Abstract
PURPOSE
We aimed to validate actually achieved macroscopic ablation volumes in relation to calculated target volumes using four different radiofrequency ablation (RFA) systems operated with default settings and protocols for 3 cm and 5 cm target volumes in ex vivo bovine liver.
MATERIALS AND METHODS
Sixty-four cuboid liver specimens were ablated with four commercially available RFA systems (Radionics Cool-tip, AngioDynamic 1500X, Boston Scientific RF 3000, Celon CelonPower LAB): 16 specimens for each system; eight for 3 cm, and eight for 5 cm. Ablation diameters were measured, volumes were calculated, and RFA times were recorded.
RESULTS
For the 3 cm target ablation volume, all tested RFA systems exceeded the mathematically calculated volume of 14.14 cm3. For the 3 cm target ablation volume, mean ablation volume and mean ablation time for each RFA system were as follows: 28.5±6.5 cm3, 12.0±0.0 min for Radionics Cool-tip; 17.1±4.9 cm3, 9.36±0.63 min for AngioDynamic 1500X; 29.7±11.7 cm3, 4.60±0.50 min for Boston Scientific RF 3000; and 28.8±7.0 cm3, 20.85±0.86 min for Celon Celon-Power LAB. For the 5 cm target ablation volume, Radionics Cool-tip (48.3±9.9 cm3, 12.0±0.0 min) and AngioDynamic 1500X (39.4±16.2 cm3, 19.59±1.13 min) did not reach the mathematically calculated target ablation volume (65.45 cm3), whereas Boston Scientific RF 3000 (71.8±14.5 cm3, 9.15±2.93 min) and Celon CelonPower LAB (93.9±28.1 cm3, 40.21±1.78 min) exceeded it.
CONCLUSION
While all systems reached the 3 cm target ablation volume, results were variable for the 5 cm target ablation volume. Only Boston Scientific RF 3000 and Celon CelonPower LAB created volumes above the target, whereas Radionics Cool-tip and AngioDynamic 1500X remained below the target volume. For the 3 cm target ablation volume, AngioDynamic 1500X with 21% deviation was closest to the target volume. For the 5 cm target volume Boston Scientific RF 3000 with 10% deviation was closest.
Radiofrequency ablation (RFA) is a minimally invasive technique for eliminating both primary tumors and metastases. It may be particularly useful for treating patients with inoperable lesions or contraindications to open surgery. Since its introduction, percutaneous ablation has been established as an effective and safe treatment (1, 2), especially in patients with primary and secondary malignancies of the liver (3, 4), the kidney (5, 6), the lung (7, 8), and the breast (9, 10).
As radiofrequency (RF) energy can only be deployed in a closed electrical circuit, monopolar RFA devices may require up to four neutral electrodes (grounding pads), commonly placed on the thighs. The large surface of the grounding pads (manufacturer-specific, up to 200 cm2) is intended to prevent excessive heating at the skin level; the surface of the active part(s) of the RF electrode(s) is about 100 times smaller (manufacturer-specific, usually 1–5 cm2) than the grounding pad surface area.
Instead of monopolar systems with grounding pads, a different technique to apply RF energy is to use bipolar or multipolar devices (3, 11, 12). In bipolar devices, both the cathode and the anode are positioned within the active tip of the electrode, separated by an insulator. The current is applied between the electrodes; no grounding pads are needed. Multipolar systems induce synergetic heat effects by using a switching algorithm between two or more electrodes to induce synergetic heat effects (3).
The volume and shape of the coagulation necrosis (due to possibly different diameter extensions in the three spatial dimensions) achievable with standard clinical RF generators (apart from the generators’ monopolar, bipolar or multipolar nature) depend especially on the impact of the energy applied, probe geometry, duration of heat exposure, fluid content of the target tissue, organ perfusion, and blood vessel density (13). Additionally, in in vivo settings, the so-called heat-sink effect has to be taken into account. The fluid content and perfusion of the tissue and blood vessel density in the target organ have been described as the main factors dissipating heat from the target site and thereby resulting in a smaller ablation volume (6).
For hepatocellular carcinoma for example, based on commonly accepted patient selection criteria, only some patients are suited for conventional surgery, mainly because patients present with poor Child-Pugh status and/or metastases in both hepatic lobes at diagnosis. Delis and Dervenis (14) report that less than 30% of hepatocellular carcinoma patients are eligible for liver resection; thus, approximately 70% require different treatment approaches.
RFA may be regarded as the most commonly used interventional modality in clinical practice, either for sole intervention or in combination with other methods, such as transarterial chemoembolization.
In a patient, the actual volume of an induced RFA can usually not be dissected and assessed macroscopically after the procedure. Interventionalists have to rely on imaging to assess the ablation volume and geometry of the induced coagulation necrosis after ablation, and hence therapeutic success. With the different RFA systems available on the market, it is valuable to have a sound understanding of the systems’ behavior, especially in terms of ablation volume and geometry the specific RFA system creates, that one intends to clinically use.
The objective of this study was to validate the measured size of actual ablation volumes in relation to mathematically calculated expected ablation volumes of four different RFA systems using default settings and protocols for 3 cm and 5 cm target ablation volumes in bovine ex vivo liver.
