Abstract
PURPOSE
We aimed to compare two different methods of region of interest (ROI) demarcation and determine interobserver variability on apparent diffusion coefficient (ADC) in breast lesions.
METHODS
Thirty-two patients with 39 lesions were evaluated with a 3.0 Tesla scanner using a diffusion-weighted sequence with several b-values. Two observers independently performed the ADC measurements using: 1) a small fixed area of 10 mm2 ROI within the area with highest restriction; 2) a large ROI so as to include the whole lesion. Differences were assessed using the Wilcoxon-rank test. Bland-Altman method and Spearman coefficient were applied for interobserver variability and correlation analysis.
RESULTS
ADC values measured using the two ROI demarcation methods were significantly different for both observers (P = 0.026; P = 0.033). There was no interobserver variability in ADC values using either method (large ROI, P = 0.21; small ROI, P = 0.64). ADC values of malignant lesions were significantly different between the two methods (P < 0.001). Variability in ADC was ≤0.008×10−3 mm2/s for both methods. When using the same method, ADC values were significantly correlated between the observers (small ROI: r=0.990, P < 0.001; large ROI: r=0.985, P < 0.001).
CONCLUSION
The choice of ROI demarcation method influences ADC measurements. Small ROIs show less overlap in ADC values and higher ADC reproducibility, suggesting that this method may improve lesion discrimination. Interobserver variability was low for both methods.
Diffusion-weighted imaging (DWI) of the breast has been used to improve lesion diagnosis. However, due to lesion heterogeneity, differences between acquisition protocols, and lesion demarcation strategies, there is some overlap in apparent diffusion coefficient (ADC) values of different lesion types (1).
Regarding lesion demarcation, different strategies can be found in the literature. Pereira et al. (2) suggest delimiting the whole lesion area, whereas others consider only its most solid part representing viable tumor (3, 4).
A study focusing on the use of ADC minimum, average, and maximum to characterize breast lesions (5) has indirectly investigated the influence of region of interest (ROI) on ADC quantification, but has not specifically compared these two methods of demarcation. Here, we compare these two ROI demarcation methods and determine their interobserver variability in ADC quantification.
Methods
Study population
During a five-month period, 38 women with clinical indications to perform breast magnetic resonance imaging (MRI) were studied following approval from the Ethics Committee (code number 276/13).
Exclusion criteria were breast surgery within six months, chemotherapy or radiotherapy within 24 months, no lesions in dynamic contrast-enhanced (DCE) image, breast implants, and examinations presenting artefacts. Only lesions ≥7 mm in the DCE having histological results or a minimum two-year follow-up were included.
MRI data acquisition
Patients underwent examinations at 3.0 T system (Magnetom® Tim Trio, Siemens Medical Solutions) using a four-channel phased-array coil (Invivo Corporation) with patients in the prone position.
Acquisition protocol included the following images: axial T2-weighted, sagittal T1-weighted, sagittal T2-weighted, sagittal DWI, axial T1-weighted in six dynamic phases, and sagittal T1-weighted post-contrast.
The DWI was acquired before DCE, using a single-shot spin-echo echo-planar imaging sequence, with gradients applied in three orthogonal directions, to generate trace-weighted images.
DWI parameters were as follows: repetition time/echo time, 4900/108 ms; field-of-view, 250×250 mm2; matrix, 84×128; slice thickness, 5 mm; 16 slices; three excitations; bandwidth, 1628 Hz/pixel; inversion time, 240 ms; and b-values 50, 200, 400, 600, 800, 1000, 2000, and 3000 s/mm2. ADC maps were generated using a mono-exponential fitting of b-values 50 to 1000 s/mm2. The higher b-value images were excluded from the analysis and used to explore non-Gaussian diffusion (6).
Image analysis
The same radiologist reported the examinations using the Breast Imaging Reporting and Data System (BI-RADS) MRI lexicon for lesion interpretation. Two researchers independently analyzed DWI datasets retrospectively, using the scanner workstation with commercial software (Syngo® Multimodality; Syngo MR B17A, Siemens Healthcare). Lesions were localized in DW images using the MRI report description and the visual inspection of T2-weighted, DCE, and postcontrast images, serving as a guide for accurate ROI placement.
ROI demarcation
Lesions were defined as regions having higher signal intensity compared to normal adjacent tissue. Blinded to histological results, readers jointly selected the slice on b=400 s/mm2 images that displayed the largest lesion dimension to draw the ROI, avoiding normal tissue, as well as necrotic and cystic areas. The b-value chosen allows high contrast between lesion core and its outer limits.
For mass and non-mass lesions, ROIs were defined using two different methodological approaches: first method considered a small fixed area of 10 mm2 (small ROI) within the area with highest signal intensity, while the second method used manually adjusted ROI within lesion borders to include the whole lesion (large ROI) resulting on variable areas depending on lesion size.
