Abstract
PURPOSE
We aimed to assess the correlation between pulmonary hemorrhage and pneumothorax in computed tomography (CT)-guided transthoracic fine needle aspiration (TTFNA), particularly its possible value as protection against the development of pneumotorax.
MATERIALS AND METHODS
We reviewed the CT images of 538 patients (364 males and 174 females, mean age 70 years, range 36–90 years) who underwent CT-guided TTFNA of pulmonary nodules between January 2008 and September 2013. The following CT findings were assessed: pulmonary hemorrhage (type 1, along the needle track; type 2, perilesional; low-grade, ≤6 mm; high-grade, >6 mm), pneumothorax, distance between the target nodule and the pleural surface, and emphysema.
RESULTS
Pneumothorax occurred in 154 cases (28.6%) and pulmonary hemorrhage occurred in 144 cases (26.8%). The incidence of pneumothorax was lower in patients showing type 1 and high-grade pulmonary hemorrhage pattern. The incidence of pneumothorax in biopsies ≥30 mm from pleural surface was 26% (12/46) in cases showing this pattern, while it was 71.4% (30/42) when this pattern was not seen. Similarly, the incidence of pneumothorax in biopsies <30 mm from the pleural surface was 0% (0/28) in cases showing this hemorrhage pattern, while it was 19% (76/394) when this pattern was not seen.
CONCLUSION
Pulmonary hemorrhage during TTFNA is a frequent event that protects against pneumothorax. A bleeding greater than 6 mm along the needle track is associated with lower incidence of pneumothorax, especially in biopsies deeper than 3 cm.
Computed tomography (CT)-guided transthoracic fine needle aspiration (TTFNA) biopsy is currently considered a reliable diagnostic technique to assess malignancy of pulmonary nodules and masses (1–4). CT-guided TTFNA is an invasive technique with low incidence of severe complications and contraindications (1).
Pneumothorax is the most common complication occurring in the range of 8%–64% (with a risk of tension pneumothorax in about 7% of cases) (1–7). The risk of pneumothorax increases in the presence of obstructive lung disease and small target lesion. Furthermore, the risk of pneumothorax is directly related to the distance of the lesion from the pleural surface, number of pleural needle passages, fissures crossing, patient’s age, and operator experience (8). Pulmonary hemorrhage is the second most frequent complication of TTFNA. Pulmonary hemorrhage is rarely the cause of death and it may be associated with hemoptysis in 4%–5% of patients, even as a postprocedure complication (9). According to recent studies, the incidence of pulmonary hemorrhage ranges 15%–26%, depending on the distance of the pulmonary nodule from the pleural surface (10). Higher incidence of pulmonary hemorrhage is related to central or cavitated lesion, presence of bronchiectasis, and larger needles (11–13).
The aim of this study was to assess the correlation between pulmonary hemorrhage and pneumothorax in CT-guided TTFNA.
Materials and methods
The institutional review board waived informed consent for this retrospective study. We reviewed the CT images of 538 patients (364 males and 174 females, mean age 70 years, range 36–90 years) who underwent CT-guided TTFNA of pulmonary nodules between January 2008 and September 2013, a reasonable time to have an adequate number of cases to be studied.
Pulmonary nodules were evaluated for either primary or secondary malignancy and their diameter ranged 7–30 mm. Pulmonary masses >30 mm, chest wall lesions, and mediastinal lesions were excluded from this study.
Two radiologists (M.D.F. and C.R.), both with 11 years of experience in thoracic radiology and CT-guided pulmonary biopsies, evaluated the CTs for the presence of pneumothorax (Fig. 1) and pulmonary hemorrhage. For our statistics, we only considered images from the first pass of the needle during TTFNA.