Materials and methods
Study design
The study was designed to test four different RFA systems (three monopolar and one bipolar/multipolar) in terms of their ability to consistently achieve two different target ablation volumes (3 cm and 5 cm) in bovine ex vivo livers. RFA volumes were created in a total of 64 cuboid liver specimens (n=16 for each generator; n=8 for 3 cm and n=8 for 5 cm). For every test series, cuboids of the same liver (previously warmed to physiological body temperature of 37°C) were used for all four generators to most accurately ensure comparable tissue conditions (including impedance). All ablations were performed according to the manufacturers’ protocols and/or on the basis of personal consultation with the manufacturers (details are provided in the sections on the individual RF generators below). To ensure that the entire coagulation volume could be measured, the electrodes were placed in the center of the specimen in order to provide sufficient liver tissue for the coagulation necrosis. The samples were comfortably larger than the expected lesion size. All trials were performed without repositioning the electrodes. The liver specimens were transected and inspected after RFA. Ablation diameters were recorded and volumes calculated. Temperatures inside the ablation volume during intervention and RF times were recorded.
RFA systems
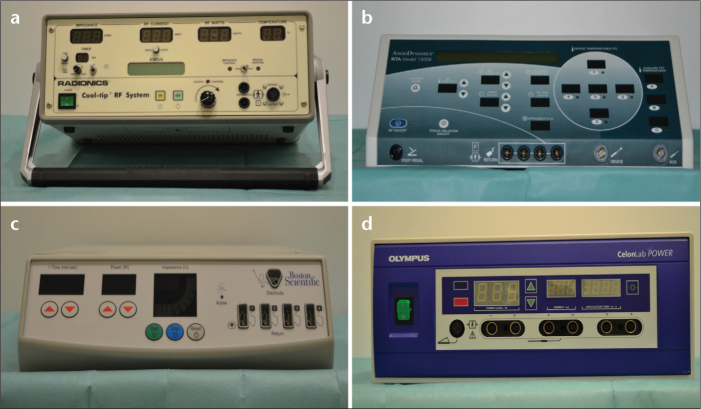
The following four RFA systems, three monopolar and one bipolar/multipolar (Fig. 1, Table 1), were used as follows:
Cool-Tip by Radionics/Valleylab/Covidien, Mansfield, Massachusetts, USA ;
1500X RF by AngioDynamics, Latham, New York, USA ;
RF 3000 by Boston Scientific, Natick, Massachusetts, USA ;
CelonPower LAB by Celon, Teltow, Germany.
Figure 1. a–d.
Photographs of the four RFA generators used. Cool-Tip by Radionics/Valleylab/Covidien (a), 1500X RF by AngioDynamics (b), RF 3000 by Boston Scientific (c), and CelonPower LAB by Celon (d).
Table 1.
Overview of the four RFA systems used
| Cool-Tip | 1500X RF | RF 3000 | CelonPower LAB | |
|---|---|---|---|---|
| Manufacturer | Radionics | AngioDynamics | Boston Scientific | Celon |
| Energy transmission | Monopolara | Monopolar | Monopolar | Bi-, multipolar |
| Frequency | 480 kHz | 460 kHz | 480 kHz | 470 kHz |
| Maximum power | 200 W | 250 W | 200 W | 250 W |
| Applicators | 1a | 1 | 1 | 1–3 |
| MR-compatible electrodeb | - | + | - | + |
| Active tipc | 3/2.5 cm | 3/5 cm | 3/5 cm | 3/4 cm |
| Induced energy control mechanism | Impedance-controlled | Temperature-controlled | Impedance-controlled | Impedance-controlled |
The cluster electrode contains three electrodes in one applicator.
The RF generators must under any circumstance remain outside the scanner room.
For the 3/5 cm target ablation volumes, respectively.
RFA, radiofrequency ablation.
The three monopolar generators have one (Radionics), two (AngioDynamics), and four (Boston Scientific) input plugs for the grounding pads. For all monopolar devices, a current balancer provided by Boston Scientific was used to evenly distribute the current to all input plugs in order to avoid unequal resistance caused by unequal distribution of current (Fig. 2). For the bipolar Celon system, the use of the balancer was not necessary.
Figure 2.
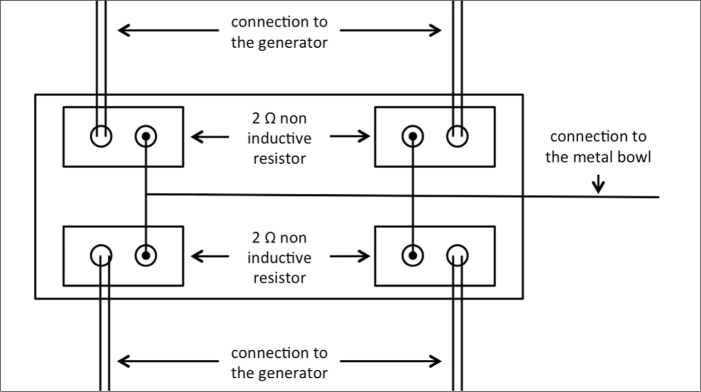
Schematic diagram for the mode of operation of the current balancer provided by Boston Scientific. The current balancer was used to evenly distribute the current to all input plugs, irrespective of the monopolar RF device used.