Small ROI demarcation, for mass and non-mass lesions with evident homogeneous signal intensity in DW images, was set in the area that firstly enhanced in DCE. If the lesion presented an area with higher signal intensity in DW images, then it was chosen instead.
For both methods ROIs were propagated to all DW images and to the ADC map to verify the positioning. Mean value and standard deviation of ADC within both ROIs were recorded. The standard deviation of the mean ADC measurements was considered an index of lesion heterogeneity. Additionally large ROI size was recorded.
Fig. 1 illustrates the two methods of ROI demarcation in ADC map in a woman with a suspected mass.
Figure 1. a, b.
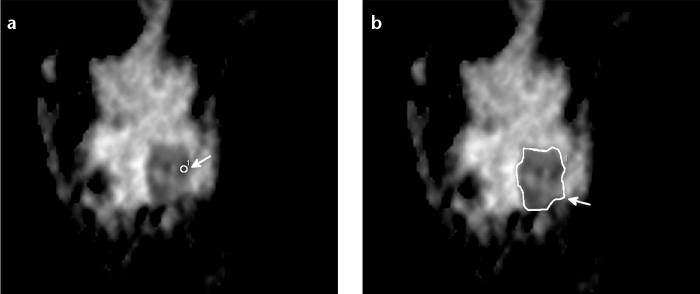
A 32-year-old woman with a suspected mass lesion of 28 mm in the lower outer quadrant of the left breast. Small ROI superimposed with the ADC map in the area with the most restricted diffusion (a, white arrow). Large ROI ADC including the whole lesion (b, white arrow). Histological result was malignant lesion not otherwise specified.
Statistical analysis
For each observer and ROI protocol ADC values were calculated for all the lesions and by lesion type. Differences in median ADCs and mean large ROI sizes were evaluated with Wilcox-on-rank test and the Mann-Whitney test, respectively.
For further analysis, mean ADC values were calculated considering the average of the measurements of the two observers. Variability in ADC measurements for each ROI method was assessed with the Bland-Altman method and the agreement using the intraclass correlation coefficient (ICC).
The correlation between measurements was assessed using the Spearman coefficient. Statistical Package for Social Sciences (SPSS) Statistics for Windows version 20.0 was used. Statistical significance was considered if P < 0.05.
Results
Six women did not fulfil the inclusion criteria. The final sample included 32 women (age range, 30–64 years) with 39 lesions (identified by both observers). Mean size was 13±10 mm and 24±12 mm for benign and malignant lesions, respectively. Lesion characteristics are presented in Table 1.
Table 1.
Lesions characteristics
| Lesion characteristics | n (%) |
|---|---|
| Size (mm) | |
| ≥7–10 mm | 5 (12.8) |
| 11–20 mm | 13 (33.3) |
| ≥ 21 mm | 21 (53.8) |
| Lesion type | |
| Mass | 36 (92.4) |
| Non-mass | 3 (7.6) |
| Malignant histopathological subtype | |
| LCIS | 1 (3.7) |
| IDC | 18 (66.7) |
| ILC | 4 (14.8) |
| Mucinous carcinoma | 1 (3.7) |
| Other malignant (NOS) | 3 (11.1) |
| Benign histopathological subtype | |
| Fibroadenoma | 6 (50.0) |
| Epithelial proliferative lesion | 3 (25.0) |
| Fibrocystic changes | 2 (16.7) |
| Complex cystic lesion | 1 (8.3) |
| Diagnostic source | |
| Biopsy | 37 (94.9) |
| Follow-up | 2 (5.1) |
LCIS, lobular carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; NOS, not otherwise specified.
A descriptive analysis of ADC values obtained using large and small ROIs and differences in median ADC values between ROI methods are presented on Table 2.
Table 2.
Comparison of ADC values for all lesions using both ROI demarcation methods by observer
| ADC measurements (×10−3 mm2/s) | |||
|---|---|---|---|
|
| |||
| Large ROI | Small ROI | p* | |
| Observer 1 | 0.026 | ||
| Mean | 1.19 | 1.16 | |
| Median | 1.03 | 0.99 | |
| Minimum | 0.69 | 0.55 | |
| Maximum | 2.55 | 2.53 | |
| SD | 0.39 | 0.29 | |
| Observer 2 | 0.033 | ||
| Mean | 1.19 | 1.16 | |
| Median | 1.12 | 0.98 | |
| Minimum | 0.67 | 0.56 | |
| Maximum | 2.54 | 2.53 | |
| SD | 0.42 | 0.30 | |
Wilcoxon rank test for median ADC values.
ADC, apparent diffusion coefficient; ROI, region of interest; SD, standard deviation.
