Abstract
PURPOSE
We aimed to evaluate the safety and effectiveness of percutaneous transluminal angioplasty (PTA) for dysfunctional femoral arteriovenous graft and analyze clinical or anatomic predictors of graft patency.
METHODS
The records of 45 patients who underwent PTA or thromboaspiration for dysfunctional or thrombosed femoral arteriovenous graft from 2005 to 2012 were reviewed retrospectively. Primary and secondary patency rates were determined at three, six, and 12 months after PTA. The primary patency rate was analyzed according to the presence of diabetes mellitus, graft age from the time of creation to the first intervention (<12 months or ≥12 months), presence of thrombus, shape of graft (U-shape vs. straight-shape), anastomosis type of graft (femoral-femoral vs. femoral-saphenous), location of stenosis (central vs. peripheral), length of stenosis (<2 cm vs. ≥2 cm), degree of stenosis severity (<70% vs. ≥70%), and stent insertion.
RESULTS
A total of 124 PTAs were performed in 45 patients. The primary patency rate at three, six, and 12 months was 84.8%, 63.6%, and 24.2%, respectively. The secondary patency rate at three, six, and 12 months was 95.2%, 95.2%, and 85.7%, respectively. The mean duration of primary and secondary patency was 13.2 and 35.7 months, respectively. No significant clinical or anatomical predictors of primary patency could be identified. Stent placement had a negative effect on primary patency.
CONCLUSION
PTA is a safe and effective treatment for dysfunctional femoral arteriovenous grafts. Stent placement seems to improve technical success, but does not enhance the primary patency rate of dysfunctional femoral arteriovenous grafts.
In general, the upper extremity arteriovenous (AV) graft is the first choice for hemodialysis patients, but not all patients are indicated, and there is a limitation to maintain patency. Therefore, new vascular access is needed on other areas including the lower extremities (1). The lower extremity AV graft is not preferred due to high rate of infection (2), but recent studies report that the incidence of infection on a lower extremity AV graft is similar to an upper extremity AV graft (3–5).
Maintaining the patency of an AV graft in hemodialysis patients is very important. Thrombosis and venous stenosis are as likely to occur in a lower extremity AV graft as in an upper extremity AV graft (6). Percutaneous transluminal angioplasty (PTA) may be useful for a dysfunctional lower limb AV graft as well as an upper limb AV graft, but a definite conclusion is hindered by the lack of direct studies. In 2004, Ryan et al. (1) described the outcomes of a percutaneous declotting technique used in 110 PTAs of 30 femoral AV grafts in 25 patients. They evaluated outcomes of percutaneous declotting procedures in patients with prosthetic femoral dialysis grafts. In 2001, Regina et al. (7) reported clinical and radiologic predictors of prosthetic AV grafts after PTA in 500 patients. However, their study was limited by the inability to classify the location of grafts in the upper or lower extremities. The purpose of our study was to retrospectively evaluate the safety and efficacy of PTA for dysfunctional femoral AV graft. We also analyzed the clinical and anatomic predictors of graft patency after PTA.
Methods
Patient population
Institutional review board approval was obtained for this study (no. 2014-01-002) and the requirement for informed consent was waived. A retrospective analysis was performed with 45 patients (15 males, 30 females; age range, 42–87 years; mean age, 64.0±11.9 years) with a femoral AV graft, who were referred for PTA or thromboaspiration due to dysfunction or thrombosis of the graft, between January 2005 and December 2012. The institute’s picture archiving and communication system was searched for this timeframe. The most common cause of chronic renal failure was diabetes mellitus in 18 (40%) patients, followed by hypertension in 13 (28.8%), unknown in 11 (24.4%), chronic glomerulonephritis in two (4.4%), and polycystic kidney disease in one (2.2%). A total of 43 patients underwent femoral AV graft due to central occlusions bilaterally or unilaterally along with inadequate or exhausted peripheral veins in bilateral upper limbs. The remaining two patients required new access creation due to infection or abnormal sense on existing hemodialysis access.
Procedures and techniques
Diagnostic imaging was performed by two interventional radiologists with nine and 17 years of experience. Diagnostic fistulography was performed with a 21-gauge needle (Becton Dickinson). Fistulography was performed from an arterial anastomotic site through the right atrium. The arterial anastomotic site was checked through reflux of contrast material by means of hard compression supplied by the operator’s hand, when the arterial inflow was decreased.