Cool-Tip
The Cool-Tip generator yields a maximum output of 200 W and can be operated in a multipolar mode in combination with an optional switching controller, which was not used in our study. The system uses an impedance-controlled algorithm. For ablation, it is important to use chilled saline in order to avoid carbonization at the electrode. Hence, the temperature at the measurable tip of the electrode should not exceed 12°C, whereas the temperature that induces coagulation necrosis in the RFA volume is, of course, much higher. If the impedance rises, the system automatically stops further delivery of energy and starts again automatically after an internally predefined decrease in impedance. For the 3 cm ablation volume, a single Cool-Tip RF electrode was used, and for the 5 cm ablation volume, the Cool-Tip cluster electrode was used. To create the latter volume, three electrodes were positioned in a prefabricated triangle with an interelectrode distance of 0.5 cm. In both cases, the ablation time was set to 12 min.
1500X RF
The monopolar generator 1500X RF has a maximum output of 250 W. Unlike the Radionics Cool-Tip, this model is temperature controlled. Every second tine of the electrode serves as a temperature probe for monitoring the inner temperature of the parenchyma during the RFA procedure. According to the protocol of AngioDynamics, a power of 150 W and a target temperature of 105°C were used for all lesions; RF time was set to 9 min to create the 3 cm ablation volume and to 15 min to create the 5 cm ablation volume. Based on the temperature-controlled mechanism, the generator only started to count off the time once the target temperature was actually reached. The RFA procedure may thus take longer than the target RF time (Tables 2 and 3). The StarBurst XL RF electrode was used for the creation of both 3 cm and 5 cm ablation volumes. This electrode can be adjusted/expanded from 2 to 5 cm. The electrode has a nine-tine Christmas tree configuration, with the option of introducing fluids, such as saline, into the tissue via an open perfusion system.
Table 2.
Performance parameters of the four RFA systems for 3 cm target ablation volume
| Cool-Tip | 1500X RF | RF 3000 | CelonPower LAB | |
|---|---|---|---|---|
| Manufacturer | Radionics | AngioDynamics | Boston Scientific | Celon |
| x-axisa (cm) | 4.0±0.5 | 3.3±0.3 | 3.7±0.6 | 4.0±0.5 |
| y-axisb (cm) | 3.7±0.4 | 3.0±0.6c | 3.8±0.7c | 3.5±0.4 |
| x/y quotientd | 1.09±0.14 | 1.14±0.23 | 0.98±0.18 | 1.17±0.16 |
| z-axise (cm) | 3.8±0.5 | 3.2±0.4 | 3.9±0.5 | 3.9±0.6 |
| Volume (cm3) | 28.5±6.5 | 17.1±4.9f | 29.7±11.7f | 28.8±7.0 |
| Ablation timeg (min) | 12.0±0.0 | 9.36±0.63 | 4.60±0.50 | 20.85±0.86 |
| Temperatureg (°C) | 72.5±14.9 | 60.8±10.9 | 61.6±22.0 | 54.2±15.0 |
| P h | < 0.001 | 0.135 | 0.007 | 0.001 |
| Deviationi (%) | 101 | 21 | 110 | 103 |
Overall P values for ANOVA are significant (P ≤ 0.043): i.e., x-, y-, and z-axis, and volume.
Diameter in the longitudinal axis along the electrode track.
Diameter in the perpendicular axis in the plane created by cutting along the electrode track.
P = 0.04 for AngioDynamics vs. Boston Scientific.
Degree of sphericity: the more spherical, the more the ratio approaches 1.
Diameter in the axis orthogonal to the x-y plane, at the midpoint of the x-axis on both sides, summated.
P = 0.03 for AngioDynamics vs. Boston Scientific.
Values for ablation time and temperature have descriptive character and were not used for ANOVA.
Double t test for paired samples: a value P < 0.05 implies a significant difference between the actual and the mathematically calculated volume.
Percent deviation of the actual volume from the mathematically calculated volume (14.14 cm3).
Data are presented as mean±standard deviation for the 3 cm RF-induced coagulation necrosis.
Table 3.
Performance parameters of the four RFA systems for 5 cm target ablation volume
| Cool-Tip | 1500X RF | RF 3000 | CelonPower LAB | |
|---|---|---|---|---|
| Manufacturer | Radionics | AngioDynamics | Boston Scientific | Celon |
| x-axisa (cm) | 4.4±0.5b | 4.5±0.6b | 4.1±0.5b | 5.4±0.5b |
| y-axisc (cm) | 4.6±0.4d | 4.2±1.0d | 5.9±1.0d | 5.3±0.7 |
| Mean x/y quotiente | 0.96±0.13 | 1.11±0.24 | 0.73±0.23 | 1.02±0.14 |
| z-axisf (cm) | 4.6±0.5g,h | 3.9±0.5g,h | 5.8±0.5h | 6.1±1.1g |
| Volume (cm3) | 48.3±9.9i | 39.4±16.2i,j | 71.8±14.5j | 93.9±28.1i |
| Ablation timek (min) | 12.0±0.0 | 19.59±1.13 | 9.15±2.93 | 40.21±1.78 |
| Temperaturek (°C) | 60.0±23.2 | 57.6±23.0 | 84.5±7.7 | 79.6±18.6 |
| P l | 0.002 | 0.003 | 0.25 | 0.024 |
| Deviationm (%) | 26 | 40 | 10 | 43 |
Overall P values for ANOVA are significant (P ≤ 0.004): i.e., x-, y-, z-axis, x/y quotient, and volume.
