Abstract
PURPOSE
We aimed to assess the correlation between renal apparent diffusion coefficient (ADC) values measured by diffusion-weighted imaging (DWI) and the clinical stages of diabetic nephropathy.
MATERIALS AND METHODS
DWI (b value, 0 and 600 s/mm2) was performed in 78 patients with clinically confirmed diabetic nephropathy (study group) and 22 volunteers without diabetes mellitus or any renal disease (control group). The mean ADCs were calculated from multiple region-of-interest circles positioned in the renal cortex. Diabetic nephropathy was clinically categorized into five stages based on the values of urinary albumin excretion and glomerular filtration rate (GFR).
RESULTS
Mean renal ADC values of patients with stage 3 or 4 disease were significantly lower than those in patients with stage 1 or 2 disease and the control group (P < 0.001). ADC values of patients with stage 5 disease were significantly lower than those in patients with stage 4 (P = 0.003), stage 3 (P = 0.020), stages 2 and 1, and the control group (P < 0.001). Significant correlations were found between mean renal ADC values and clinical stages of diabetic nephropathy (r=−0.751, P < 0.001), between mean renal ADC values and estimated GFR values (r=0.642, P < 0.001), and between mean renal ADC values and urinary albumin excretion (r=−0.419, P < 0.001).
CONCLUSION
Renal ADC values show a significant correlation with clinical stages of diabetic nephropathy. As a relatively simple and noninvasive tool without contrast media administration, renal quantitative DWI may potentially play a role in making clinical decisions in the follow-up of diabetic patients.
Diabetic nephropathy is classically defined as a clinical syndrome characterized by persistent albuminuria, a relentless decline in glomerular filtration rate (GFR) progressing to end-stage renal disease, raised arterial blood pressure, and enhanced cardiovascular morbidity and mortality (1). In diabetic patients, renal functional deterioration is the result of heterogeneous renal structural changes, including glomerular basal membrane thickening and mesangial expansion, extracellular matrix accumulation, mesangiolysis, reduced podocyte number, microaneurysm formation, arteriolar hyalinosis which ultimately leads to glomerulosclerosis, tubular atrophy, interstitial expansion, and fibrosis (2). Renal damage occurs in multiple stages. Throughout its early stages, diabetic nephropathy has no symptoms. Persistent microalbuminuria is a predictor of the development of clinical nephropathy. Microalbuminuria has been proposed as a marker of widespread endothelial dysfunction and indicates microvascular damage (3). Better understanding of the mechanisms that lead to structural and functional changes in the diabetic kidney may facilitate the development of more effective follow-up and treatment modalities. Diagnostic tests that help identify early microvascular damage at an early stage will provide significant benefits to get the disease under control. Quantitative diffusion-weighted magnetic resonance imaging (MRI) may offer this opportunity and can play a role in the evaluation of renal disease. Several studies have indicated the potential use of the apparent diffusion coefficient (ADC) as a marker of renal function, showing lower renal ADC in kidney dysfunction (4–9). Yet, there are only a few studies concerning the use of renal quantitative diffusion-weighted imaging (DWI) in diabetic nephropathy (5, 9–11). In this study, our aim was to assess the correlation between renal ADC values from quantitative DWI of kidneys and the clinical stages of nephropathy in diabetic patients.