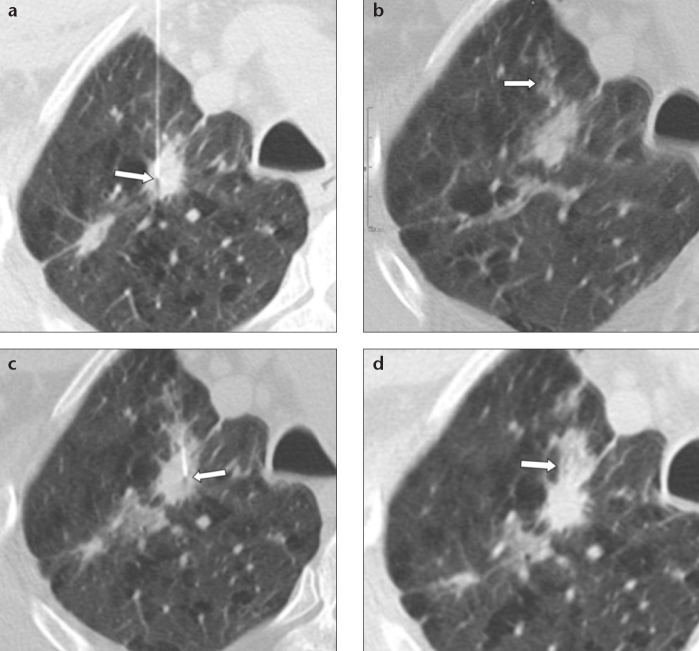
Figure 1. a–c.
CT images of a 63-year-old male smoker with a cavitated noncalcified pulmonary nodule (a, arrow) who underwent TTFNA (b, arrow). Post-biopsy pneumothorax (c, arrow) with patient in prone position; note the absence of pulmonary hemorrhage. Cytological result of TTFNA was pulmonary adenocarcinoma.
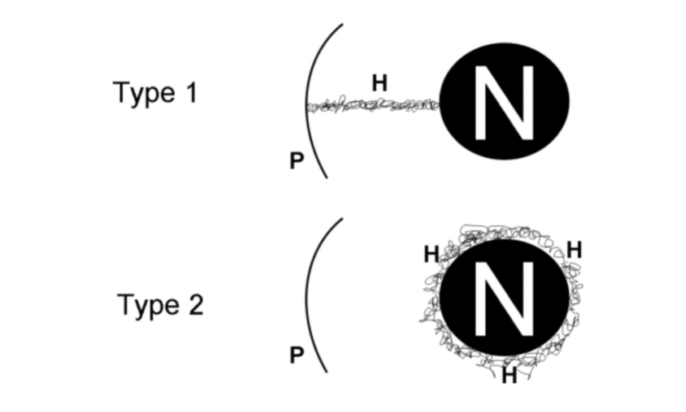
Pulmonary hemorrhage was defined by the appearance of ground-glass opacity, as described by the Fleischner Society (14). Pulmonary hemorrhage was classified arbitrarily according to location, as follows: type 1, ground-glass opacity along the needle track; type 2, ground-glass opacity around the target nodule (Fig. 2). The maximum diameter of the ground-glass opacity was used to score the amount of bleeding: low-grade hemorrhage, for opacities ≤6 mm; high-grade hemorrhage, for opacities >6 mm (Figs. 2–4).
Figure 2.
Types of pulmonary hemorrhage: type 1, hemorrhage developing along the needle track; type 2, perilesional hemorrhage. Hemorrhagic lesions were further classified as low-grade (diameter ≤6 mm), and high-grade (diameter >6 mm). H, pulmonary hemorrhage; P, pleural plane; N, pulmonary nodule.
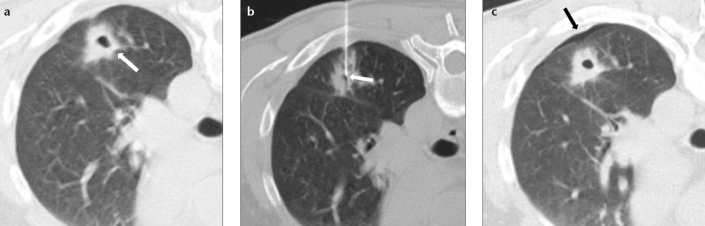
Figure 4. a–c.
