Abstract
Sarcoidosis is a multisystem inflammatory disease of unknown etiology. The lungs and the lymphoid system are the most commonly involved organs. Extrapulmonary involvement is reported in 30% of patients, and the abdomen is the most common extrapulmonary site with a frequency of 50%–70%.
Although intra-abdominal sarcoidosis is usually asymptomatic, its presence may affect the prognosis and treatment options. The lesions are less characteristic and may mimick neoplastic or infectious diseases such as lymphoma, diffuse metastasis, and granulomatous inflammation. The liver and spleen are the most common abdominal sites of involvement. Sarcoidosis of the gastrointestinal system, pancreas, and kidneys are extremely rare. Adenopathy which is most commonly found in the porta hepatis, exudative ascites, and multiple granulomatous nodules studding the peritoneum are the reported manifestations of abdominal sarcoidosis. Since abdominal sarcoidosis is less common and long-standing, unrecognized disease can result in significant morbidity and mortality. Imaging contributes to diagnosis and management of intra-abdominal sarcoidosis. In this report we reviewed the cross-sectional imaging findings of hepatobiliary, gastrointestinal, and genitourinary sarcoidosis.
Sarcoidosis is a multisystemic inflammatory disease of unknown etiology. It is characterized by noncaseating epithelioid cell granulomas in the absence of other granulomatous diseases such as tuberculosis, fungal infections, autoimmune processes, or delayed-type hypersensitivity reaction to foreign antigens (1). Epidemiology of the disease varies among racial groups. The global prevalence is reported to be 2–60 per 100,000 people (2). Higher rates are detected in people of Scandinavian and Irish descent (2).
Sarcoidosis is usually diagnosed between 20 and 40 years of age (3). Women are more frequently affected than men (3). The etiology of the disease is still not exactly clarified. The lungs and the lymphoid system are the most commonly involved sites with a frequency of 90% and 30%, respectively (4). Extrapulmonary involvement of sarcoidosis is reported in 30% of patients and the abdomen is the most common extra-thoracic site with a frequency of 50%–70%. Liver (50%–80%), spleen (40%–80%), lymph nodes (30%), and kidney are frequently involved abdominal sites, sometimes without symptoms. Cardiopulmonary involvement is the main cause of death.
Diagnosis of sarcoidosis is based upon clinical features and demonstration of noncaseating granulomas in at least two different organs. A negative staining and culture for acid fast bacilli, exclusion of drug-induced disease and exposure to toxins are also included in the diagnostic criteria. The overall prognosis is good, although most patients would have permanent organ impairment. Corticosteroids and steroid-sparing agents such as methotrexate, azathioprine, and anti-TNF-α are instituted for immunosuppressive treatment when major organs are involved or organ function is threatened.
Abdominal sarcoidosis can occur in the absence of lymphatic or pulmonary disease (4). Although usually asymptomatic, the presence of symptomatic abdominal involvement may affect the prognosis and treatment options. Symptomatic abdominal sarcoidosis requires treatment with immunosuppressant agents. Surgical interventions may be required in the presence of gastrointestinal complications such as massive hemorrhage, strictures, obstruction, or perforation. Splenectomy can be performed for symptomatic relief in splenic involvement or as prophylaxis for splenic rupture.
The lesions in abdominal sarcoidosis are less characteristic, mimicking more common neoplastic or infectious diseases such as lymphoma, diffuse metastasis, granulomatous or mycobacterial infections (5). Furthermore, it is reported that a significant fraction (26%) of subjects may have hepatic sarcoidosis without pulmonary involvement (6). In this article we aimed to review the imaging manifestations of abdominal sarcoidosis.
Liver and biliary tract
Hepatic involvement of sarcoidosis follows lymph nodes and lung in frequency. It has been reported in 50%–79% of patients by biopsy, and 67%–70% in autopsy series (7). Despite this common hepatic involvement, the frequency of liver function test abnormalities is about 35% (8). Liver biopsy is the only way to make a definitive diagnosis of hepatic involvement (4). Less than 5% of patients with sarcoidosis suffer from symptomatic liver disease.
Hepatomegaly is the most common imaging finding of hepatic sarcoidosis, detected on computed tomography (CT) of the abdomen in about more than half of the patients (9). It is often associated with splenomegaly. Other findings range from asymptomatic incidental granulomas becoming more confluent with increasing size, to portal hypertension and cirrhosis because of chronic inflammation, granuloma formation, and fibrosis in the portal triads. Focal nodules are seen in the range of 0%–19% (10–12). Another frequent complication of hepatic sarcoidosis is the portal vein thrombosis as a result of stasis from obliterated small portal veins (13). It has also been proposed that there is a correlation between chronic hepatic sarcoidosis and hepatocellular carcinoma (14).
Granulomatous inflammation of the gallbladder or extrinsic compression of the cystic duct by enlarged lymph nodes may lead to acute cholecystitis (15). Subacute or chronic cholecystitis due to granulomas in the gallbladder wall has also been reported as a complication of biliary sarcoidosis (16). Granulomatous involvement of the common hepatic duct and accompanying enlarged granulomatous lymph nodes are reported leading to obstructive jaundice (17). Sarcoidosis may cause strictures in extrahepatic bile ducts mimicking cholangiocarcinoma. Several case series have implied that, there is an association between sarcoidosis and sclerosing cholangitis (18).
Ultrasonography (US) findings of hepatic sarcoidosis include increased parenchymal echogenicity and coarsening of the parenchymal appearance with or without discrete nodules (Fig. 1) (19). Calcification can rarely be seen if the disease is long-standing (19). The nodules which represent the coalescence of small granulomas are typically innumerable, diffusely distributed, and range from 1–2 mm to several centimeters in size. They have been reported to be hypoechoic on US and hypodense on CT scans, relative to the liver parenchyma (Fig. 2) (19). However, they may also be hyperechoic depending on background liver echogenicity and the degree of fibrosis present in the granuloma. Because of these nonspecific imaging findings, tissue biopsy may be necessary to differentiate hepatic sarcoidosis from metastases and lymphoma (20). Focal nodules are identified in 5% of patients at imaging examinations (Fig. 3) (21). Contour irregularity can also be seen in sarcoidosis (Fig. 4) (19).