PTA was performed when there was >70% narrowing of the lumen compared to baseline (8) or in the presence of a clinical dysfunction, such as high pressure of the AV graft, and unstoppable bleeding. If the AV graft was thrombosed, thrombolysis using urokinase and/or mechanical thrombolysis were used for declotting without fistulography (7).
Once a stenosis was identified on fistulography, the puncture sites were anesthetized with lidocaine hydrochloride. General or regional anesthesia was not applied. The AV graft was accessed using an 18-gauge needle and 0.018-inch guidewire directed toward the stenotic site. A 7 F or 8 F vascular introducer sheath (Terumo and Cook Medical) was placed. After roadmap imaging was obtained using a collateral pore of the vascular introducer sheath, lesion degree, length, and location were identified. Heparin was not routinely infused into the graft. For stenoses at venous anastomotic site and central vein, 4 cm × 7–14 mm balloon catheters (Blue Max/XXL/Cutting Balloon, Boston Scientific and Conquest/Atlas, BARD) were used. The balloon was inflated using an Encore 26 manual inflation device (Boston Scientific) to a pressure of 5–30 atm for 1–2 min (Fig. 1). After PTA, an immediate roadmap imaging was obtained and evaluated for residual stenosis. In cases where the residual stenosis exceeded 30%, 1–2 mm larger balloon catheters were used along with the cutting balloon catheter or high-pressure balloon. After the procedure, the puncture site was sutured (9).
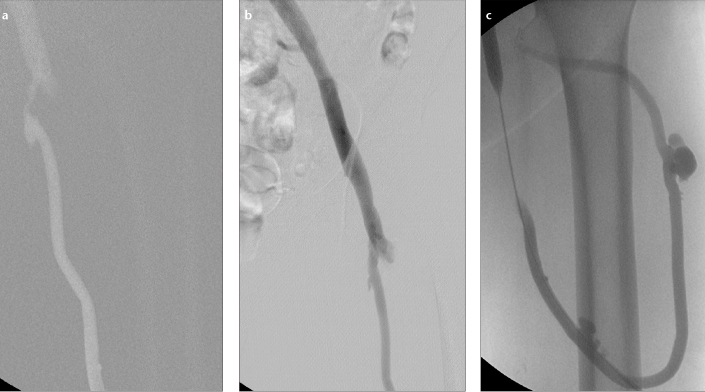
Figure 1. a–c.
Routine percutaneous transluminal angioplasty for dysfunctional femoral arteriovenous graft. Fistulography (a) before the procedure shows severe stenosis in venous anastomotic site. Postinterventional fistulography (b) shows no residual stenosis. Reflux fistulography (c) shows an intact arterial anastomotic site and a pseudoaneurysm at the needle puncture site in the graft.
If complete graft thrombosis was identified on physical examination, the apex puncture technique was used for U-loop hemodialysis grafts and the crossed-catheter technique was used for straight hemodialysis grafts. The graft loop was punctured with a micropuncture set (Cook Medical) and the Desilets-Hoffman sheath (Cook Medical) was advanced to the venous limb along a hydrophilic guidewire for thromboaspiration. While applying constant manual suction with a 10 mL syringe connected to the rear end of the sheath, the Desilets-Hoffman sheath was slowly withdrawn for thromboaspiration. A small amount of contrast was repeatedly injected and the thrombi were aspirated, during back and forth manipulation of the Desilets-Hoffman sheath within the hemodialysis graft. Aspiration was repeated until no further thrombi could be aspirated. Then, a 7/8 mm by 4 cm balloon catheter was advanced into the draining vein and 5000 IU of heparin was administered. Angiography of the draining vein was performed to assess the outflow stenosis and angioplasty was performed as required. After treatment of the venous limb was completed, thromboaspiration of the arterial limb was performed in the same manner. If an arterial plug remained at the arterial anastomosis, a 5 F Fogarty thrombectomy balloon catheter (Baxter) was passed through the arterial anastomosis, and the plug was pulled back into the midarterial limb of the hemodialysis graft. An angioplasty balloon was then placed over the plug to break it. Angiography of the entire hemodialysis graft was performed, and any residual stenosis or adherent thrombus was treated as required (10). Stent placement was indicated for >30% residual stenosis after PTA.