CT images of a 77-year-old male smoker with a solid noncalcified pulmonary nodule (a, arrow) who underwent TTFNA (b, arrow). The arrows in (c) indicate perilesional pulmonary hemorrhage (Type 2) without pneumothorax, with patient in supine position. Cytological result of TTFNA was colorectal metastasis.
The known risk factors for pneumothorax were also scored (4). The distance between the nodule and the pleural surface (depth) was measured according to the needle trajectory. Five groups were established according to nodule depth: adjacent to the pleura (<1 mm), 1–10 mm, 11–20 mm, 21–30 mm, and >30 mm.
Emphysema was scored as mild, moderate, or severe using Goddard classification, which is a visual scale where area of vascular disruption and low attenuation value are scored for each lung field. Emphysema was considered mild for ≤25%, moderate for 25%–50%, and severe for >50% score. Only patients with moderate/severe score were deemed positive for emphysema (15).
Biopsy procedure
All CT-guided TTFNA were performed with a multidetector CT helical scanner (Somatom Emotion 6, Siemens, Erlangen, Germany). The technical parameters were as follows: 120 mAs, 100 kV, collimation 6×2 mm, slice thickness 2.5 mm, reconstruction increment of 1 mm.
All patients who underwent CT-guided TTFNA of pulmonary nodules were administered a solution of lidocaine 1%, as local anesthetic. After skin disinfection with iodopovidone 1%, a 22-gauge Chiba point needle with centimeter mark (Hospital Service SPA, Aprilia, Italy) (total length 7–15 cm, according to nodule depth), was introduced through the skin, as perpendicularly as possible to the pleural surface. The shortest trajectory was chosen according to large vessels, main and lobar bronchi, pleural fissures, skeletal structures, and calcified areas. A centimeter-marked wire netting, laser lights, and markers on the skin were used to determine the entry point of the needle. The angle and depth for the needle path was evaluated and controlled by CT scan and multiplanar reformation (16). We used a 20 mL syringe mounted on a Franzen pistol handle (Syring pistol; Cameco LTD-Medical supplies, London, United Kingdom) to withdraw the biological material.
The cytological sample was assessed in real time by a cytologist. It was then scraped on a glass slide, immersed in 95% alcohol and stained with May Grunwald Giemsa (rapid coloring MGG (cod. 090805); Bio-Optica, Milano, Italy). Samples with a cell count too small for cytological characterization were considered insufficient. Samples that did not allow cell characterization, despite a sufficient number of cells, were considered inadequate.
Statistical analysis
Demographics, pulmonary hemorrhage and its features (type and grade), pneumothorax, nodule depth, and emphysema were statistically tested using categorical statistics.
We used descriptive statistical tests for nominal data such as Pearson’s chi-square and the binary logistic regression analysis, using the onset of pneumothorax as the dependent variable. The statistical program used was SPSS 19 (IBM SPSS Statistics for Windows; IBM Corporation, Armonk, New York, USA). A P value less than 0.05 was considered significant.
Results
Pneumothorax occurred in 154 of 538 cases (28.6%). All patients with pneumothorax (100%) were asymptomatic and did not require pleural drainage. Pulmonary hemorrhage occurred in 106 of 154 cases with pneumothorax (69%).
Pulmonary hemorrhage occurred in 144 of 538 cases (26.8%); notably, 112 of 144 (78%) were type 1 and 32 of 144 (22%) were type 2. The amount of bleeding was low-grade in 46 of 144 cases (32%) and high-grade in 98 of 144 cases (68%). Among 112 type 1 cases, 38 (34%) were low-grade and 74 (66%) were high-grade. Hemoptysis was found in two subjects. In both cases type 2 pulmonary hemorrhage had developed. Among 32 type 2 cases, eight (25%) were low-grade and 24 (75%) were high-grade.