Materials and methods
The local institutional review board approved this study. Informed consent was obtained from each subject before study participation. Between May 2008 and June 2009, a total of 94 consecutive patients with diabetic nephropathy and no other systemic disease except diabetes mellitus were referred for radiologic examination. Patients with congenital hypoplastic kidney (n=1), hydronephrosis (n=2), simple cysts larger than 3 cm in diameter or presence of more than three cysts (n=5), history of obstructive uropathy (n=1), pyelonephritis (n=1), any renal operation (n=1), and patients with pacemaker (n=1) or claustrophobia (n=3) were excluded from the study. None of the subjects had renal emphysema, which is a rare condition mostly seen in diabetic patients (12). A total of 78 patients (43 male, 35 female; age range, 26–70 years) were evaluated by MRI. A total of 22 subjects (11 male, 11 female; age range, 34–70 years) without diabetes mellitus or any renal diseases were enrolled as the control group. Three subjects in the study group (3.8%) and two subjects in the control group (9.0%) were older than 65 years of age. Diabetic nephropathy was clinically categorized into five stages based on the values of urinary albumin excretion in 24-hour urine collection and glomerular filtration rate (GFR) which was estimated by 24-hour urine creatinine clearance: stage 1 (hyperfiltration), albuminuria <30 mg/24 h and GFR >100 mL/min/1.73 m2; stage 2 (microalbuminuria), albuminuria 30–300 mg/24 h and GFR 90–100 mL/min/1.73 m2; stage 3 (overt proteinuria), albuminuria >300 mg/24 h and GFR 60–89 mL/min/1.73 m2; stage 4 (progressive nephropathy), GFR 15–59 mL/min/1.73 m2 with increasing urine albumin excretion; stage 5 (end-stage kidney disease), GFR <15 mL/min/1.73 m2 with massive urine albumin excretion (13).
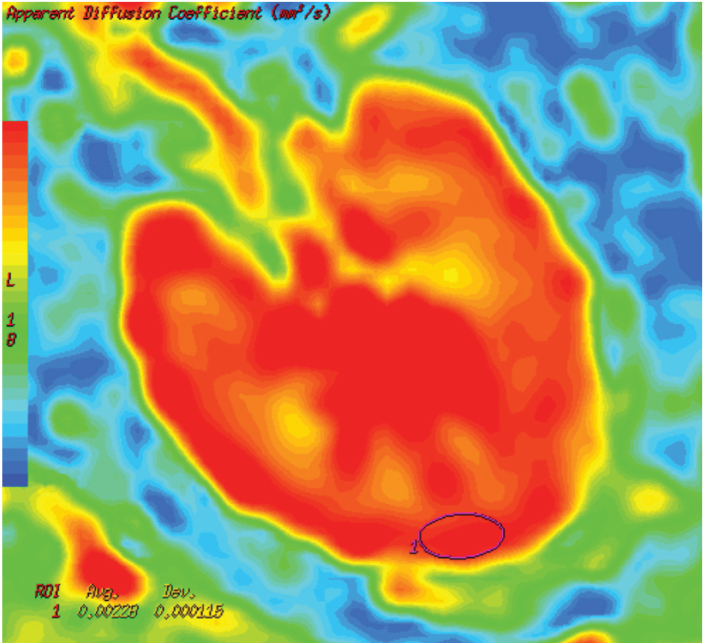
MRI was performed using a 1.5 Tesla system with an 8-channel body array coil (Signa Excite HD; GE Healthcare, Milwaukee, Wisconsin, USA). The maximum gradient amplitude and slew rate were 33 mT/m and 120 mT/m/s, respectively. Axial single-shot echo-planar DWI was performed during normal respiration using the following parameters: b value, 0 and 600 s/mm2; TR/TE, 6000/81.8; bandwidth, 250 kHz; number of excitations, 6; slice thickness, 6 mm; field of view, 40 cm; matrix, 128×128. Total scan time was 2 min 24 s for the DWI image set. A single radiologist reviewed all images on a workstation (Advantage Workstation 4.3; GE Healthcare). Each kidney was divided into three regions as upper, mid, and lower thirds. The ADC maps were constructed using image analysis software (FuncTool 2.6.9, GE Healthcare). ADC values of each region were measured with placement of at least three region-of-interest (ROI) ovoid circles (area, 80–100 mm2) on the renal cortical areas in the ADC map images (Fig. 1). In subjects with poor corticomedullary differentiation, ROIs were placed on the outer one third region of renal parenchyma, corresponding to the kidney contours. After ROI measurements, the mean ADC values were calculated for each region, each kidney, and each patient.
Figure 1.
Axial color apparent diffusion coefficient (ADC) map image of a 47-year-old male patient with stage 1 diabetic nephropathy. An ovoid region-of-interest (ROI) was placed in the cortex at the midsection of the left kidney. ROI revealed an ADC value of 2.28×10−3 mm2/s.