Figure 1.
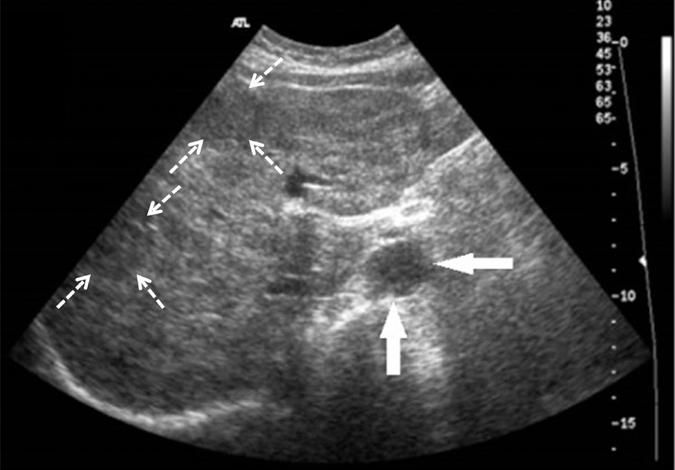
Axial US image of a 59-year-old female with biopsy-proven hepatic sarcoidosis demonstrates innumerable, diffusely distributed hypoechoic granulomas (dashed arrows) in an enlarged hyperechoic liver. A periportal hypoechoic enlarged lymph node (arrows) is also detected at the hilum of the liver.
Figure 2.
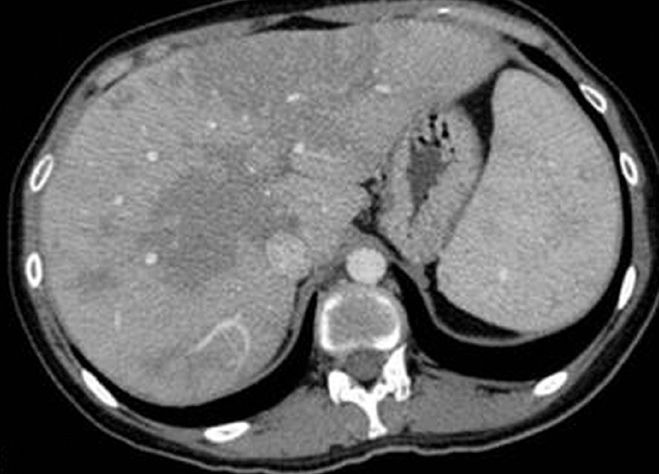
Axial contrast-enhanced CT image of a 23-year-old female with biopsy-proven hepatosplenic sarcoidosis shows coalescence of granulomas in the form of large, geographic, hypoechoic, hypodense areas in an enlarged liver and the spleen.
Figure 3.
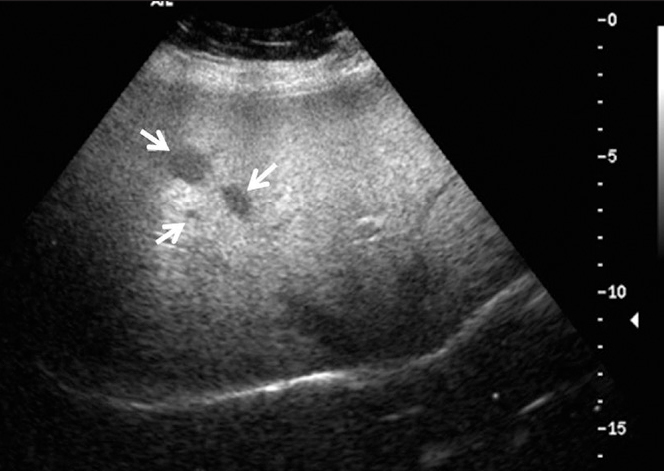
Liver US of a 52-year-old female demonstrates three hypoechoic nodules (arrows) in the right lobe of the liver representing noncaseating granulomas proven by biopsy.
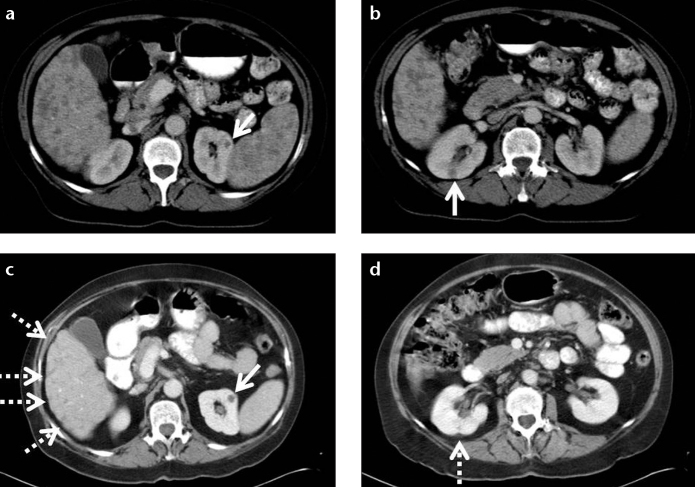
Figure 4. a, b.
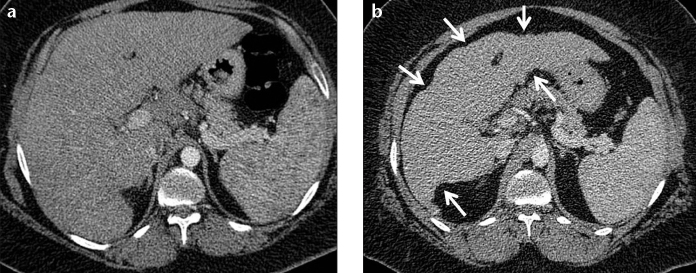
A 41-year-old female with biopsy-proven hepatosplenic sarcoidosis. Contrast-enhanced axial CT image (a) and follow-up CT examination after three years (b). Contour irregularity of the shrinking liver became prominent within three years (b, arrows).