The incidence of bleeding, according to the distance from the pleural surface, was as follows: 4 of 144 adjacent (<1 mm) lesions (3%), 12 of 144 lesions 1–10 mm deep (8%), 22 of 144 lesions 10–20 mm deep (15%), 32 of 144 lesions 20–30 mm deep (22%), and 74 of 144 lesions >30 mm deep (52%). The presence of bleeding was associated with the nodule depth (P < 0.001).
The features of pulmonary hemorrhage showed correlation with the incidence of pneumothorax. Pneumothorax was seen in 12 of 74 cases with type 1 and high-grade hemorrhage (16%) and in 14 of 24 cases with type 2 and low-grade hemorrhage (58%) (P = 0.011). In the subset of nodules ≤30 mm deep (422 cases), pneumothorax was seen in none of 28 cases with type 1 and high-grade hemorrhage (0%) and in 76 of 394 cases without type 1 and high-grade hemorrhage (19%) (P < 0.001). In the subset of nodules >30 mm deep (116 cases), pneumothorax was seen in 12 of 46 (26%) cases with type 1 plus high-grade hemorrhage and in 30 of 42 cases (71%) without pulmonary hemorrhage (P < 0.001) (Table).
Table.
Summary of relationship between development of pneumothorax and presence/absence of type 1 high-degree pulmonary hemorrhage in relation to nodule depth
| Development of pneumothorax | P | ||
|---|---|---|---|
|
| |||
| Presence of type 1 high-grade pulmonary hemorrhage | Absence of type 1 high-grade pulmonary hemorrhage | ||
| Lesions <3 cm from the pleural plane | 0% (0/28) | 26.9% (76/394) | < 0.001 |
| Lesions >3 cm from the pleural plane | 26% (12/46) | 71.4% (30/42) | < 0.001 |
Emphysema was diagnosed in 192 of 538 cases (36%): its severity was mild in 10 (2%), moderate in 73 (14%), and severe in 110 cases (20%). Emphysema was significantly related with pneumothorax (P = 0.001). Pneumothorax occurred in 72 of 192 cases (38%) of emphysema and in 82 of 346 cases (24%) without emphysema.
A binary logistic regression analysis was performed, using the pneumothorax occurrence as a dependent variable and age, gender, presence of emphysema, type and grade of pulmonary hemorrhage, and nodule depth as covariates. Pneumothorax showed positive correlation with nodule depth (r=0.7) and emphysema (r=0.62), but negative correlation with high-grade hemorrhage (r=−0.9) (the development of pneumothorax is reduced with increasing pulmonary hemorrhage). The overall model fit was significant (P = 0.023).
In 24 cases, biopsy did not provide a sufficient sample for cytological assessment. In these cases, a second biopsy was performed and a valid sample was achieved in all (100%). Notably, none of these 24 cases presented pulmonary hemorrhage or pneumothorax, despite repetition of the procedure (Fig. 5).
Figure 5. a–d.
CT images of an 86-year-old male smoker with solid noncalcified pulmonary nodule in the right upper lung lobe, in the presence of diffuse centrilobular and paraseptal emphysema. Panel (a) shows TTFNA of the pulmonary nodule (arrow). Panel (b) shows pulmonary hemorrhage along the needle track in the absence of pneumothorax (arrow). In the absence of a cytological diagnosis “on site”, a second sampling is carried out with reasonable safety in a different area of the pulmonary nodule (c, arrow). Note how pulmonary hemorrhage along the needle tract is increased after the second cytology (d, arrow) and pneumothorax is not present. Cytological result of TTFNA was pulmonary adenocarcinoma.
Discussion
In this study, pneumothorax occurred in 29% of cases, within the range of 8%–64% reported in the literature (1–7, 17–20). Pulmonary hemorrhage was relatively frequent in our experience (27%) compared to the incidence reported in the literature (15%–26%) (8). Pulmonary hemorrhage did not cause interruption of the procedure and hemoptysis was extremely rare, regardless of the amount of hemorrhage. Pulmonary hemorrhage was related to lower incidence of pneumothorax.