Definitions and complications
Primary and secondary patency rates of the AV graft after PTA were defined in accordance with reporting standards and quality improvement guidelines of the Society of Interventional Radiology (11). Primary patency was defined as patency from the time of primary intervention until fistula thrombosis or repeated radiologic intervention. Secondary patency was defined as patency from the time of primary intervention until the fistula is surgically declotted, revised, or abandoned; until the patient receives a renal transplant; or until the patient is lost to follow-up. The technical success of angioplasty was defined as a reduction of the stenosis to <30% of the luminal diameter for underlying significant stenosis (12). Central area stenoses were defined as those located in the native external iliac vein or common iliac vein. Peripheral area stenoses were defined as those located in the venous anastomotic site, arterial anastomotic site, and intragraft. Complications were categorized as major and minor in accordance with the published guidelines of the Society of Interventional Radiology (13).
Follow-up
All patients were recommended to visit the vascular surgery outpatient clinic every three months for a physical examination. If a clinical abnormality was identified, diagnostic fistulography was performed to check for AV graft dysfunction. Patient follow-up was terminated when the fistula was surgically revised, new AV access was made, or the patient died.
Statistical analysis
All available clinical and radiological records were reviewed retrospectively. Overall primary and secondary patency rates were obtained. Predictors of the primary patency were investigated based on the following variables: the presence of diabetes mellitus, graft age from the time of creation to the first intervention (<12 months or ≥12 months), presence of thrombus, shape of graft (U-shape vs. straight-shape), anastomosis type of graft (femoral-femoral vs. femoral-saphenous), location of stenosis (central area vs. peripheral area), length of stenosis (<2 cm vs. ≥2 cm), degree of stenosis severity (<70% vs. ≥70%), and presence of stent. Technical success rate and complications were investigated. Survival analysis was performed using the Kaplan-Meier curve and the log-rank test. P < 0.05 was considered to be statistically significant. The software used for statistical analysis was SPSS version 14.0 (SPSS Inc.).
Results
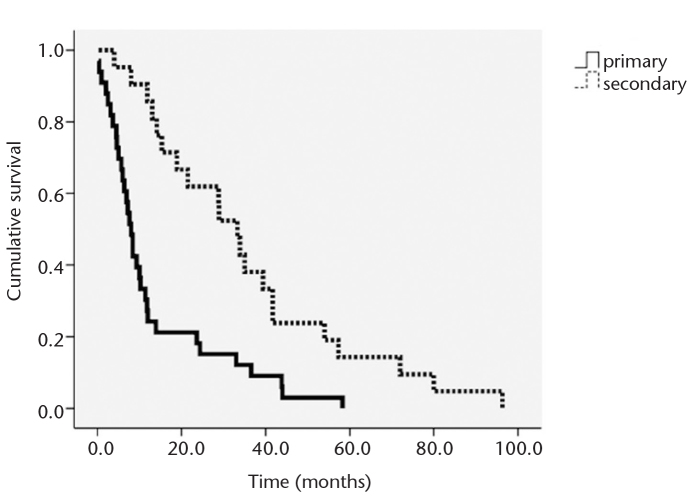
A total of 124 PTAs were performed in 45 patients as follows: one PTA in 21 patients (47%), two PTAs in four patients (9%), three PTAs in nine patients (20%), and four or more PTAs in 11 patients (24%). Technical success rate was 100%. The complication rate was 0.8%, representing one of 124 interventions, an anastomotic site dissection treated with stent insertion. The mean follow-up was 35.7±24.6 months (range, 4–96.3 months). For the femoral AV graft, the primary patency rate at three, six, and 12 months was 84.8%, 63.6%, and 24.2%, respectively. The secondary patency rate at three, six, and 12 months was 95.2%, 95.2%, and 85.7%, respectively (Fig. 2). Mean duration of primary patency was 13.2 months (median time, 8.0 months), while mean duration of secondary patency was 35.7 months (median time, 33.3 months). Predictors of graft patency are summarized in Table 1.
Figure 2.
Graph depicting the results of survival analysis for primary and secondary patency rates after percutaneous transluminal angioplasty on dysfunctional femoral arteriovenous graft.
Table 1.
