Abstract
Hepatocellular adenoma (HCA) is a generally benign liver tumor with the potential for malignancy and bleeding. HCAs are categorized into four subtypes on the basis of genetic and pathological features: hepatocyte nuclear factor 1α-mutated HCA, β-catenin-mutated HCA, inflammatory HCA, and unclassified HCA. Magnetic resonance imaging (MRI) plays an important role in the diagnosis, subtype characterization, and detection of HCA complications; it is also used to differentiate HCA from focal nodular hyperplasia. In this review, we present an overview of the genetic abnormalities, oncogenesis, and typical and atypical MRI findings of specific subtypes of HCA using contrast-enhanced MRI with or without hepatobiliary contrast agents (gadobenate dimeglumine and gadoxetate disodium). We also discuss their different management implications after diagnosis.
Hepatocellular adenoma (HCA) is a rare, benign tumor of the liver that occurs predominantly in young and middle-aged women (1). In contrast to focal nodular hyperplasia (FNH), HCA may involve complications, such as a life-threatening bleeding and malignant degeneration (1–3). The strong association between the occurrence of HCA and the use of oral contraceptives was first acknowledged in 1970s (4), and the incidence of HCA is now thought to be 30 times greater in oral contraceptive users compared to nonusers (5, 6). A dose-dependent association and spontaneous regression following the withdrawal of estrogens have also been described (4, 7). However, the exact role of estrogen in HCA is still poorly understood.
In this review, we present an overview of the typical and atypical magnetic resonance imaging (MRI) findings of different HCAs compared to FNH, and discuss various pitfalls that may be encountered with MRI.
The new classification of hepatocellular adenoma
A molecular and immunohistochemical classification of HCA has been introduced by the Bordeaux group (Table 1) (8, 9); in this classification, HCAs are divided into four subgroups based on clear genetic differences.
Table 1.
Immunohistochemical and MRI signs used for differentiating the HCA subtypes
| HNF1A-mutated HCA | β-catenin- mutated HCA | Inflammatory HCAa | Unclassified HCA | |
|---|---|---|---|---|
| Immunohistochemical staining | ||||
| Glutamine synthetaseb | − | +/− | +/− | − |
| β-catenin | − | + | +/− | − |
| C-reactive protein | − | − | + | − |
| Serum amyloid A | − | − | + | − |
| LFABP | − | + | + | + |
| Typical MRI findingsc | Diffuse homogenous lesional steatosis | Faint scar strong, diffuse, | Atoll sign and hyperintense signal on T2-weighting |
Inflammatory HCAs may show β-catenin positivity, in these cases most lesions may also show homogenous glutamine synthetase staining.
In exceptional cases, glutamine synthetase can be normal in β-catenin-mutated HCAs.
HCA, hepatocellular adenoma; HNF1A, hepatocyte nuclear factor 1α; MRI, magnetic resonance imaging; LFABP, liver fatty acid binding protein.
The first group accounts for 30% to 40% of cases and is defined by the presence of hepatocyte nuclear factor 1α (HNF1A) mutations (10). The HNF1A gene controls lipid metabolism and mediates the downregulation of liver fatty acid binding protein (LFABP). LFABP downregulation is typically observed using LFABP staining, which is 100% accurate (8, 11). The most typical presentation of group 1 (i.e., HNF1A-mutated) HCA lesions is the aberrant presence of internal steatosis. It should be noted, however, that internal steatosis is not sufficient for diagnosing this HCA subtype, as other subtypes may also exhibit internal steatosis (12). We prefer to avoid the term steatotic HCA, which is used in some literature on this particular subtype (13). Some patients with HNF1A-mutated HCA have an associated mutation that is thought to be responsible for maturity-onset noninsulin-dependent diabetes (14). Therefore, once this HCA subtype has been diagnosed, the clinician should be warned of the possibility of underlying diabetes.