Several authors showed that most common factors of post-biopsy pneumothorax are: emphysema, needle trajectory non-perpendicular to the pleural surface, and transpleural multiple needle passages (10). Furthermore, Yeow et al. (13), demonstrated a nodule diameter ≤20 mm and increasing nodule depth predict higher likelihood of pneumothorax. In our study, we also observed a positive correlation between nodule depth and pneumothorax, with an overall pneumothorax incidence of 36% for nodules >30 mm deep.
In this study, 34% of patients showed moderate-severe emphysema which is a well-known risk factor for pneumothorax during TTFNA. To our knowledge, the prevalence of emphysema was not reported in previous studies (17, 21). Interestingly, our data show that type 1 and high-grade pulmonary hemorrhage is a protective factor for pneumothorax (P < 0.001). This was true in all clinical situations, notably for lesions >30 mm deep. The incidence of pneumothorax for nodules >30 mm deep was 71% in those cases without pulmonary hemorrhage, while it decreased to 26% in the presence of type 1 and high-grade hemorrhage (Table). Also for nodules ≤30 mm deep, pneumothorax was more frequent in cases without type 1 and high-grade of pulmonary hemorrhage, whereas no pneumothorax was seen in the 28 cases with type 1 and high-grade hemorrhage. Type 2 and low-grade pulmonary hemorrhage were not related to lower incidence of pneumothorax.
The binary logistic regression showed that high-grade pulmonary hemorrhage, nodules ≤30 mm deep, and no emphysema were associated with the absence of pneumothorax in 92% of cases (P = 0.023).
To our knowledge, this is the first study testing the correlation between pulmonary hemorrhage and pneumothorax in TTFNA. We speculate that this correlation might be related to a reduction of ventilation caused by free blood in alveolar space. This patch-like alteration might reduce the airflow from airspace to pleural space, namely, the cause of pneumothorax in TTFNA (Fig. 5). We suggest that the presence of type 1 and high-grade pulmonary hemorrhage might be a marker of low likelihood of pneumothorax, and allow for multiple biopsies in the same session when cytology is not sufficient.
The limitation of our study was the lack of a prospective evaluation and the absence of long-term follow-up in patients who developed pulmonary hemorrhage during CT-guided biopsy.
In conclusion, we showed that pulmonary hemorrhage occurs rather frequently in CT-guided TTFNA and it is a protective factor for pneumothorax when the CT pattern suggests high-grade hemorrhage along the needle track (type 1). The evaluation of the CT pattern of pulmonary hemorrhage should be included in the management of multiple biopsy sessions.
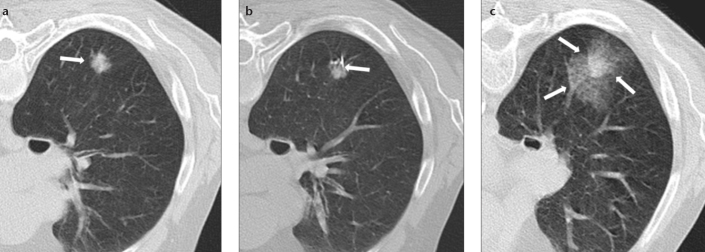
Figure 3. a–c.