Diameter in the longitudinal axis along the electrode track.
P = 0.01 for Radionics vs. Celon; P = 0.001 for AngioDynamics vs. Celon; P < 0.001 for Boston Scientific vs. Celon.
Diameter in the perpendicular axis in the plane created by cutting along the electrode track.
P = 0.03 for Radionics vs. Boston Scientific; P = 0.003 for AngioDynamics vs. Boston Scientific.
Degree of sphericity: the more spherical, the more the ratio approaches 1.
Diameter in the axis orthogonal to the x-y plane, at the midpoint of the x-axis on both sides, summated.
P = 0.002 for Radionics vs. Celon; P < 0.001 for AngioDynamics vs. Celon.
P = 0.02 for Radionics vs. Boston Scientific; P < 0.001 for AngioDynamics vs. Boston Scientific.
P < 0.001 for Radionics vs. Celon; P < 0.001 for AngioDynamics vs. Celon.
P = 0.02 for AngioDynamics vs. Boston Scientific.
Values for ablation time and temperature measurement have descriptive character and were not used for ANOVA.
Double t test for paired samples: a value P < 0.05 implies a significant difference between the actual and the mathematically calculated volume.
Percentage deviation of the actual volume from the mathematically calculated volume (65.45 cm3).
Data are presented as mean±standard deviation for the 5 cm RF-induced coagulation necrosis.
RF 3000
The impedance-controlled RF 3000 yields a maximum output of 200 W. To create a 3 cm ablation volume, according to the protocol provided, the initial output was set to 40 W and increased every 30 s by 10 W, up to a final output of 90 W. In case of an impedance increase ahead of schedule (before reaching the 90 W level, the so-called roll-off), power delivery was automatically discontinued for 30 s and started again with 50% of the roll-off power. To create a 5 cm ablation volume, according to the protocol provided, the initial output was set to 100 W and increased every 30 s by 10 W, up to a final output of 150 W. As for the 3 cm ablation volume, in case of an impedance increase ahead of schedule (before reaching the 150 W level), power delivery was automatically discontinued for 30 s and started again with 50% of the roll-off power. To create the respective ablation volumes, the umbrella-shaped twelve-tine expandable LeVeen electrode was expanded according to the manufacturer’s ablation protocol to a diameter of 3 cm for the 3 cm or to a diameter of 5 cm for the 5 cm ablation volume.
CelonPower LAB
The CelonPower LAB yields a maximum output of 250 W and can be used as a bipolar and multipolar device. Up to three electrodes can be connected to one generator. The electrode contains the electric plus and minus poles at an uninsulated active tip, divided by an insulator. Hence, no dispersive pad is needed. To avoid an impedance rise, 15 current flows among the three electrodes are possible through permutations of the respective plus and minus poles. If the impedance increases between the two active tips, the generator stops power delivery to these active parts (with an automatic algorithm switching to other current pathways), preventing the use of this constellation until local impedance has decreased sufficiently. For the 3 cm lesion, two internally cooled Celon ProSurge electrodes (T30) with a 3 cm active tip were used. According to the protocol, the distance between the two electrodes was set to 1 cm. The power was set to 60 W, and the time was set to 20 min. For the 5 cm lesion, three internally cooled Celon ProSurge electrodes (T40) with a 4 cm active tip were used. According to the protocol, the distance between the three electrodes was set to 2.5 cm in an equilateral triangular configuration. In this case, the power was set to 120 W. The time was set to 40 min.
Liver specimen storage and preparation
The ex vivo trials were performed with fresh bovine livers provided overnight from the local slaughterhouse. A total of 16 fresh livers with peritonea were used, eight livers for the 3 cm target ablation volume and eight livers for the 5 cm target ablation volume. Approximately 10×10×8 cm or larger cuboids of the livers were prepared to ensure that the whole coagulation necrosis after RFA would easily be located inside the parenchyma. Before the specimen preparation for RFA, all liver specimens remained in a closed cold chain of <4°C from the time of slaughter in order to prevent premature denaturation and dehydration. Before RFA, the still-sealed <4°C liver specimens were placed in a plastic tub containing 60 L of water equipped with a heating rod (Eheim Jaeger, Finsterrot, Germany) with maximum power of 200 W and a recirculation pump (Fig. 3). The temperature in the recirculating water was set to 37°C to simulate the physiological body temperature just before RFA.
Figure 3.