On MRI, nodular lesions of 5–20 mm are characteristically hypointense on all sequences which are most apparent on the T2-weighted fat-saturated images (Fig. 5a) (22). This finding helps excluding metastases and inflammatory diseases which are usually hyperintense on T2-weighted fat-saturated images. They enhance less than the background liver on gadolinium-enhanced T1-weighted images (Fig. 5b) (22). Contour irregularity of the liver and high periportal signal intensity are the other reported MRI findings of hepatic sarcoidosis (19).
Figure 5. a, b.
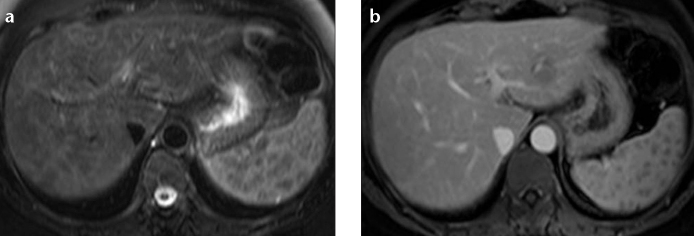
A 50-year-old female with biopsy-proven hepatosplenic sarcoidosis. T2-weighted fat-saturated images show hypointense lesions in the liver and spleen (a). The lesions enhance less than the background liver on gadolinium-enhanced T1-weighted images (b).
Spleen
Splenic involvement has been reported in 24%–53% of cases (23). Splenomegaly due to sarcoidosis is commonly associated with involvement of the lungs and the liver. Splenic infiltration can be homogeneous or in the form of multiple discrete nodules (24, 25). Splenic nodules can be seen in 15% of the abdominal CT scans and are more common than the hepatic nodules (21). The differential diagnosis of hypodense nodules in both liver and spleen includes tuberculosis, lymphoma, metastasis, and abscess. Infections such as candidiasis should also be considered if the patient is immunocompromised.
Similar to hepatic nodules, the splenic nodules are seen as multiple, hypoechoic, hypodense, hypointense, and nonenhancing lesions, scattered in the spleen (Figs. 6, 7a). They tend to be discrete, but may coalesce as they increase in size (22). Contour irregularity is another abnormal finding of splenic sarcoidosis (Fig. 7b) (19).
Figure 6.
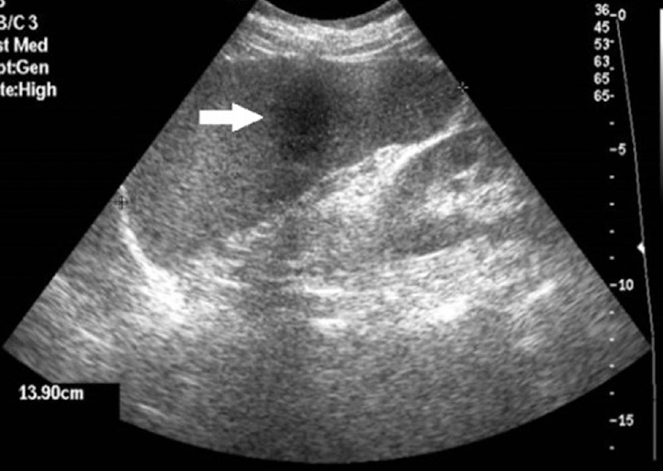
Sagittal US image of a 59-year-old female with proven splenic sarcoidosis shows a 3.5 cm hypoechoic, focal lesion (arrow) in an enlarged spleen, due to a noncaseating granuloma.
Figure 7. a, b.
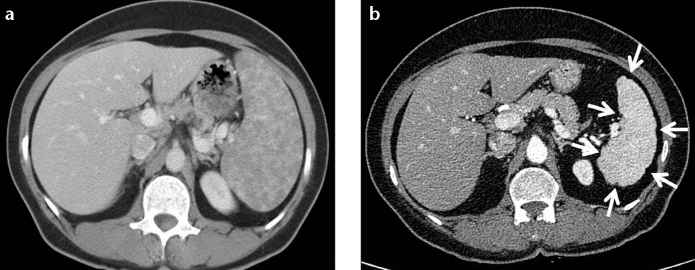
A 45-year-old female with proven splenic sarcoidosis. Contrast-enhanced CT of the abdomen shows multiple hypodense granulomas infiltrating the enlarged spleen (a). Contour irregularity of the shrinking spleen became prominent within six-years follow-up (b, arrows).
Gastrointestinal tract
Gastrointestinal (GI) tract involvement in sarcoidosis is extremely rare and the majority of the cases are asymptomatic. On autopsy series, about 10% of patients with systemic sarcoidosis were found to have gastric granulomatous mucosal infiltration, most commonly in the antrum (26). Differential diagnosis of gastrointestinal sarcoidosis includes Crohn’s disease, malignancies, gastritis, and infectious diseases such as tuberculosis and fungal infections, and a definitive diagnosis requires biopsy (26).
Gastric involvement in sarcoidosis can be localized or diffuse. In the localized form, granulomatous infiltration of the mucosa leading to ulcer formation, gastritis, hyperemia with friable mucosa, or nodular irregularities resembling polyps are seen (4). In diffuse form, fibrosis due to granulomatous infiltration, widespread mucosal thickening, rigidity, and reduction in lumen size have been reported (26–28). Antral narrowing and deformity owing to gastric mucosal fold enlargement may lead to gastric outlet obstruction mimicking Menetrier’s disease (29, 30). The most common imaging finding of upper GI involvement is a segmental linitis plastica-type appearance which should be differentiated from gastric carcinoma (29). Extrinsic compression from extensively enlarged retroperitoneal lymph nodes may also occur throughout the GI tract.