Statistical analyses were performed using Statistical Package for the Social Sciences version 13.0 (SPSS Inc., Chicago, Illinois, USA). Values are expressed as mean±standard deviation. Male-to-female ratio of the groups was compared using the chi-square test. Oneway ANOVA test was used to determine significant differences in mean age and mean ADC values between groups. Post hoc comparison Tukey test was used to compare subgroups. Relationships between mean ADC values and GFR values, amount of albuminuria, and the stage of diabetic nephropathy were assessed using Pearson’s correlation test. A P value of less than 0.05 was considered statistically significant for all analyses.
Results
There was no significant difference between the patient and control groups in terms of mean age (56.2±8.7 vs. 52.0±11.1 years, P = 0.055), and sex distribution (P = 0.670). Diabetic subjects had an estimated GFR range of 8.2–183.6 (mean, 71.8±42.4) mL/min/1.73 m2 and an albuminuria range of 2.0–8082.1 mg/24 h (mean, 663.7±1341.3 mg/24 h). Fifty-four (69.2%) diabetic patients had hypertension, while none of the control subjects had previously known hypertension.
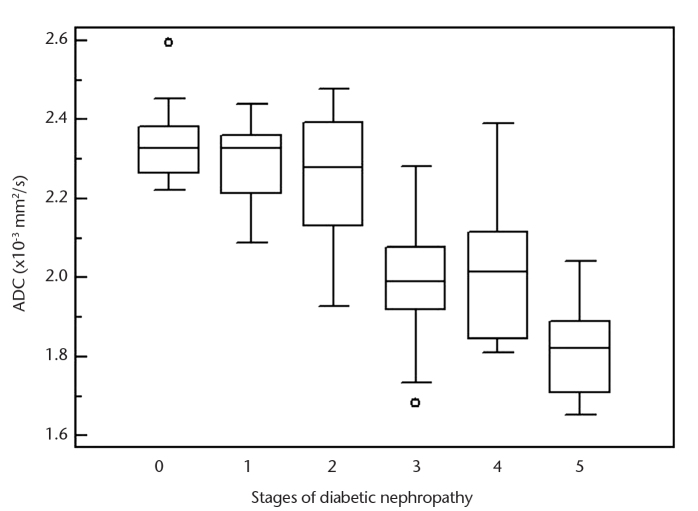
Table 1 shows mean renal ADC values for control and diabetic groups according to disease stage. The graph illustrates the spread of renal ADC values in healthy control subjects and in patients at different stages of diabetic kidney disease (Fig. 2). There was no significant difference between right and left renal ADC values in patient and control groups (P = 0.855 and P = 0.756, respectively). There were no significant differences between mean renal ADC values of control group and stage 1 patients (P = 0.944), control group and stage 2 patients (P = 0.613), stage 1 and stage 2 patients (P = 0.977), and stage 3 and stage 4 patients (P = 0.990). Mean renal ADC values of stage 3 and stage 4 patients were significantly lower compared with the control group, stage 1 patients and stage 2 patients (P < 0.001, for all comparisons). Similarly, mean renal ADC values of stage 5 patients were significantly lower compared with stage 4 (P = 0.003), stage 3 (P = 0.020), stages 2, 1, or control group (P < 0.001, for all three). According to these results, patients were categorized as group 1 (stage 1+2, n=34), group 2 (stage 3+4, n=34), and group 3 (stage 5, n=10). In post hoc comparisons group 3 and group 2 had significantly lower mean renal ADC than group 1 (P < 0.001, for both) or the control group (P < 0.001, for both), and group 3 had significantly lower mean renal ADC values than group 2 (P = 0.001), but there was no significant difference between group 1 and the control group (P = 0.522) (Table 2).
Table 1.