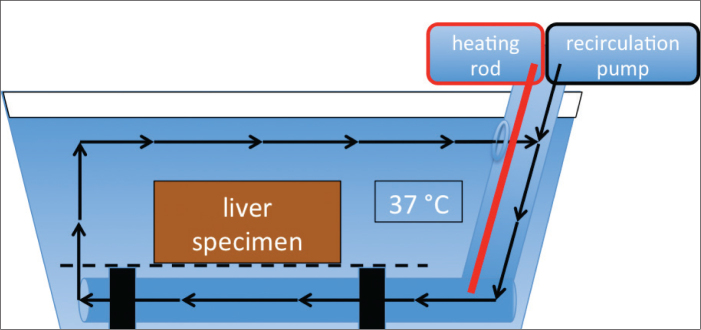
Diagram of 60 L water plastic tub equipped with a heating rod and recirculation pump used to warm the liver specimen to physiological body temperature of 37°C before RFA.
After reaching physiological body temperature, the specimens were transferred into a metal bowl (filled with 37°C 0.9% saline to simulate physiological current flow and heat conduction) for RFA. The metal bowl, connected to the generators input plugs, served as the counter electrode for the current and replaced the dispersive pads used in patients. The shape of the bowl guaranteed a current flow to all sides of the specimen. For each RFA, the electrode(s) was/were maneuvered into the center of the specimen and securely fixed (Fig. 4).
Figure 4.
RFA setup: metal bowl filled with 0.9% saline, heated to 37°C to simulate physiological body temperature, current flow and heat conduction, cuboid liver specimen, and RF electrode (white arrow). To further validate the temperature inside the parenchyma, a fiberoptic measuring system (Neoptix, Québec, Canada; black arrow) was positioned inside the parenchyma 1 cm from the expected center of each lesion.
Lesion size measurement
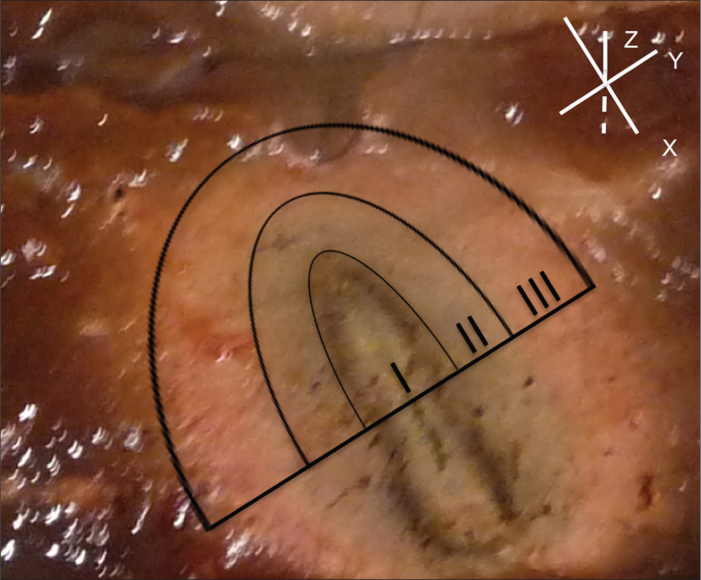
After each RFA, the specimen was cut along its electrode track. This longitudinal axis of the necrosis was defined as the x-axis. The axis perpendicular to the plane created in this way was defined as y-axis. To more accurately characterize the shape and volume of the coagulation necrosis, each created plane was again cut orthogonally to the x-y plane at the midpoint of the x-axis; the resultant cut was measured and summed to obtain the z-axis as a further measure.
Targeted spherical ablation volume is calculated as π×d3/6, so that targeted Vspherical=14.14 cm3 for d=3 cm and targeted Vspherical=65.45 cm3 for d=5 cm. Actual ablation volume is calculated as π×x×y×z/6, in order to account for the possibility of ellipsoid ablation volumes.
All diameters of the ablations were measured, including the transitional zone (Fig. 5). The tissue up to the macroscopically visible hemorrhagic rim has repeatedly been postulated in the literature to correspond to a transitional zone from inner coagulation necrosis to outer normal hepatic tissue (15). Other previous studies likewise included the transitional zone of necrosis in the calculation of necrotic lesion volumes because the histopathological correlation with NADH dehydrogenase, as the vitality marker revealed irreversible cell damage at the necrotic margins and the histological assessment of in vivo specimens revealed irreversibly damaged cells within the hemorrhagic margins (16).
Figure 5.
RFA performed under ex vivo conditions: (I) Necrosis/carbonization zone; (II) coagulation zone; (III) transitional zone. The upper right aspect depicts the three spatial dimensions according to the defined ablation volume alignment in the x-, y-, and z-axes. Note the central indentation of the RF electrode.
Statistical analysis
Descriptive statistical data are presented as mean±standard deviation and included data on diameters in the x-, y-, and z-axes of the RFA as well as the calculated volumes, average temperature measured 1 cm from the RFA center, and duration of RFA.
Differences in each group and between groups of the diameter measurement and volume calculation were evaluated by means of one-way analysis of variance (ANOVA). RFA time and temperature measurement were excluded from calculations in the analysis of variance.
For the analysis, a computer software (Statistical Package for Social Sciences, Version 19.01, SPSS Inc., Chicago, Illinois, USA) was used. To compare the test settings, ANOVA, followed by post-hoc tests with Scheffé’s method, was performed. A double t-test for paired samples was performed to compare actual vs. mathematically calculated volumes Statistical level of significance was set to P < 0.05.