Kaplan-Meier analysis for clinical and anatomic predictors of patency rate after percutaneous transluminal angioplasty
| Variable | n | Patency rate (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 3-month | 6-month | 12-month | P | |||
| Diabetes mellitus | Present | 18 | 83.3 | 66.7 | 25.0 | 0.876 |
| Absent | 27 | 76.9 | 76.9 | 15.4 | ||
| From surgery to first PTA | <12 months | 20 | 76.9 | 61.5 | 7.7 | 0.139 |
| ≥12 months | 25 | 88.9 | 61.1 | 27.8 | ||
| Thrombosis | Present | 24 | 81.3 | 62.5 | 25.0 | 0.886 |
| Absent | 21 | 88.2 | 64.7 | 23.5 | ||
| Graft shape | U-shaped | 36 | 88.5 | 65.4 | 30.8 | 0.313 |
| Straight | 9 | 71.4 | 57.1 | 0.0 | ||
| Anastomosis type | FE-FE | 30 | 83.3 | 58.3 | 20.8 | 0.654 |
| FE-SA | 15 | 88.9 | 77.8 | 33.3 | ||
| Stenosis location | Central* | 8 | 87.5 | 50.0 | 25.0 | 0.339 |
| Peripheral | 37 | 84.0 | 68.0 | 24.0 | ||
| Stenosis length | ≤2 cm | 17 | 80.0 | 50.0 | 20.0 | 0.654 |
| >2 cm | 28 | 90.5 | 71.4 | 28.6 | ||
| Stenosis severity | <70% | 20 | 81.8 | 72.7 | 18.2 | 0.347 |
| ≥70% | 25 | 90.0 | 60.0 | 30.0 | ||
| Stent insertion | Present | 9 | 88.9 | 44.4 | 0.0 | 0.016 |
| Absent | 36 | 83.3 | 70.8 | 33.3 | ||
PTA, percutaneous transluminal angioplasty; FE-FE, femoral-femoral type; FE-SA, femoral-saphenous type.
External iliac vein or common iliac vein.
Stents were inserted in nine of 124 procedures. Indications of stent placement were elastic recoils in eight cases and anastomotic site dissection in one case. Location of stent placement was venous anastomotic site in six cases, external iliac vein in two cases, and common iliac vein in one case. All venous anastomotic sites requiring stent insertion were of femoral-femoral anastomosis graft type .
Presence of diabetes mellitus (P = 0.876), graft age from the time of creation to the first intervention (P = 0.139), presence of thrombus (P = 0.886), shape of graft (P = 0.313), anastomosis type of graft (P = 0.654), location of stenosis (P = 0.339), length of stenosis (P = 0.654), or degree of stenosis (P = 0.347) were not significant predictors of primary patency. Stent placement after PTA was determined to have a negative predictive value on primary patency (P = 0.016).
Discussion
Our study shows that after PTA, primary and secondary patency rates were similar to the outcomes observed by previous studies on upper limb hemodialysis access (Table 2) (12, 14–17). Therefore, PTA is an effective treatment for dysfunctional femoral AV graft, as it is for the upper limb AV graft.
Table 2.
Review of reported patency rates after percutaneous transluminal angioplasty of nonthrombosed and thrombosed hemodialysis fistulas in upper extremities
| Fistula type | No. of fistulas | Success rate (%) | Primary patency (%) | Secondary patency (%) | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 6-month | 12-month | 6-month | 12-month | ||||
| Nonthrombosed hemodialysis fistulas | |||||||
| Clark et al. (14) | Forearm, upper arm | 53 | 94 | 55 | 26 | 82 | 82 |
| Manninen et al. (12) | Forearm | 53* | 91 | 58 | 44 | 90 | 85 |
| Lay et al. (15) | Forearm | 31 | 90 | 77 | 64 | 85 | 81 |
| Turmel-Rodrigues et al. (16) | Forearm | 155 | 95 | 67 | 51 | ND | 85 |
| Upper arm | 65 | 97 | 57 | 35 | ND | 82 | |
| Thrombosed hemodialysis fistulas | |||||||
| Turmel-Rodrigues et al. (16) | Forearm | 54 | 94 | 74 | 47 | ND | 80 |
| Upper arm | 9 | 78 | 27 | 27 | ND | 65 | |
| Haage et al. (17) | Forearm, upper arm | 54 | 89 | 52 | 27 | ND | ND |
ND, not determined.
Includes 12 thrombosed fistulas.