Mean renal ADC values according to stages of diabetic nephropathy
| Control group (n=22) | Stage 1 (n=16) | Stage 2 (n=18) | Stage 3 (n=18) | Stage 4 (n=16) | Stage 5 (n=10) | |
|---|---|---|---|---|---|---|
| Right renal mean ADC | 2.33±0.11 | 2.27±0.11 | 2.26±0.20 | 1.99±0.19 | 1.99±0.19 | 1.84±0.13 |
| Left renal mean ADC | 2.34±0.09 | 2.31±0.12 | 2.25±0.16 | 1.98±0.17 | 2.04±0.18 | 1.78±0.15 |
| Bilateral renal mean ADC | 2.33±0.09 | 2.29±0.10 | 2.25±0.16 | 1.98±0.16 | 2.01±0.17 | 1.81±0.12 |
Values indicate the mean±standard deviation (×10−3 mm2/s).
ADC, apparent diffusion coefficient.
Figure 2.
Box plot shows range of renal apparent diffusion coefficient (ADC) values according to stages of diabetic kidney disease. Stage 0 indicates the control group.
Table 2.
Mean renal ADC values according to grouped stages of diabetic nephropathy
| Control group | Group 1 (stages 1 and 2) | Group 2 (stages 3 and 4) | Group 3 (stage 5) | |
|---|---|---|---|---|
| Bilateral renal mean ADC | 2.33±0.09 | 2.28±0.14a | 2.00±0.17a | 1.81±0.12a |
Values indicate the mean±standard deviation (×10−3 mm2/s).
ADC, apparent diffusion coefficient.
P < 0.001, when comparing groups by ANOVA.
In patients with diabetic nephropathy, a significant negative correlation was found between the mean renal ADC values and the disease stage (r=−0.751, P < 0.001), a significant positive correlation was found between the mean renal ADC values and estimated GFR values (r=0.642, P < 0.001), and a significant negative correlation was found between the mean renal ADC values and urinary albumin excretion in 24-hour urine collection (r=−0.419, P < 0.001).
Discussion
In this study, we found a significant negative correlation between the mean renal ADC values and clinical stages of diabetic nephropathy, using quantitative DWI. Also, we found a significant positive correlation with GFR value and a significant negative correlation with urinary albumin excretion in diabetic patients. Additionally, we identified significantly lower mean renal ADC values in stage 3, 4 and 5 diabetic patients compared to healthy control subjects, but no significant differences in earlier diabetic stages.
Similarly, previous studies have shown that renal ADC values are reduced in a variety of acute and chronic kidney diseases, and there is a significant correlation between ADC and GFR values (4–11). Namimoto et al. (4) reported that ADC values measured in the cortex and the medulla in kidneys of patients with chronic or acute renal failure were significantly lower than the values in normal kidneys. In an experimental rat diabetic nephropathy study investigating the combined use of DWI and blood oxygen level-dependent imaging, which can detect parenchymal hypoxia, Ries et al. (5) found significantly lower renal ADC values that correlated with histopathologic findings and concluded that ADC measurements may be a sensitive indicator of the severity of ischemic lesions. Carbone et al. (6) reported a good correlation between GFR and renal ADC values in a preliminary study with 14 patients. Xu et al. (7) also showed significantly lower renal ADC values in impaired kidneys compared with normal kidneys, and found a positive correlation between GFR and renal ADC values. In a study by Xu et al. (8), the ADC values of kidneys were significantly lower than normal at most stages of chronic kidney disease, except stage 1, and a negative correlation was shown between the renal ADC values and serum creatinine levels of the patients. In a study using diffusion tensor imaging (DTI) in 16 diabetic patients, Lu et al. (10) reported significantly lower renal medullary ADC and fractional anisotropy (FA) and cortical ADC values in diabetics with estimated GFR <60 mL/min/1.73 m2, and lower medullary ADC and FA values in diabetics with estimated GFR ≥60 mL/min/1.73 m2, probably related to interstitial fibrosis, glomerulosclerosis, and tubular damage, as shown in a rat model of diabetic nephropathy (11). In a recent human study with DWI, Inoue et al. (9) showed a statistically significant correlation between renal ADC and estimated GFR values in the chronic kidney disease patients with diabetes (n=43) and without diabetes (n=76). On the contrary, Gaudiano et al. (14) found no significant correlation between medullary and cortical FA and ADC, and estimated GFR, in a preliminary DTI study with marked heterogeneous chronic renal disease patient population (n=45). These results point to the potential role of renal ADC measurements in the evaluation of nephropathy. However, these studies had heterogeneous groups and/or smaller patient populations compared with our study. To our knowledge, this is the first study to correlate all the clinical stages of diabetic nephropathy with renal ADC values.