Results
The induced coagulation necroses were homogeneous and continuous for both 3 cm and 5 cm target lesion volumes and for all four generators. Sixty-four thermal ablation volumes were created in 16 livers. For each generator, 16 ablation volumes were produced (eight for 3 cm ablation volumes, and eight for 5 cm ablation volumes).
The performance parameters for the four RFA systems are presented in Table 2 for the 3 cm target ablation volume and in Table 3 for the 5 cm target ablation volume.
3 cm target ablation volume
As indicated in Table 2, all of the RFA systems exceeded 3 cm in diameter in the x-, y-, and z-axes and achieved the expected target volume for 3 cm ablation volume.
The AngioDynamics system was closest to the target volume, with a percentage deviation of 21%. The Radionics (101%), Celon (103%), and Boston Scientific (110%) systems had a greater percentage deviation from the prescribed target volume.
Considering ablation sphericity, the most spherical 3 cm lesion, as indicated by the x/y quotient, was produced with the Boston Scientific system (0.98±0.18 [reciprocal equivalent to 1.02±0.21]). The longest mean ablation diameters of each RFA system in the three spatial dimensions (dominant ablation axis) were reached along the x-axis with the single Cool-Tip electrode for the Radionics, with the nine-tine expandable electrode for the AngioDynamics, and with the Celon ProSurge electrodes for the Celon systems (4.0±0.5, 3.3±0.3, and 4.0±0.5 cm, respectively); along the z-axis as the dominant ablation axis, the twelve-tine expandable electrode for the Boston Scientific system was longest (3.9±0.5 cm). The most ellipsoid ablation volumes in the x-y plane were created by the Celon system (x/y, 1.17±0.16), followed by the AngioDynamics and Radionics systems (x/y, 1.14±0.23 and 1.09±0.14).
The largest mean ablation volume was produced with the monopolar Boston Scientific system with expandable electrodes (29.7±11.7 cm3; mean ablation time, 4.6±0.50 min), followed by the bipolar Celon (28.8±7.0 cm3; mean ablation time, 20.85±0.86 min) and the Radionics systems (28.5±6.5 cm3; mean ablation time, 12±0.00 min; used in monopolar mode). The smallest mean ablation volume was reached with the AngioDynamics system (17.1±4.9 cm3; mean ablation time, 9.36±0.63 min). There was a significant difference in volume between the Boston Scientific and AngioDynamics systems (P = 0.03).
Accounting for the possibility of ellipsoid volumes (Vellipsoid=π*x*y*z/6; with x, y, and z being the mean ablation diameters of the respective spatial axes), all of the tested RFA systems exceeded the mathematically calculated target ablation volume of 14.14 cm3.
5 cm target ablation volume
As indicated in Table 3, only the Celon system achieved at least 5 cm diameters in the x-, y-, and z-axes. The Radionics, Boston Scientific and AngioDynamics systems did not achieve the preset diameters in all three axes.
With a percentage deviation of 10% the Boston Scientific system was closest to the set volume. The Radionics (26%) the AngioDynamics (40%), and the Celon system (43%) had greater percentage deviations from the prescribed target volume.
In terms of ablation sphericity, the most spherical 5 cm lesion, as indicated by the x/y quotient, was produced with the Celon system, using three electrodes in a triangular configuration (x/y, 1.02±0.14). The longest mean ablation diameters within each RFA system in the three spatial dimensions (dominant ablation axis) were reached along the x-axis with the expandable nine-tine electrode for the AngioDynamics system (4.5±0.6 cm), along the y-axis with the Cool-Tip cluster electrode for the Radionics system or with the 12-tine expandable electrode for the Boston Scientific system (4.6±0.4 cm; 5.9±1.0 cm), and along the z-axis with the Celon ProSurge electrodes for the Celon system (6.1±1.1 cm). The most ellipsoid ablation volumes in the x/y plane were created by the Boston Scientific system (x/y, 0.73±0.23 [reciprocal equivalent to 1.37±0.33]).
The largest mean ablation volume was produced with the Celon system (93.9±28.1 cm3; mean ablation time, 40.21±1.78 min), followed by the Boston Scientific (71.8±14.5 cm3; mean ablation time, 9.15±2.93 min) and the Radionics systems (48.3±9.9 cm3; mean ablation time, 12.0±0.0 min; used in monopolar mode). The smallest mean ablation volume was reached with the AngioDynamics system (39.4±16.2 cm3; mean ablation time, 19.59±1.13 min). There was a significant difference in the volumes between the Celon system and both the Radionics and AngioDynamics systems (each P < 0.001), as well as between the AngioDynamics and Boston Scientific systems (P = 0.02).
Accounting for the possibility of ellipsoid volumes, the Radionics system (V=48.3±9.9 cm3) and the AngioDynamics system (V=39.4±16.2 cm3) did not reach the mathematically calculated target ablation volume of 65.45 cm3, whereas the Celon system (V=93.9±28.1 cm3) and the Boston scientific system (V=71.8±14.5 cm3) exceeded it.