Small bowel is the least common site of involvement in GI sarcoidosis (27). Reported manifestations of intestinal sarcoidosis include intestinal obstruction due to extrinsic compression by enlarged mesenteric and abdominal lymph nodes or reduction of the lumen size secondary to cicatrizing constriction of mucosal granulomatous lesions (Fig. 8) (4, 31). Differential diagnosis of small bowel sarcoidosis includes Crohn’s disease, carcinoma, foreign body reactions, intestinal tuberculosis, Whipple’s disease, and celiac disease. Elevated angiotensin converting enzyme levels, superficial mucosal involvement instead of transmural inflammation, absence of perianal disease, fistulas, or strictures may favor the diagnosis of sarcoidosis (32).
Figure 8. a–c.
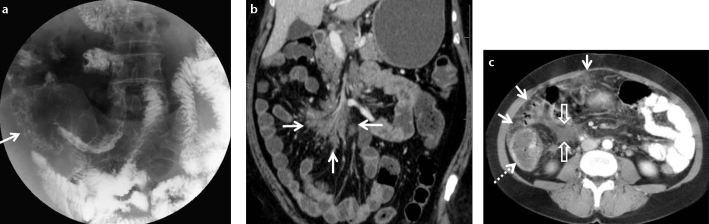
Barium small bowel follow-through (a) shows mucosal irregularity, nodularity, and angulation of terminal ileum and ileocecal region (arrow) in a 62-year-old female patient with biopsy-proven sarcoidosis. Subsequent contrast-enhanced CT examination (b) demonstrates multiple lymph nodes in the mesenteric root (arrows). Panel (c) shows wall thickening of the caecum (dashed arrow), heterogeneous hyperdensity of the omentum (arrows) and mesenteric free fluid (open arrows).
Sigmoid colon is the most frequently involved site of large bowel (27). Colorectal sarcoidosis can occur despite the presence of a grossly normal appearing mucosa. Multiple nodules, polyps, aphthous erosions, ulcers, obstructive lesions, or stenosis are the imaging findings of manifest colonic sarcoidosis (Fig. 8) (33–36). External compression by enlarged lymph nodes is the most common reason of intestinal obstruction.
Sarcoidosis has been reported to be one of the causes of noninfectious granulomatous appendicitis, along with foreign body reaction and Crohn’s disease. A markedly enlarged, unopacified appendix of soft tissue density, larger than that is seen in “simple acute appendicitis” and indistinguishable walls may be the findings on CT (Fig. 9). The absence of fat stranding or periappendiceal fluid and the presence of enlarged lymph nodes in a subject with distended appendix should alert radiologists for the possibility of a granulomatous appendicitis, carcinoma, or lymphoma (37).
Figure 9. a–c.
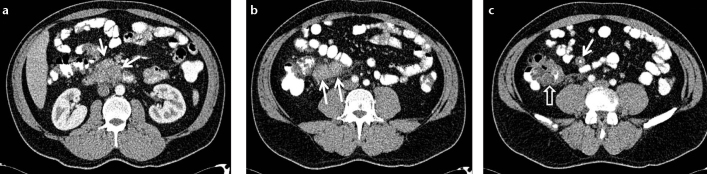
Contrast-enhanced abdominal CT of a 38-year-old male suffering from cough and abdominal pain. Enlarged lymph nodes in the mesenteric root (a, arrows), a markedly enlarged appendix with thickening of the wall (b, c, arrows) and thickening of the caecal apex (c, open arrow) are seen. Bronchoscopic and colonoscopic biopsies revealed chronic granulomatous inflammation confirming the diagnosis of pulmonary and gastrointestinal sarcoidosis.
Peritoneum and lymph nodes
Peritoneal involvement is extremely rare in sarcoidosis (38, 39). The most frequent clinical presentations of peritoneal sarcoidosis are exudative ascites, multiple granulomatous nodules studding the peritoneum, or a single peritoneal lesion (Fig. 8). Peritoneal biopsy is required to rule out carcinomatosis, tuberculosis, and fungal infections (4, 31). Symptoms usually regress without any treatment or with short-term treatment of corticosteroids (39).
Enlarged lymph nodes are detected in approximately 30% of patients particularly in the porta hepatis, para-aortic region, and the celiac axis (9, 21). Unlike lymphoma, the lymph nodes are tpically smaller than 2 cm in diameter and more discrete rather than confluent. Compared to lymphoma, involvement of the retrocrural area is less common (9). Affected lymph nodes show soft tissue attenuation on CT (Fig. 10). They appear homogenously hyperintense or may resemble central hypointensity surrounded by peripheral high signal intensity on T2-weighted fat-saturated images. They are mildly enhanced on gadolinium-enhanced T1-weighted images (Fig. 11) (40).
Figure 10. a, b.
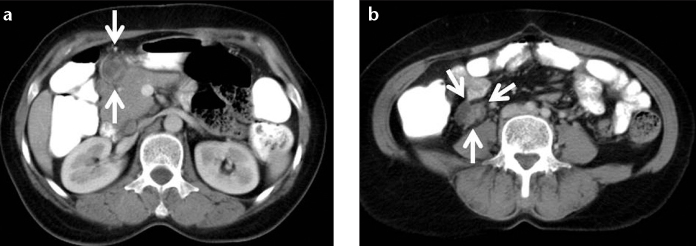
A 62-year-old female with biopsy-proven abdominal sarcoidosis. Contrast-enhanced CT of the abdomen shows peripancreatic (a) and pericecal (b) enlarged hypodense lymph nodes with peripheral and heterogeneous enhancement.
Figure 11.
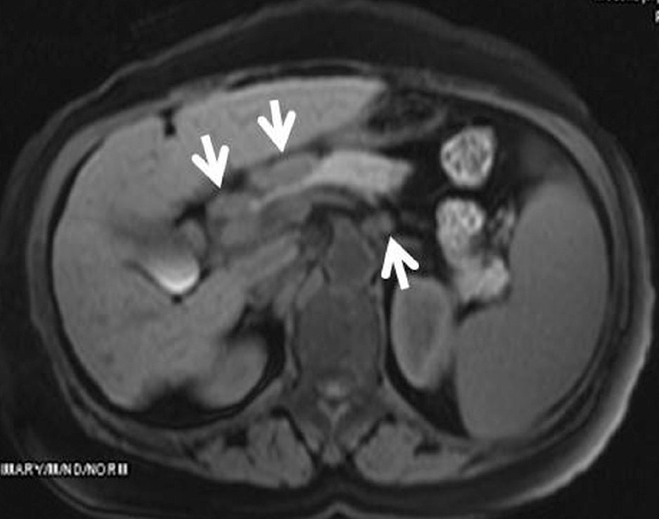
A 58-year-old female with biopsy-proven abdominal sarcoidosis. Gadolinium-enhanced T1-weighted image shows enlarged and discrete lymph nodes smaller than 2 cm in diameter detected in the porta hepatis, peripancreatic region, and celiac axis (arrows).