In addition, our results show that dysfunctional AV grafts treated with PTA have better technical success and patency rates than previously published. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-DOQI) guideline recommends that, after PTA, primary patency rates should be at least 50% at six months for dysfunctional AV grafts with only stenosis and at least 40% at three months for dysfunctional AV grafts with thrombus (18). The present finding of six-month primary patency rate of 64.7% for dysfunctional femoral AV grafts with stenosis and 62.5% for those with thrombosis exceeds the National Kidney Foundation expectations. These results likely reflect the innovations in the interventional devices (e.g., high pressure balloon or cutting balloon) and the medical treatments for prevention of infection over the decade since the study of Peirce et al. (1).
Permanent vascular access is very important for hemodialysis in patients with chronic renal failure. In general, radiocephalic AV fistula is the first vascular access, followed by brachiocephalic AV fistula (19–22). If there is no suitable superficial vein, prosthetic AV fistula or brachiobasilic AV fistula with basilic vein transposition are required (23, 24). However, if the upper limb use is not appropriate, axillary-axillary AV access (necklace graft) (25), superficial femoral vein transposition (26), axillary artery to right atrial graft (27), hemodialysis reliable outflow graft (28), and femoral AV graft could be used as alternative choices for vascular access (19–21). Autogenous AV fistula or graft AV fistula are available for femoral AV access, although the former is not preferred because of wide incision and associated complications (19, 29, 30).
The femoral AV graft is longer than the upper arm graft and is easy to cannulate (1). Blood flow is increased in the femoral AV graft, and the incidence of thrombosis or stenosis is low with maintenance of patency (31). The location of the femoral AV graft is usually invisible, which preserves patients privacy (2).The femoral AV graft has good durability demonstrating longer primary patency after surgery than upper extremity grafts (4, 32, 33).
Early after the introduction of the femoral AV graft, the mortality rate was high and amputation of lower extremities due to infection was frequent. Morgan et al. (2) described their experience using femoral vessels for dialysis access in 1980. In that study, 27 of 161 patients experienced infection that resulted in lower limb amputation in 22% and mortality in 18%. At our institution, there have been no infections of femoral AV grafts. Generally, the infection rate has declined because of the prophylactic use of antibiotics prior to surgery or procedure (1). Furthermore, for sterilization of the femoral triangle, topical disinfectant is used before surgery, procedure, and dialysis. These efforts have progressively improved femoral AV graft viability (1). The incidence of infection and thrombus has become similar in lower and upper extremity grafts (3–5).
Lilly et al. (7) reported that primary patency rate was significantly higher in AV grafts with stenosis rather than thrombus. In addition, the degree of stenosis and the ratio of intragraft to systolic blood pressure were factors affecting the primary patency rate in their study. Rajan et al. (34) reported that no clinical or anatomic variables affected the patency outcome. In our study, there was no significant difference in the primary patency rate based on various clinical and anatomic predictors.
Metallic stents may be used in case of elastic recoil of the vessel wall or dissection after PTA. In our study, stents were placed in nine of 124 procedures. The indications of stent placement were elastic recoil in eight cases and anastomotic site dissection in one case. Location of stent was venous anastomotic site in six cases, external iliac vein in two cases, and common iliac vein in one case. Funaki et al. (6) reported that stent placement is useful in lower extremity elastic vein stenoses in patients with thigh hemodialysis grafts. In this study, stent placement improved the technical success, but it was not effective to maintain the patency on dysfunctional femoral AV grafts. This was because patients requiring stent placement had more elastic lesions than the PTA-only group. However, the small number of patients with stents and patient selection bias may have influenced the results.
To our knowledge, there have been no reports on stent graft placement for dysfunctional femoral AV graft until now. In the upper extremities, the use of stent graft provides longer term and superior patency than standard balloon angioplasty contrary to our findings (35).
The main limitation of the present study is the inappropriate setting of the comparison group. Patients included in the stent insertion group were refractory to PTA and naturally registered a poor outcome after stent insertion. Another limitation is that we did not use the same type of balloon in all patients, because the data were collected over a long stretch of time. Other limitations include the retrospective design and the small sample size.