Both observers determined significantly higher median ADCs using large ROIs compared with small ROIs (P = 0.026; P = 0.033). Median ADCs compared between observers using the same method were not significantly different for large (P = 0.21) or small ROIs (P = 0.64).
Median ADC for benign and malignant lesions were 1.70×10−3 mm2/s and 0.91×10−3 mm2/s using small ROIs and 1.71×10−3 mm2/s and 0.97×10−3 mm2/s for large ROIs, with significant difference between methods for malignant lesions (P < 0.001). Interobserver differences in median ADC measurement by either demarcation method were not significantly different when considering benign (small ROI, P = 0.46; large ROI, P = 0.90) or malignant (small ROI, P = 0.98; large ROI, P = 0.20) lesions. ADC measurements were highly correlated between the ROI demarcation methods for each observer (r=0.97, P < 0.001; r=0.95, P < 0.001) and between observers for the same method (small ROI: r=0.990, P < 0.001; large ROI: r=0.985, P < 0.001).
Mean size for large ROIs for both observers was 179.0 mm2. ROI size was not significantly different between observers when considering all lesions (P = 0.65), benign lesions (P = 0.23), or malignant lesions (P = 0.29). Size measurements were highly correlated between observers (r=0.90; P < 0.001). A moderate correlation was found between size and ADC (r=0.43; P = 0.006). The standard deviation of ADC measurements between ROI methods were significantly different for both observers (P < 0.001; P < 0.001), with no correlation between standard deviation and ADC for large (r=−0.006; P = 0.97) or small ROIs (r=0.13; P = 0.45).
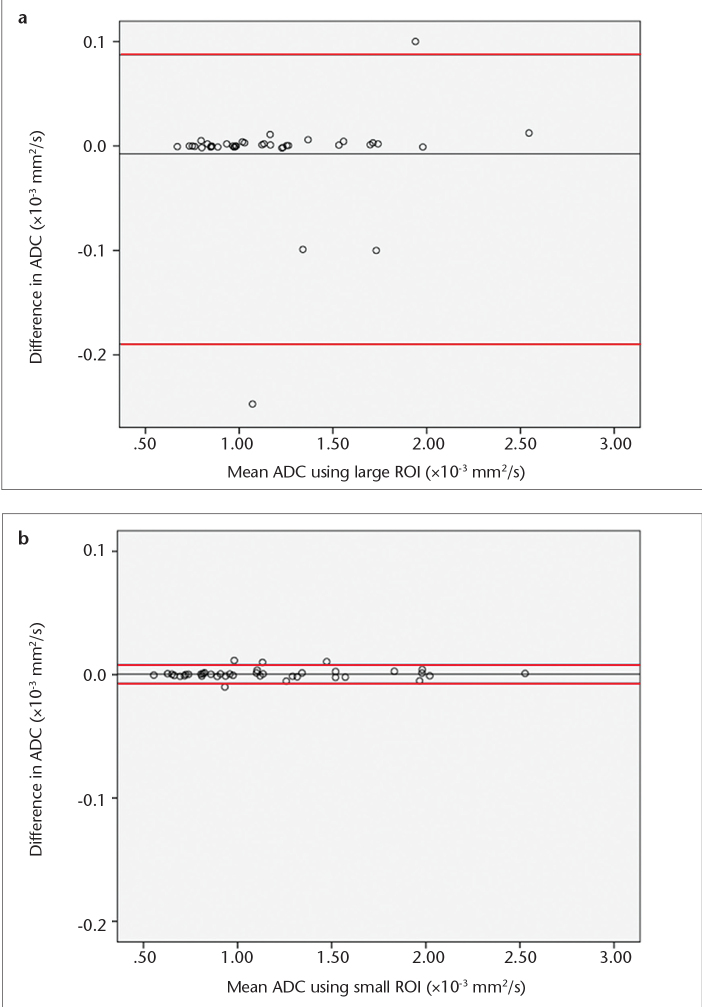
The mean difference in ADC was 0.008×10−3 mm2/s ([−0.190; +0.087]×10−3 mm2/s) for large ROIs (Fig. 2a), and 0.0005×10−3 mm2/s ([−0.007; +0.008]×10−3 mm2/s) for small ROIs (Fig. 2b). The mean variation was 1.4% for large and 0.2% for small ROIs.
Figure 2. a, b.
Interobserver variability of ADC measurements using large (a) and small ROIs (b). The mean values of individual measurements for both observers are plotted on the horizontal axis. The differences of individual measurements are plotted on the vertical axis. Central line represents the mean absolute difference in ADC between the observers. Red lines represent the limits of agreement (1.96 times the standard deviation) below and above the mean absolute difference.
ADC agreement was excellent for large (ICC=0.97 [0.984; 0.996]) and small ROIs (ICC=0.98 [0.986; 0.997]).