Pancreas
Pancreatic sarcoidosis has been detected in 1%–3% of cases with systemic disease on autopsy series. It may lead to symptoms such as abdominal pain, nausea and vomiting, weight loss, and obstructive jaundice; however, it is rarely symptomatic during life (41). Sarcoidosis of pancreas may be in the form of direct tissue infiltration, duct obstruction, or constrictive peripancreatic lymphadenopathy. Tissue infiltration has been reported to be diffusely nodular in about half of the cases (42). In the other half, a focal pancreatic mass is seen usually in the head of the pancreas; this mass mimics pancreatic cancer and requires surgical resection for differential diagnosis (Fig. 12) (42). However, cholangiograms typically show a long, smoothly tapered narrowing rather than the more abrupt termination associated with tumor (43).
Figure 12. a, b.
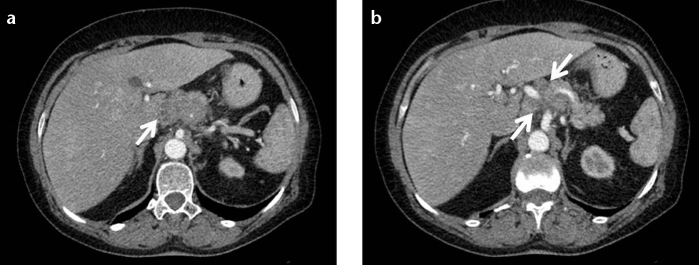
A 63-year-old female with biopsy-proven pancreatic sarcoidosis. Contrast-enhanced CT of the abdomen (a) shows enlarged lobulated pancreas. Ill-defined multiple small hypodense lesions are detected in the pancreatic head and body (b, arrows).
On CT scans, pancreatic sarcoidosis manifests as solitary ill-defined pancreatic masses, dilatation of the common bile duct and pancreatic duct, or peripancreatic lymphadenopathy. On MRI, T1-weighted images show multiple hypointense masses, T2-weighted images show mildly elevated signal intensity, and gadolinium-based intravenous contrast administration shows reduced enhancement compared to normal pancreas (44).
Kidney
Nephrocalcinosis, nephrolithiasis, and interstitial calcium deposition which may lead to renal failure have been reported in sarcoidosis. Interstitial nephritis is a possible manifestation, and may demonstrate a striated nephrogram on contrast-enhanced CT (45). The most frequently detected effect of sarcoidosis on the kidney is nephrocalcinosis. It results from hyper-calcemia and hypercalciuria secondary to production of calcitriol typically by the extrarenal granulomata (20, 46). In the presence of nephrocalcinosis, corticosteroids are used in addition to management of hypercalcemia and hypercalciuria.
Direct granulomatous involvement of the kidneys is rarely observed; however, it is indistinguishable from lymphoma or metastasis (47). Glomerular nephritis from direct infiltration may also occur, although it usually does not impair renal function (46). Granulomatous pseudotumors are exceedingly rare presentations of renal sarcoidosis with only a few pathologically-proven case reports (48, 49). These pseudotumors are focal, exophytic nodules which may be singular or multiple, unilateral or bilateral and echogenic on US (42). They may be hypo-, iso-, or hyperdense on unenhanced CT relative to the normal renal parenchyma, and but are hypo-enhancing on contrast-enhanced CT (Fig. 13) (48–50). On MRI, poor circumscription of the mass or masses from the renal parenchyma is demonstrated, indicating interstitial infiltration. On unenhanced T1- and T2-weighted imaging, the pseudotumor may be homogenous or slightly heterogenous, predominantly remaining isointense to the surrounding renal parenchyma (48–50). There is less early and delayed enhancement relative to the normal renal cortex following gadolinium-based intravenous contrast administration (46). Although renal carcinoma, metastasis, lymphoma, xantogranulomatous pyelonephritis, angiomyolipoma, and oncocytoma are included in the differential diagnosis of such focal renal lesions, the clinical history of sarcoidosis and involvement of other organs would prevent unnecessary surgical interventions for the diagnosis.
Figure 13. a–d.
A 57-year-old female with biopsy-proven hepatic, splenic, and renal sarcoidosis. Contrast-enhanced CT of the abdomen (a, b) shows multiple hypodense granulomas infiltrating the liver and spleen. Small hypodense granulomas are also detected in both kidneys (a, b, arrows). Contour irregularity of the shrinking liver became prominent (c, dashed arrows) and contour irregularity of the right kidney at the site of granuloma occurred (d, dashed arrow) at five-month follow-up.
Genital tract
The diagnosis of genital tract sarcoidosis was made on surgical specimens in previously reported cases. Uterus is the most commonly involved site of the female genital system (51). It is usually detected while investigating the cause of abnormal uterine bleeding in patients with a previous history of sarcoidosis at other anatomic sites (52). Ovarian sarcoidosis is extremely rare and known to mimic ovarian malignancy with soft tissue nodules (51). There is no specific radiological finding in the literature to describe ovarian sarcoidosis (51).