Discussion
The aim of this study was to validate the agreement of mathematically calculated ablation volumes with the actual ablation volumes produced with four RFA systems using the default settings. This was achieved by evaluating how accurately the four systems tested achieved the predefined ablation diameters of 3 cm and 5 cm and the associated volumes of 14.14 and 65.45 cm3. Mathematical volumes of 3 cm and 5 cm were tested to challenge the four RFA systems with a common lesion size, as well as with the maximum comparable lesion sizes.
Achieving an adequate predefined ablation volume may be regarded as an important prerequisite for the safe and successful RFA of a tumor. It is therefore essential to most accurately obtain this desired ablation volume without overly exceeding it (and thereby possibly damaging too much healthy tissue or important nearby structures), as well as to be able to reproduce the results with little variability.
Having a sound understanding, not only of the three-dimensional shape but also of the orientation of the (possibly ellipsoid) ablation volume to be induced by the RF device inside the organ is crucial for preventing tumor progression and achieving therapeutic success. In a 5 cm target ablation volume, for instance, it is crucial to be aware that the 12-tine expandable electrode of the Boston Scientific system creates its longest ablation diameter along the y-axis, whereas the ablation diameter along the x-axis is considerably shorter.
For all RFA systems, a homogeneous and continuous target ablation volume with 3 cm in diameter (equivalent to 14.14 cm3) was comfortably achieved. A rather spherical ablation zone was observed with the Boston Scientific system. The other three devices created more ellipsoid ablation volumes.
For the 3 cm ablation, the AngioDynamics system was closest to the mathematically calculated volume, with a deviation of 21% (actual volume of 17.1 cm3 in comparison to the mathematically calculated volume of 14.14 cm3). The other three systems deviated from the target volume by over 100%: Radionics, 101% (actual volume, 28.5 cm3), Celon, 103% (28.8 cm3), and Boston Scientific, 110% (29.7 cm3).
In contrast, the 5 cm diameter target ablation volume (equivalent to 65.45 cm3) was still comfortably exceeded with the Celon system and the Boston Scientific system, whereas the Radionics and the AngioDynamics systems failed to achieve the target volume.
In this regard, several factors have to be taken into account. The Cool-Tip cluster electrode for the Radionics system recently received a modified approval for ablations of up to 4.2×4.5 cm in diameter (tested by the manufacturer in 20°C bovine livers without repositioning of the electrode; manufacturer’s data). For larger target ablation volumes, the use of a switching controller in combination with the RF generator and three separate monopolar electrodes is recommended. An alternative option is to reposition the electrode after the first ablation to expand the target volume.
To obtain an impression of which maximum diameters and volumes the Radionics system in combination with the cluster electrode is able to create in our standardized test set-up, the trials were performed without switching the controllers.
With the temperature-controlled AngioDynamics system, the achieved ablation diameters for the 5 cm target ablation volume ranged from 3.5 to 6.3 cm. In this RF series, the first two and again the last two ablation diameters exceeded 5 cm. We assumed that this is a coincidence or an unidentified malfunction of the generator, as no other explanation is apparent for this variation.
The large target ablation volume achieved with the Celon system (93.9±28.1 cm3) may be favored by the long ablation time of 40 min in comparison to 20, 12, and 9 min for the other generators (AngioDynamics, Radionics, Boston Scientific, respectively). The long ablation time has the potential to smoothen and to more consistently secure a higher energy deposit in the parenchyma. In any case, the tendency to create large volumes with this RFA system in 5 cm target diameter ablations should be taken into account in order to most accurately obtain the desired ablation volume without exceeding. With the 12-tine monopolar LeVeen electrode of the Boston Scientific system, the ablation volume (71.8±14.5 cm3) was still above the target volume of 65.45 cm3. For the 5 cm target volume, the Boston Scientific system created a doughnut-like outer ablation shape (no central area of sparing inside the produced lesion). The Radionics system created a spherical coagulation, while the AngioDynamics and Celon systems created ellipsoid coagulations. One factor contributing to the dimensions of the produced ablation volumes may also be heat trapping between the electrodes (17, 18).
For the 5 cm ablation, the Boston Scientific system, with a 10% deviation and an actual volume of 71.8 cm3, was closest to the mathematically calculated volume of 65.45 cm3. The Radionics system (26%, 48.3 cm3) and the AngioDynamics system (40%, 39.4 cm3) remained below the target volume. The Celon system created a volume 93.9 cm3, corresponding to a 43% deviation from the target volume.
Increased vascularization, large vessels in the vicinity (heat sink effect), or changes in the parenchymal consistency (e.g., cirrhosis or chemotherapy) may have different effects on the ablation volumes achieved in patients that were not accounted for in this ex vivo study design. Additionally, the ablations were performed in disease- and tumor-free parenchyma, which may render different volumes compared to the RFA of a lesion.
A comparison of the energy deposited in the tissue by the four generators investigated was not possible, as the Celon and the AngioDynamics systems do, but the Radionics and Boston Scientific systems do not, feature the required software. Calculating the electricity consumption was not considered appropriate, as the generators (as well as possible supplements such as saline pumps) require different amounts of power, precluding a straightforward comparison of the energy delivered to the tissue from an electricity consumption approach.