In conclusion, PTA is an effective treatment for dysfunctional femoral AV grafts. Stent placement seems to improve the technical success, but does not improve the primary patency of dysfunctional femoral AV grafts. No clinical or anatomic factor affected the patency outcome.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Peirce RM, Funaki B, Van Ha TG, Lorenz JM. Percutaneous declotting of virgin femoral hemodialysis grafts. AJR Am J Roentgenol. 2005;185:1615–1619. doi: 10.2214/AJR.04.0693. [DOI] [PubMed] [Google Scholar]
- 2.Morgan AP, Knight DC, Tilney NL, Lazarus JM. Femoral triangle sepsis in dialysis patients: frequency, management, and outcome. Ann Surg. 1980;191:460–464. doi: 10.1097/00000658-198004000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korzets A, Ori Y, Baytner S, et al. The femoral artery-femoral vein polytetrafluoroethylene graft: a 14-year retrospective study. Nephrol Dial Transplant. 1998;13:1215–1220. doi: 10.1093/ndt/13.5.1215. [DOI] [PubMed] [Google Scholar]
- 4.Tashjian DB, Lipkowitz GS, Madden RL, et al. Safety and efficacy of femoral-based hemodialysis access grafts. J Vasc Surg. 2002;35:691–693. doi: 10.1067/mva.2002.121750. [DOI] [PubMed] [Google Scholar]
- 5.Flarup S, Hadimeri H. Arteriovenous PTFE dialysis access in the lower extremity: a new approach. Ann Vasc Surg. 2003;17:581–584. doi: 10.1007/s10016-003-0052-8. [DOI] [PubMed] [Google Scholar]
- 6.Funaki B, Szymski GX, Leef JA, et al. Treatment of venous outflow stenoses in thigh grafts with Wallstents. AJR Am J Roentgenol. 1999;172:1591–1596. doi: 10.2214/ajr.172.6.10350295. [DOI] [PubMed] [Google Scholar]
- 7.Lilly RZ, Carlton D, Barker J, et al. Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis. 2001;37:945–953. doi: 10.1016/s0272-6386(05)80010-1. [DOI] [PubMed] [Google Scholar]
- 8.Polytimi L, Sofia G, Paris P. Close to transplant renal artery stenosis and percutaneous transluminal treatment. J Transplant. 2011;2011:219109. doi: 10.1155/2011/219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesely TM. Use of a purse string suture to close a percutaneous access site after hemodialysis graft interventions. J Vasc Interv Radiol. 1998;9:447–450. doi: 10.1016/s1051-0443(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 10.Goo DE, Kim YJ, Park ST, Yang SB, Yoon SC, Song D. Thromboaspiration of arteriovenous hemodialysis graft thrombosis using Desilets-Hoffman sheath: single-center experience. J Vasc Access. 2014;15:401–408. doi: 10.5301/jva.5000221. [DOI] [PubMed] [Google Scholar]
- 11.Gray RJ, Sacks D, Martin LG, Trerotola SO. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol. 2003;14:S433–S442. doi: 10.1097/01.rvi.0000094618.61428.58. [DOI] [PubMed] [Google Scholar]
- 12.Manninen HI, Kaukanen ET, Ikaheimo R, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas--initial success and long-term results. Radiology. 2001;218:711–718. doi: 10.1148/radiology.218.3.r01mr38711. [DOI] [PubMed] [Google Scholar]
- 13.Sacks D, Marinelli DL, Martin LG, Spies JB. Reporting standards for clinical evaluation of new peripheral arterial revascularization devices. Technology Assessment Committee. J Vasc Interv Radiol. 1997;8:137–149. doi: 10.1016/s1051-0443(97)70530-x. [DOI] [PubMed] [Google Scholar]
- 14.Clark TW, Hirsch DA, Jindal KJ, Veugelers PJ, LeBlanc J. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol. 2002;13:51–59. doi: 10.1016/s1051-0443(07)60009-8. [DOI] [PubMed] [Google Scholar]
- 15.Lay JP, Ashleigh RJ, Tranconi L, Ackrill P, Al-Khaffaf H. Result of angioplasty of brescia-cimino haemodialysis fistulae: medium-term follow-up. Clin Radiol. 1998;53:608–611. doi: 10.1016/s0009-9260(98)80155-4. [DOI] [PubMed] [Google Scholar]
- 16.Turmel-Rodrigues L, Pengloan J, Baudin S, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant. 2000;15:2029–2036. doi: 10.1093/ndt/15.12.2029. [DOI] [PubMed] [Google Scholar]