Sarcoidosis involving the male reproductive tract has rarely been reported. Granulomas may be detected in the epididymis, testis, and prostate gland, in order of decreasing frequency. Spermatic cord, scrotum, and penis are rarely involved (53, 54). Testicular (Fig. 14) and extratesticular (Figs. 15, 16) involvement need to be differentiated from other granulomatous diseases such as tuberculosis, syphilis, Wegener’s granulomatosis, granuloma inguinale, lymphogranuloma venereum, filariasis, coccidioidomycosis, blastomycosis, actinomycosis, and schistosomiasis (55). The lesions are hypoechoic on US and generally hypointense on T2-weighted images. They exhibit enhancement on gadolinium-enhanced T1-weighted images (56). Open testicular biopsy needs to be performed to exclude malignancy, on account of coexisting testicular tumors reported with sarcoidosis (57–59).
Figure 14.
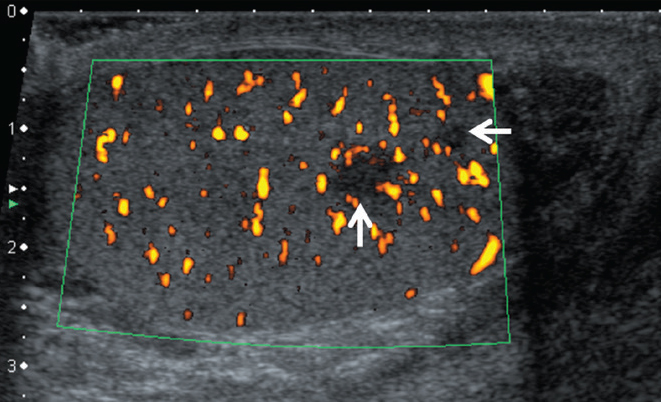
A 30-year-old male with testicular sarcoidosis proven by partial removal of the testis. B-mode and color Doppler US shows scrotal swelling and hypoechoic nodular areas in the left testis (arrows).
Figure 15. a, b.
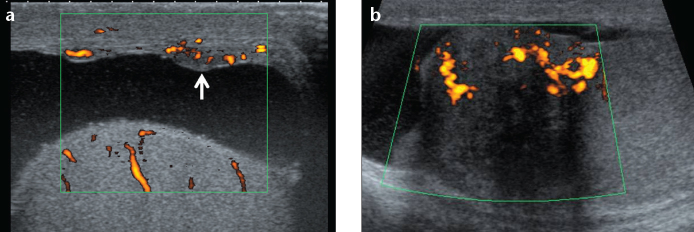
A 30-year-old male with biopsy-proven sarcoidosis. B-mode and color Doppler US of the testis shows nodular thickening of the tunica vaginalis (a, arrow) and of the head of the epidydimis (b).
Figure 16.
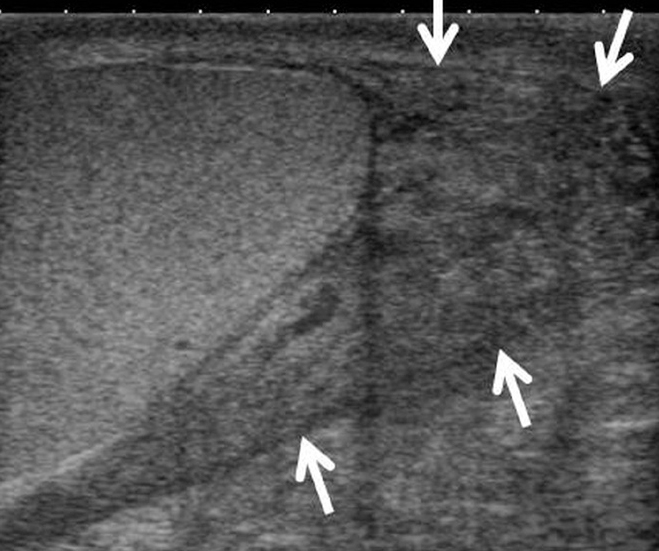
A 21-year-old male with extra-testicular sarcoidosis proven by biopsy. US shows infiltration around the vas deferens (arrows), at the lower pole of the scrotum, which disappeared after treatment. The patient also had neurosarcoidosis.
Conclusion
Sarcoidosis is a multisystemic inflammatory disease which has been reported in every system and organ of the human body. Abdominal sarcoidosis is less common and most often asymptomatic. It may mimic more common infectious diseases or neoplasms. Awareness of the imaging findings of intra-abdominal sarcoidosis would help to prevent long-standing unrecognized disease.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Chesnutt MS, Gifford AH, Prendergast TJ. Pulmonary disorders. In: McPhee SJ, Papadakis MA, Tierney LM, editors. Current medical diagnosis & treatment. 49th ed. New York: McGraw-Hill; 2010. [Google Scholar]
- 2.Baughman RP, Lower EE. Sarcoidosis. In: Fauci A, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. 17th ed. New York: McGraw Hill; 2008. [Google Scholar]
- 3.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 4.MacArthur KL, Forouhar F, Wu GY. Intra-abdominal complications of sarcoidosis. J Formos Med Assoc. 2010;109:484–492. doi: 10.1016/S0929-6646(10)60082-4. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard JA, Aquino SL. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol. 2008;190:1–6. doi: 10.2214/AJR.07.7001. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy PT, Zakaria N, Modawi SB, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006;18:721–726. doi: 10.1097/01.meg.0000223911.85739.38. [DOI] [PubMed] [Google Scholar]
- 7.Hercules HD, Bethlem NM. Value of liver biopsy in sarcoidosis. Arch Pathol Lab Med. 1984;108:831–834. [PubMed] [Google Scholar]
- 8.Judson MA. Hepatic, splenic, and gastrointestinal involvement with sarcoidosis. Semin Respir Crit Care Med. 2002;23:529–541. doi: 10.1055/s-2002-36517. [DOI] [PubMed] [Google Scholar]
- 9.Warshauer DM, Molina PL, Hamman SM, et al. Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology. 1995;195:757–762. doi: 10.1148/radiology.195.3.7754007. [DOI] [PubMed] [Google Scholar]
- 10.Deutch SJ, Sandler MA, Tankanow LB. Abdominal lymphadenopathy in sarcoidosis. J Ultrasound Med. 1987;6:237–242. doi: 10.7863/jum.1987.6.5.237. [DOI] [PubMed] [Google Scholar]
- 11.Britt AR, Francis IR, Glazer GM, Ellis JH. Sarcoidosis: abdominal manifestations at CT. Radiology. 1991;178:91–94. doi: 10.1148/radiology.178.1.1984330. [DOI] [PubMed] [Google Scholar]
- 12.Folz SJ, Johnson CD, Swensen SJ. Abdominal manifestations of sarcoidosis in CT studies. J Comput Assist Tomogr. 1995;19:573–579. doi: 10.1097/00004728-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Merlo F, Wanless IR, Shimamatsu K, Sherman M, Greig P, Chiasson D. The role of granulomatous phlebitis and thrombosis in the pathogenesis of cirrhosis and portal hypertension in sarcoidosis. Hepatology. 1997;26:554–560. doi: 10.1002/hep.510260304. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani P, Vohra M, Sheagren JN. An association of sarcoidosis with hepatocellular carcinoma. Ann Oncol. 2005;16:1714–1715. doi: 10.1093/annonc/mdi306. [DOI] [PubMed] [Google Scholar]