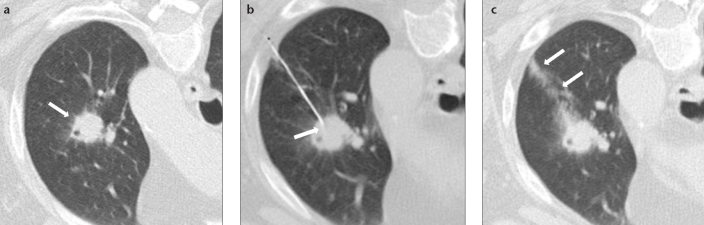
CT images of a 73-year-old female nonsmoker with solid noncalcified pulmonary nodule (a, arrow) who underwent TTFNA (b, arrow). The arrows in (c) indicate high-grade (>6 mm) pulmonary hemorrhage along the needle tract (Type 1), with patient in prone position. Cytological result of TTFNA was pulmonary adenocarcinoma.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Poulou LS, Tsagouli P, Ziakas PD, Politi D, Trigidou R, Thanos L. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol. 2013;54:640–645. doi: 10.1177/0284185113481595. [DOI] [PubMed] [Google Scholar]
- 2.Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol. 2012;46:19–22. doi: 10.2478/v10019-012-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley JH, Fish GD, Andriole JG, et al. Lung lesions: cytologic diagnosis by fine-needle biopsy. Radiology. 1987;162:389–391. doi: 10.1148/radiology.162.2.3797651. [DOI] [PubMed] [Google Scholar]
- 4.Loh SE, Wu DD, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singapore. 2013;42:285–290. [PubMed] [Google Scholar]
- 5.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167:105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- 6.Larscheid RC, Thorpe PE, Scott WJ. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest. 1998;114:704–709. doi: 10.1378/chest.114.3.704. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol. 2000;175:239–243. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin Radiol. 2013;68:43–48. doi: 10.1016/j.crad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 10.Lucidarme O, Howarth N, Finet JF, Grenier PA. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology. 1998;207:59–65. doi: 10.1148/radiology.207.3.9609901. [DOI] [PubMed] [Google Scholar]
- 11.Laspas F, Roussakis A, Efthimiadou R, Papaioannou D, Papadopoulos S, Andreou J. Percutaneous CT-guided fine-needle aspiration of pulmonary lesions: results and complications in 409 patients. J Med Imaging Radiat Oncol. 2008;52:458–462. doi: 10.1111/j.1440-1673.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 12.Yildirim E, Kirbas I, Harman A, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: factors effecting risk of complications. Eur J Radiol. 2009;70:57–60. doi: 10.1016/j.ejrad.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Yeow KM, Su IH, Pan KT, et al. Risk factors of bleeding and pneumothorax. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 15.Asai N, Kawamura Y, Yamazaki I, et al. Is emphysema a risk factor for pneumothorax in CT-guided lung biopsy? Springer-plus. 2013;30:196. doi: 10.1186/2193-1801-2-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Filippo M, Saba L, Concari G, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med. 2013;118:1071–1081. doi: 10.1007/s11547-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 17.Boiselle PM, Shepard JAO, Mark EJ, et al. Routine additions of an automated biopsy device to fine-needle aspiration of the lung: a prospective assessment. AJR Am J Roentgenol. 1997;169:661–666. doi: 10.2214/ajr.169.3.9275873. [DOI] [PubMed] [Google Scholar]
- 18.Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology. 1997;202:97–103. doi: 10.1148/radiology.202.1.8988197. [DOI] [PubMed] [Google Scholar]
- 19.Arslan S, Yilmaz A, Bayramgürler B, Uzman O, Nver E, Akkaya E. CT-guided transthoracic fine needle aspiration of pulmonary lesions: Accuracy and complications in 294 patients. Med Sci Monit. 2002;8:493–497. [PubMed] [Google Scholar]
- 20.Rizzo S, Preda L, Raimondi S, et al. Risk factors for complications of CT-guided lung biopsies. Radiol Med. 2011;116:548–563. doi: 10.1007/s11547-011-0619-9. [DOI] [PubMed] [Google Scholar]
- 21.Dennie CJ, Matzinger FR, Marriner JR, Maziak DE. Transthoracic needle biopsy of the lung: Results of early discharge in 506 outpatients. Radiology. 2001;219:247–251. doi: 10.1148/radiology.219.1.r01ap11247. [DOI] [PubMed] [Google Scholar]