In the last decades, tremendous increases in the number of diabetics with end-stage kidney disease makes it necessary to establish more efficient monitoring and treatment modalities in these patients. Currently, it is understood that diabetic nephropathy is a more heterogeneous disease than initially thought, in terms of progression of renal dysfunction. Different clinical scenarios may occur in diabetic nephropathy setting. Some patients may have significant loss of GFR while still normoalbuminuric. On the other hand, some patients with microalbuminuria may not progress to the level of significant proteinuria and GFR loss, and may even show some reversal in renal dysfunction (15). Therefore, we need better parameters to predict progression of nephropathy in diabetic patients.
With advances in MRI hardware and new sequences, DWI has become a useful tool for the detection and characterization of tumors and treatment monitoring in oncologic patients (16). For the evaluation of functional status of the kidney in diffuse parenchymal pathologies, renal ADC and FA measurements appear to be feasible and promising (17). Since the ADC is dependent on capillary perfusion and molecular diffusion of water in biologic tissues, alteration of the ADC provides information about microstructural changes in the tissue of interest. Changes in the water content of the renal tissue and intrarenal blood and tubular flow may affect the renal ADC values (18). In the earlier stages of diabetic nephropathy with microalbuminuria, GFR is normal or increased. A reduction in GFR reflects reduction of hyperfiltration. In this way, a lower rate of water transfer across the interstitial space leads to reduced diffusion. When overt proteinuria occurs, histopathologic abnormalities are often far advanced. Progressive glomerulosclerosis and tubulointerstitial fibrosis formed in the later stages may also restrict water diffusion (19). In line with these changes in renal tissue composition, in our study, we found that significant decrease in renal ADC values appear in stage 3 of diabetic nephropathy, and diffusion restriction in renal parenchyma becomes more prominent in end-stage kidney disease. Our results suggest that renal quantitative DWI may be helpful in the follow-up of diabetic patients on serial studies and in predicting the progression or regression of the kidney disease. However, further studies are necessary to establish the significance and potential practicality of using DWI.
This study has some limitations. Although our study has the largest number of patients in the literature, relatively small number of stage 5 diabetic nephropathy may be a limitation. Absence of histopathologic correlation may be another limitation; however, clinical staging is a widely accepted method for detection and prediction of diabetic nephropathy. Also, as ROI placement and reading was performed by one reader only, interobserver variability was not assessed. ROI measurements were restricted to the renal cortex; renal medullary changes were not evaluated in this study. Although multiple ROIs were placed in the cortical regions, there may have been a slight interference with medullary areas, which may have affected our results. Further functional MRI, including DTI, studies on both cortical and medullary changes in diabetic nephropathy may contribute to the understanding of the disease process. On the other hand, we ignored the influence of hypertension, since hypertension was reported to have no effect on renal ADC value, even though it causes end-organ damage (20). Additionally, there may be some other factors, such as age or environmental effects, which may be associated with alterations in renal morphology or function (21, 22). Although the proportion of our geriatric patients was very small, renal aging may potentially influence our outcomes. Furthermore, DWI itself also has some limitations, including lower signal-to-noise ratio and image distortions.
In conclusion, our study demonstrates that renal ADC values correlate negatively with the stages of diabetic nephropathy. As a relatively simple and noninvasive tool without contrast media administration, renal quantitative DWI may potentially play a role in making clinical decisions in the follow-up of diabetic nephropathy.
Acknowledgments
The authors thank Dr. Mehtap Türkay from the Department of Public Health, Faculty of Medicine, Akdeniz University, Antalya for her valuable contributions during statistical analysis of the data.