Discussion
Breast DWI has an important role in oncological imaging. ADC measurements help distinguish between benign and malignant lesions, although some overlap in ADC values is generally observed. Since the estimated ADC can be influenced by the methodological procedures adopted for ROI demarcation, its choice can help decrease the overlap.
In this study, large ROIs encompassing the whole lesion were compared with small fixed-sized ROIs. Using both methods, ADC estimates were within the range previously reported by other groups regarding lesion type (1). However, ADC estimates obtained using smaller ROIs were lower and the overlap between lesion types was also reduced compared with ADC estimates obtained using large ROIs. A likely explanation is that small ROIs include only the most solid portion of the lesions, corresponding to viable tumor. As this area represents the most cellular part of the lesion, the estimated ADC may be more appropriate for lesion differentiation. Also, when using small ROIs it is possible to exclude necrotic and cystic areas and the outer edges of the lesions which present lower restriction. Inclusion of the whole lesion in ROI demarcation contributes to an increased mean ADC estimate. By using small ROIs, partial volume effects in the ADC calculation would be expected to decrease.
Comparison of median ADC values between ROI methods showed significant differences for malignant lesions, indicating that the choice of ROI method for ADC quantification could condition false negative results.
Interobserver variability of ADC measurements in our study was lower for both ROI methods than previously reported using a 1.5 T scanner (7). Small ROIs showed lower interobserver variability and more reproducible ADC estimates, favoring its use in the clinical practice. An important advantage of using fixed-sized small ROIs is that its placement is much less time consuming compared to having to delimit the whole lesion. Differences in how the precise delimitation is done by different observers will have an impact on ADC values, increasing its variability and resulting in a slightly lower interobserver agreement.
The nature of lesions, the criteria adopted for ROI demarcation, and the use of a 3.0 T scanner may explain the interobserver agreement differences in our study compared with Petralia et al. (7). Higher magnetic field strength can be used to increase the signal-to-noise ratio and spatial resolution to achieve better visibility for lesions smaller than 10 mm (8). Improvement in visibility facilitates ROI definition within lesion margins and better identification of high signal intensity areas, which results in lower ADC variability and therefore, higher reproducibility of measurements. Moreover, both researchers in this study had extensive experience in ROI delineation for breast DWI, which could have contributed to the lower variability.
Limitations of this study include the small number and unbalanced distribution of lesions and the fact that most lesions were homogeneous apart from obvious easy-to-pinpoint areas of diffusion restriction.
In conclusion, ROI demarcation method affects the estimated ADC measurements. Small fixed-size ROIs resulted in smaller ADC overlap between benign and malignant lesions and higher ADC reproducibility. Although interobserver variability was low for both ROI methods, meaning that either could be used in the clinical setting, small ROI would be more efficient for measuring ADC, since its drawing is less time consuming. Studies with larger samples should be performed to confirm these findings.
Acknowledgments
This work was sponsored by the Foundation of Science and Technology, Polytechnic Institute of Porto (grant no: SFRH/BD/50027/2009 and Pest-OE/SAU/UI0645/2011).
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Vermoolen M, Nievelstein K. Apparent diffusion coefficient measurements in the differentiation between benign and malignant lesions: a systematic review. Insights Imaging. 2012;3:395–409. doi: 10.1007/s13244-012-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira FP, Martins G, Carvalhães de Oliveira R de V. Diffusion magnetic resonance imaging of the breast. Magn Reson Imaging Clin N Am. 2011;19:95–110. doi: 10.1016/j.mric.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Baltzer PA, Renz DM, Herrmann KH, et al. Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol. 2009;19:1612–1620. doi: 10.1007/s00330-009-1326-5. [DOI] [PubMed] [Google Scholar]
- 4.Ei Khouli RH, Jacobs MA, Mezban SD, et al. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010;256:64–73. doi: 10.1148/radiol.10091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano M, Satake H, Ishigaki S, Ikeda M, Kawai H, Naganawa S. Diffusion-weighted imaging of breast masses: comparison of diagnosis performance using various apparent diffusion coefficient parameters. AJR Am J Roentgenol. 2012;198:717–722. doi: 10.2214/AJR.11.7093. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira L, Brandão S, Matos E, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014;24:1197–1203. doi: 10.1007/s00330-014-3146-5. [DOI] [PubMed] [Google Scholar]
- 7.Petralia G, Bonello L, Summers P, et al. Intraobserver and interobserver variability in the calculation of apparent diffusion coefficient (ADC) from diffusion-weighted magnetic resonance imaging (DW-MRI) of breast tumours. Radiol Med. 2011;116:466–476. doi: 10.1007/s11547-011-0616-z. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka A, Minato M, Harada M, et al. Comparison of 3.0- and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiat Med. 2008;26:15–20. doi: 10.1007/s11604-007-0187-6. [DOI] [PubMed] [Google Scholar]