447–453. doi: 10.1007/s00261-009-9551-6. [DOI] [PubMed] [Google Scholar]
- 22.Schacherer D, Girlich C, Zorger N, et al. Sono-hepatic-arteriography (Sono-HA) in the assessment of hepatocellular carcinoma in patients undergoing transcatheter arterial chemoembolization (TACE) Ultraschall Med. 2010;31:270–275. doi: 10.1055/s-0029-1245242. [DOI] [PubMed] [Google Scholar]
- 23.Uller W, Wiggermann P, Gössmann H, et al. Evaluation of the microcirculation of hepatocellular carcinomas using contrast-enhanced ultrasound with intraarterial and intravenous contrast application during transarterial chemoembolization with drug-eluting beads (DEB-TACE): preliminary data. Clin Hemorheol Microcirc. 2011;49:55–66. doi: 10.3233/CH-2011-1457. [DOI] [PubMed] [Google Scholar]