- 17.Haage P, Vorwerk D, Wildberger JE, Piroth W, Schurmann K, Gunther RW. Percutaneous treatment of thrombosed primary arteriovenous hemodialysis access fistulae. Kidney Int. 2000;57:1169–1175. doi: 10.1046/j.1523-1755.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48:S1–S322. [Google Scholar]
- 19.Antoniou GA, Lazarides MK, Georgiadis GS, Sfyroeras GS, Nikolopoulos ES, Giannoukas AD. Lower-extremity arteriovenous access for haemodialysis: a systematic review. Eur J Vasc Endovasc Surg. 2009;38:365–372. doi: 10.1016/j.ejvs.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–610. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GJ, White SA, Nicholson ML. Vascular access for haemodialysis. Br J Surg. 2000;87:1300–1315. doi: 10.1046/j.1365-2168.2000.01579.x. [DOI] [PubMed] [Google Scholar]
- 22.Ascher E, Hingoran A, Gunduz Y, et al. The value and limitations of the arm cephalic and basilic vein for arteriovenous access. Ann Vasc Surg. 2001;15:89–97. doi: 10.1007/s100160010002. [DOI] [PubMed] [Google Scholar]
- 23.Rivers SP, Scher LA, Sheehan E, Lynn R, Veith FJ. Basilic vein transposition: an underused autologous alternative to prosthetic dialysis angioaccess. J Vasc Surg. 1993;18:391–397. [PubMed] [Google Scholar]
- 24.Dagher FJ, Gelber RL, Ramos EJ, Sadler JH. Basilic vein to brachial artery fistula: a new access for chronic hemodialysis. South Med J. 1976;69:1438–1440. doi: 10.1097/00007611-197611000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Morsy MA, Khan A, Chemla ES. Prosthetic axillary-axillary arteriovenous straight access (necklace graft) for difficult hemo-dialysis patients: a prospective single-center experience. J Vasc Surg. 2008;48:1251–1254. doi: 10.1016/j.jvs.2008.06.064. [DOI] [PubMed] [Google Scholar]
- 26.Chemla ES, Morsy M, Anderson L, Makanjuola D. Complex bypasses and fistulas for difficult hemodialysis access: a prospective, single-center experience. Semin Dial. 2006;19:246–250. doi: 10.1111/j.1525-139X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 27.Kopriva D, Moustapha A. Axillary artery to right atrium shunt for hemodialysis access in a patient with advanced central vein thrombosis: a case report. Ann Vasc Surg. 2006;20:418–421. doi: 10.1007/s10016-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 28.Steerman SN, Wagner J, Higgins JA, et al. Outcomes comparison of HeRO and lower extremity arteriovenous grafts in patients with long-standing renal failure. J Vasc Surg. 2013;57:776–783. doi: 10.1016/j.jvs.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 29.Gradman WS, Cohen W, Haji-Aghaii M. Arteriovenous fistula construction in the thigh with transposed superficial femoral vein: our initial experience. J Vasc Surg. 2001;33:968–975. doi: 10.1067/mva.2001.115000. [DOI] [PubMed] [Google Scholar]
- 30.Gradman WS, Laub J, Cohen W. Femoral vein transposition for arteriovenous hemodialysis access: improved patient selection and intraoperative measures reduce postoperative ischemia. J Vasc Surg. 2005;41:279–284. doi: 10.1016/j.jvs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Culp K, Flanigan M, Taylor L, Rothstein M. Vascular access thrombosis in new hemodialysis patients. Am J Kidney Dis. 1995;26:341–346. doi: 10.1016/0272-6386(95)90655-x. [DOI] [PubMed] [Google Scholar]
- 32.Huber TS, Carter JW, Carter RL, Seeger JM. Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg. 2003;38:1005–1011. doi: 10.1016/s0741-5214(03)00426-9. [DOI] [PubMed] [Google Scholar]
- 33.Khadra MH, Dwyer AJ, Thompson JF. Advantages of polytetrafluoroethylene arteriovenous loops in the thigh for hemodialysis access. Am J Surg. 1997;173:280–283. doi: 10.1016/S0002-9610(96)00405-9. [DOI] [PubMed] [Google Scholar]
- 34.Rajan DK, Bunston S, Misra S, Pinto R, Lok CE. Dysfunctional autogenous hemodialysis fistulas: outcomes after angioplasty--are there clinical predictors of patency? Radiology. 2004;232:508–515. doi: 10.1148/radiol.2322030714. [DOI] [PubMed] [Google Scholar]
- 35.Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362:494–503. doi: 10.1056/NEJMoa0902045. [DOI] [PubMed] [Google Scholar]