A possible limitation of the RF technique in general, concerning its heat conduction in a clinical setting, is the heat-mediated dehydration of a tissue, which is followed by carbonization and an increase in impedance that may reduce the RF output into the target volume.
The wide range of achieved ablation diameters and volumes (Tables 2, 3) and the standard deviations for each of the four devices, despite the highly controlled environment, requires some explanation. In an ex vivo setting, a consistent ablation volume is only achieved if the tissue conditions are identical, especially if the impedance remains constant. Small differences in hydration or tissue properties (e.g., fatty liver, animal age) are sufficient to cause differences in impedance and hence to result in deviations from the target diameter and volume. In the devices used in our study, a marked rise in impedance shuts down the power supply to avert the carbonization caused by the dehydration of the surrounding tissue (11). This power fluctuation may result in lower or higher ablation volumes, explaining the wide range of volumes measured.
In vivo studies have shown that the RFA volumes achieved in cirrhotic livers or after chemotherapy may vary due to changes in the liver impedance (19). Therefore, the assessment of ablation-induced tissue lesions by validated imaging techniques is essential.
However, although the experiments were conducted under near physiological conditions, the heat sink effect, a main cause of inadequate ablation (3), was absent in our experimental setup. Therefore, a larger necrosis volume should not necessarily be considered a disadvantage of each respective system; rather, this may provide the reserve necessary to compensate for a high vessel density in tumor tissues.
When using one of the tested RFA systems, it is important to keep in mind that our results are ex vivo results and may differ from ablation diameters in vivo.
In conclusion, it is neither intended nor possible to make a straightforward recommendation in favor of one of the RFA systems tested in this study. The RFA systems available on the market differ. Interventionalists need to keep this in mind and gain a sound understanding, especially of the ablation volume and geometry of their specific RFA system in order to achieve therapeutic success. Other factors must also be taken into account, ranging from the patient group to be treated (tumor entity, location, configuration, volume, shape, and disease progression) to the integration into a hospital’s workflow (subjective ease of handling, acceptance by other staff working with the RFA system in daily clinical routine, RF time, and economic efficiency).
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Tacke J. Percutaneous radiofrequency ablation-clinical indications and results. Rofo. 2003;175:156–168. doi: 10.1055/s-2003-37226. [DOI] [PubMed] [Google Scholar]
- 2.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radiofrequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 3.Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT., Jr Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol. 2009;20:118–124. doi: 10.1016/j.jvir.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol. 2013 Aug 3; doi: 10.1007/s00270-013-0708-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Tacke J, Mahnken AH, Gunther RW. Percutaneous thermal ablation of renal neoplasms. Rofo. 2005;177:1631–1640. doi: 10.1055/s-2005-858819. [DOI] [PubMed] [Google Scholar]
- 6.Mahnken AH, Rohde D, Brkovic D, Gunther RW, Tacke JA. Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol. 2005;46:208–214. doi: 10.1080/02841850510015938. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35:343–350. doi: 10.1007/s00270-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 8.Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol. 2012;81:395–399. doi: 10.1016/j.ejrad.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 9.Ohtani S, Kochi M, Ito M, et al. Radiofrequency ablation of early breast cancer followed by delayed surgical resection--a promising alternative to breast-conserving surgery. Breast. 2011;20:431–436. doi: 10.1016/j.breast.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253:861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clasen S, Rempp H, Schmidt D, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol. 2012;81:111–113. doi: 10.1016/j.ejrad.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Bruners P, Schmitz-Rode T, Gunther RW, Mahnken A. Multipolar hepatic radiofrequency ablation using up to six applicators: preliminary results. Rofo. 2008;180:216–222. doi: 10.1055/s-2008-1027184. [DOI] [PubMed] [Google Scholar]
- 13.Desinger K, Stein T, Tschepe J. Investigations on radiofrequency current application in bipolar technique for interstitial thermotherapy (RF-ITT) Minimal Invasive Medizin. 1996;7:92–97. [Google Scholar]
- 14.Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. 2008;14:3452–3460. doi: 10.3748/wjg.14.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151–158. doi: 10.3348/kjr.2001.2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streitparth F, Knobloch G, Balmert D, et al. Laser-induced thermotherapy (LITT)--evaluation of a miniaturised applicator and implementation in a 1.0-T highfield open MRI applying a porcine liver model. Eur Radiol. 2010;20:2671–2678. doi: 10.1007/s00330-010-1831-6. [DOI] [PubMed] [Google Scholar]
- 17.Frericks BB, Ritz JP, Roggan A, Wolf KJ, Albrecht T. Multipolar radiofrequency ablation of hepatic tumors: initial experience. Radiology. 2005;237:1056–1062. doi: 10.1148/radiol.2373041104. [DOI] [PubMed] [Google Scholar]
- 18.Pereira PL, Trubenbach J, Schenk M, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004;232:482–490. doi: 10.1148/radiol.2322030184. [DOI] [PubMed] [Google Scholar]
- 19.Glaiberman CB, Pilgram TK, Brown DB. Patient factors affecting thermal lesion size with an impedance-based radiofrequency ablation system. J Vasc Interv Radiol. 2005;16:1341–1348. doi: 10.1097/01.RVI.0000179796.92828.54. [DOI] [PubMed] [Google Scholar]