- 15.Freed JS, Reiner MA. Acute cholecystitis as a complication of sarcoidosis. Arch Intern Med. 1983;143:2188–2189. [PubMed] [Google Scholar]
- 16.Mert A, Avsar S, Ozaras R, et al. Gallbladder involvement in sarcoidosis. J Clin Gastroenterol. 2004;38:612–613. doi: 10.1097/00004836-200408000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Bloom R, Sybert A, Mascatello VJ. Granulomatous biliary tract obstruction due to sarcoidosis. Report of a case and review of the literature. Am Rev Respir Dis. 1978;117:783–787. doi: 10.1164/arrd.1978.117.4.783. [DOI] [PubMed] [Google Scholar]
- 18.Maambo E, Brett AS, Vasudeva R, Burns RG. Hepatobiliary sarcoidosis presenting as sclerosing cholangitis: long-term follow-up. Dig Dis Sci. 2007;52:3363–3365. doi: 10.1007/s10620-006-9451-3. [DOI] [PubMed] [Google Scholar]
- 19.Kessler A, Mitchell DG, Israel HL, Goldberg BB. Hepatic and splenic sarcoidosis: Ultrasound and MR imaging. Abdom Imaging. 1993;18:159–163. doi: 10.1007/BF00198055. [DOI] [PubMed] [Google Scholar]
- 20.Kahi CJ, Saxena R, Temkit M, et al. Hepatobiliary disease in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:117–123. [PubMed] [Google Scholar]
- 21.Warshauer DM, Joseph K, Lee T. Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol. 2004;182:15–28. doi: 10.2214/ajr.182.1.1820015. [DOI] [PubMed] [Google Scholar]
- 22.Warshauer DM, Semelka RC, Ascher SM. Nodular sarcoidosis of the liver and spleen: appearance on MR images. J Magn Reson Imaging. 1994;4:553–557. doi: 10.1002/jmri.1880040407. [DOI] [PubMed] [Google Scholar]
- 23.Taavitsainen M, Koivuniemi A, Helminen J, et al. Aspiration biopsy of the spleen in patients with sarcoidosis. Acta Radiologica. 1987;28:723–725. [PubMed] [Google Scholar]
- 24.Penna C, Deroide GA. Images in clinical medicine. Splenic sarcoidosis. N Engl J Med. 2003;349:16. doi: 10.1056/ENEJMicm020110. [DOI] [PubMed] [Google Scholar]
- 25.Warshauer DM. Splenic sarcoidosis. Semin Ultrasound CT MR. 2007;28:21–27. doi: 10.1053/j.sult.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Friedman M, Ali MA, Borum ML. Gastric sarcoidosis: a case report and review of the literature. South Med J. 2007;100:301–303. doi: 10.1097/SMJ.0b013e318030ed94. [DOI] [PubMed] [Google Scholar]
- 27.Vahid B, Spodik M, Braun KN, Ghazi LJ, Esmaili A. Sarcoidosis of gastrointestinal tract: a rare disease. Dig Dis Sci. 2007;52:3316–3320. doi: 10.1007/s10620-006-9448-y. [DOI] [PubMed] [Google Scholar]
- 28.Lukens FJ, Machicao VI, Woodward TA, DeVault KR. Esophageal sarcoidosis: an unusual diagnosis. J Clin Gastroenterol. 2002;34:54–56. doi: 10.1097/00004836-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Chinitz MA, Brandt LJ, Frank MS, Frager D, Sablay L. Symptomatic sarcoidosis of the stomach. Dig Dis Sci. 1985;30:682–688. doi: 10.1007/BF01308419. [DOI] [PubMed] [Google Scholar]
- 30.Kaneki T, Koizumi T, Yamamoto H, et al. Gastric sarcoidosis—A single polypoid appearance in the involvement. Hepatogastroenterology. 2001;48:1209–1210. [PubMed] [Google Scholar]
- 31.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103:3184–3192. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 32.Noël JM, Katona IM, Piñeiro-Carrero VM. Sarcoidosis resulting in duodenal obstruction in an adolescent. J Pediatr Gastroenterol Nutr. 1997;24:594–598. doi: 10.1097/00005176-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Boyum R, Yeung KJ, Kaplan KJ, Lipton AJ, Rogers PL. Pediatric gastrointestinal sarcoidosis presenting with protein-losing enteropathy. J Ped Gastroenterol Nutr. 2007;44:152–156. doi: 10.1097/01.mpg.0000235976.52249.ec. [DOI] [PubMed] [Google Scholar]
- 34.Beniwal RS, Cummings OW, Cho WK. Symptomatic gastrointestinal sarcoidosis: Case report and review of the literature. Dig Dis Sci. 2003;48:174–178. doi: 10.1023/a:1021711204498. [DOI] [PubMed] [Google Scholar]
- 35.Veitch AM, Badger I. Sarcoidosis presenting as colonic polyposis: report of a case. Dis Colon Rectum. 2004;47:937–939. doi: 10.1007/s10350-004-0520-4. [DOI] [PubMed] [Google Scholar]
- 36.Ushiki A, Koizumi T, Kubo K, Suzawa K, Arakura N, Suzawa H. Colonic sarcoidosis presenting multiple submucosal tumor-like lesions. Intern Med. 2009;48:1813–1816. doi: 10.2169/internalmedicine.48.2427. [DOI] [PubMed] [Google Scholar]