Footnotes
Financial disclosure
Support for this study was provided by Scientific Research Projects Council of Pamukkale University (Project No. 2008TPF025).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Parving HH. Diabetic nephropathy: prevention and treatment. Kidney Int. 2001;60:2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner BM, editor. Brenner and Rector’s The Kidney. 8th ed. Philadelphia, PA: Saunders Elsevier; 2007. pp. 1265–1298. [Google Scholar]
- 3.Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent patients. Kidney Int. 1997;52:1369–1374. doi: 10.1038/ki.1997.463. [DOI] [PubMed] [Google Scholar]
- 4.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832–837. doi: 10.1002/(sici)1522-2586(199906)9:6<832::aid-jmri10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17:104–113. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 6.Carbone SF, Gaggioli E, Ricci V, Mazzei F, Mazzei MA, Volterrani L. Diffusion-weighted magnetic resonance imaging in the evaluation of renal function: a preliminary study. Radiol Med. 2007;112:1201–1210. doi: 10.1007/s11547-007-0217-6. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging. 2007;26:678–681. doi: 10.1002/jmri.20979. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Fang W, Ling H, Chai W, Chen K. Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol. 2010;20:978–983. doi: 10.1007/s00330-009-1619-8. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Sedor JR, Gulani V, et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol. 2011;34:476–482. doi: 10.1159/000333044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hueper K, Hartung D, Gutberlet M, et al. Magnetic resonance diffusion tensor imaging for evaluation of histopathological changes in a rat model of diabetic nephropathy. Invest Radiol. 2012;47:430–437. doi: 10.1097/RLI.0b013e31824f272d. [DOI] [PubMed] [Google Scholar]
- 12.Tsitouridis I, Michaelides M, Sidiropoulos D, Arvanity M. Renal emphysema in diabetic patients: CT evaluation. Diagn Interv Radiol. 2010;16:221–226. doi: 10.4261/1305-3825.DIR.2130-08.1. [DOI] [PubMed] [Google Scholar]
- 13.McGowan TA, Ziyadeh FN. Clinical course and management of diabetic nephropathy. In: Greenberg A, Cheung AK, editors. Primer on Kidney Diseases. 4th ed. Philadelphia, PA: Saunders Elsevier; 2005. pp. 249–255. [Google Scholar]
- 14.Gaudiano C, Clementi V, Busato F, et al. Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol. 2013;23:1678–1685. doi: 10.1007/s00330-012-2749-y. [DOI] [PubMed] [Google Scholar]
- 15.Najafian B, Mauer M. Morphological features of declining renal function in type 1 diabetes. Semin Nephrol. 2012;32:415–422. doi: 10.1016/j.semnephrol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Türkbey B, Aras Ö, Karabulut N, et al. Diffusion-weighted MRI for detecting and monitoring cancer: a review of current applications in body imaging. Diagn Interv Radiol. 2012;18:46–59. doi: 10.4261/1305-3825.DIR.4708-11.2. [DOI] [PubMed] [Google Scholar]
- 17.Gürses B, Kılıçkesmez O, Taşdelen N, Fırat Z, Gürmen N. Diffusion tensor imaging of the kidney at 3 Tesla MRI: normative values and repeatability of measurements in healthy volunteers. Diagn Interv Radiol. 2011;17:317–22. doi: 10.4261/1305-3825.DIR.3892-10.1. [DOI] [PubMed] [Google Scholar]
- 18.Notohamiprodjo M, Reiser MF, Sourbron SP. Diffusion and perfusion of the kidney. Eur J Radiol. 2010;76:337–347. doi: 10.1016/j.ejrad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JL, Rusinek H, Chandarana H, Lee VS. Functional MRI of the kidneys. J Magn Reson Imaging. 2013;37:282–293. doi: 10.1002/jmri.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yıldırım E, Güllü H, Çalışkan M, Karadeli E, Kırbaş I, Müderrisoğlu H. The effect of hypertension on the apparent diffusion coefficient values of kidneys. Diagn Interv Radiol. 2008;14:9–13. [PubMed] [Google Scholar]
- 21.Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int. 2008;74:710–720. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 22.Tarnoki DL, Tarnoki AD, Littvay L, et al. Genetic and environmental variance of renal parenchymal thickness: a twin study. Croat Med J. 2013;54:550–554. doi: 10.3325/cmj.2013.54.550. [DOI] [PMC free article] [PubMed] [Google Scholar]