- 37.Zissin R, Gayer G, Bernheim J, Kots E, Shapiro-Feinberg M, Hertz M. Granulomatous appendicitis presenting as right lower quadrant pain: CT findings. Abdom Imaging. 2003;28:280–283. doi: 10.1007/s00261-002-0060-0. [DOI] [PubMed] [Google Scholar]
- 38.Bernaciak J, Spina JC, Curros ML, Maya G, Venditti J, Chacon C. Case report: peritoneal sarcoidosis in an unusual location. Semin Respir Crit Care Med. 2002;23:597–600. doi: 10.1055/s-2002-36523. [DOI] [PubMed] [Google Scholar]
- 39.Uthman IW, Bizri AR, Khalifeh MJ. Peritoneal sarcoidosis. Semin Arthritis Rheum. 2002;31:353. doi: 10.1053/sarh.2002.32548. [DOI] [PubMed] [Google Scholar]
- 40.Bach DB, Vellet AD. Retroperitoneal sarcoidosis. AJR Am J Roentgenol. 1991;156:520–522. doi: 10.2214/ajr.156.3.1899748. [DOI] [PubMed] [Google Scholar]
- 41.Shukla M, Hassan MF, Toor V, Kaur J, Solomon C, Cohen H. Symptomatic pancreatic sarcoidosis. Case report and review of literature. JOP. 2007;8:770–774. [PubMed] [Google Scholar]
- 42.Bacal D, Hoshal VL, Jr, Schaldenbrand JD, Lampman RM. Sarcoidosis of the pancreas: Case report and review of the literature. Am Surgeon. 2000;66:675–678. [PubMed] [Google Scholar]
- 43.Frank JL, Goldman M, Nathanson I, et al. Surgical management of pancreatic sarcoid. Eur J Surg. 2001;167:68–72. doi: 10.1080/110241501750069864. [DOI] [PubMed] [Google Scholar]
- 44.Baroni RH, Pedrosa I, Tavernaraki E, Goldsmith J, Rofsky NM. Pancreatic sarcoidosis: MRI features. J Magn Reson Imaging. 2004;20:889–893. doi: 10.1002/jmri.20199. [DOI] [PubMed] [Google Scholar]
- 45.Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24:87–104. doi: 10.1148/rg.241035076. [DOI] [PubMed] [Google Scholar]
- 46.Goldsmith S, Harris M, Scherer K, Al-Quran S, Vorhis E. Sarcoidosis manifesting as a pseudotumorous renal mass. J Radiol Case Rep. 2013;7:23–34. doi: 10.3941/jrcr.v7i5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vagal AS, Shipley R, Meyer CA. Radiological manifestations of sarcoidosis. Clin Dermatol. 2007;25:312–325. doi: 10.1016/j.clindermatol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Lockhart ME, Smith JK, Kenney PJ, Urban DA. Pseudotumorous renal involvement of sarcoidosis. J Urol. 2001;165:895. [PubMed] [Google Scholar]
- 49.Heldmann M, Behm W, Reddy MP, et al. Pseudotumoral renal sarcoid: MRI, PET, and MDCT appearance with pathologic correlation. AJR Am J Roentgenol. 2005;185:697–699. doi: 10.2214/ajr.185.3.01850697. [DOI] [PubMed] [Google Scholar]
- 50.Herman TE, Shackelford GD, McAlister WH. Pseudotumoral sarcoid granulomatous nephritis in a child: case presentation with sonographic and CT findings. Pediatr Radiol. 1997;27:752–754. doi: 10.1007/s002470050218. [DOI] [PubMed] [Google Scholar]
- 51.Wuntakal R, Bharathan R, Rockall A, Jeyarajah A. Interesting case of ovarian sarcoidosis: the value of multidisciplinary team working. World J Surg Oncol. 2007;5:38. doi: 10.1186/1477-7819-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce KF, Nolan TE. Endometrial sarcoidosis as a cause of postmenopausal bleeding. A case report. J Reprod Med. 1996;41:878–880. [PubMed] [Google Scholar]
- 53.Handa T, Nagai S, Hamada K, et al. Sarcoidosis with bilateral epididymal and testicular lesions. Intern Med. 2003;42:92. doi: 10.2169/internalmedicine.42.92. [DOI] [PubMed] [Google Scholar]
- 54.Rudin L, Megalli M, Mesa-Tejada R. Genital sarcoidosis. Urology. 1974;3:750–754. doi: 10.1016/s0090-4295(74)80218-9. [DOI] [PubMed] [Google Scholar]
- 55.Canguven O, Balaban M, Selimoglu A, Albayrak S. Corticosteroid therapy improves the outcome of semen analysis in an oligozoospermic patient with epididymal sarcoidosis. Korean J Urol. 2013;54:558–560. doi: 10.4111/kju.2013.54.8.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics. 2002;22:189–216. doi: 10.1148/radiographics.22.1.g02ja14189. [DOI] [PubMed] [Google Scholar]
- 57.Paparel P, Devonec M, Perrin P, et al. Association between sarcoidosis and testicular carcinoma: a diagnostic pitfall. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:95–101. [PubMed] [Google Scholar]
- 58.Geller RA, Kuremskey DA, Copeland JS, Stept R. Sarcoidosis and testicular neoplasm: an unusual association. J Urol. 1977;118:487–488. doi: 10.1016/s0022-5347(17)58076-3. [DOI] [PubMed] [Google Scholar]
- 59.Blacher EJ, Maynard JF. Seminoma and sarcoidosis: an unusual association. Urology. 1985;26:288–289. doi: 10.1016/0090-4295(85)90128-1. [DOI] [PubMed] [Google Scholar]