A second group containing 10% to 15% of cases is identified by the presence of activating mutations of β-catenin (15). While β-catenin is phosphorylated and degraded by proteasomes under physiological conditions, tumors fail to downregulate β-catenin and instead show nuclear accumulation of the protein (16). This accumulation is known to trigger an important signaling pathway in several cancers but is typified by hepatocellular carcinoma (17). Although β-catenin activation is not sensitive enough for immunohistochemical classification due to its visibility in only a few sporadic nuclei (12), another product of the same β-catenin activation pathway, glutamine synthetase, is homogeneously expressed in most lesions and can therefore be used as a sensitive and specific immunohistochemical stain for β-catenin-mutated HCAs (8, 12). It should be noted that FNH also presents with diffuse glutamine synthetase staining. Differentiation between these two benign lesions (β-catenin-mutated HCA and FNH) is not difficult with large resections, as the map-like staining in FNH contrasts with the homogeneous expression in β-catenin-mutated HCA (12). Nonetheless, it can be challenging for the pathologist to make this differentiation in small biopsies.
The third group contains 40% to 50% of HCA cases and shows evidence of the inflammatory response (18). A typical feature of these lesions is the activation of acute phase inflammation proteins, such as serum amyloid A and C-reactive protein (8). This subgroup is also linked to obesity and high alcohol intake (19). Importantly, these lesions are associated with homogenous glutamine synthetase and β-catenin staining. Therefore, it has been stated that these cases could be at increased risk of malignant degeneration (12).
The final group accounts for 10% to 25% of HCA cases and shows no specific genetic alterations; this group is therefore currently referred to as “unclassified” (16).
Implications for diagnosis
While immunohistochemical staining of LFABP, β-catenin, glutamine synthetase, serum amyloid A, and C-reactive protein has proven to be very effective in differentiating between the four subtypes of HCA, it is also useful in the differentiation between HCA and FNH (9, 20). In a retrospective, multicenter study in France, Bioulac-Sage et al. (20) found that the certainty of biopsy diagnosis of FNH increased from 53% to 87% when additional immunohistochemistry markers and glutamine synthetase immunostaining were used. The certainty of biopsy diagnosis of HCA also increased from 59% to 74% when immunohistochemistry analyses were used. Prior to the introduction of these markers, HCA was often misdiagnosed as FNH during histological examination, especially when inflammatory HCA (formerly known as telangiectatic FNH) was involved (19). The latter lesions include the bile duct proliferation seen in FNH but demonstrate the behavior of a HCA (including the previously described risk of malignant degeneration and bleeding). This confusion should be taken into account when evaluating older radiologic descriptions of HCA and FNH, as the reference standard has only become significantly more accurate since the introduction of these markers.
Conventional MRI findings for hepatocellular adenoma
HCA is primarily diagnosed by noninvasive imaging techniques (1), and typical MRI characteristics can be used for differentiating between HCA and FNH. According to recent literature, some MRI findings are more typical than others (Table 2). T1-weighted hyperintensity seems to be prevalent only in HCAs and is most likely caused by blood degeneration products or glycogen storage (21–23).
Table 2.
Classical MRI signs that can be used for differentiating HCAs from FNHs
| HCA | FNH |
|---|---|
| Strong signs | Strong signs |
| Strong hyperintensity on T2-weighting | Spoke wheel appearance of scar |
| Hyperintensity on T1-weighting | |
| Cystic parts | |
| Hemorrhagic parts | |
| Diffuse intralesional steatosis | |
| Atoll sign | |
| Weak signs | Weak signs |
| Faint arterial enhancement | Scar |
| Liver steatosis | Lobular contours |
| Multiple lesions | Strong arterial enhancement |
We distinguish strong signs from weak signs. Strong signs are defined to be characteristic for that lesion. Weak signs are more common in either HCA of FNH, but can occur in both.
FNH, focal nodular hyperplasia; HCA, hepatocellular adenoma; MRI, magnetic resonance imaging.
Other signs of non-neoplastic degeneration can be appreciated in some HCAs through the visualization of internal bleeding cysts, necrosis or fluid. To the best of our knowledge, these finding have not been described for FNH (2). A strong T2-weighted hyperintense band in peripheral areas of the lesion is a typical sign of HCA and was only (possibly) visible in one FNH case in our series to date (see below) (21).
The finding of a central scar is a more commonly described imaging characteristic of FNH (2). However, we also found linear central scars in 21% of confirmed HCAs (21). This sign, which is characterized by a T2-weighted central scar with late enhancement on delayed phase, does not seem to be sufficiently robust to allow differentiation between these two lesions. Additionally, β-catenin-mutated HCAs appear to have a faint central scar in up to 75% of cases (Fig. 1) (21). On the other hand, in our experience a typical ‘spoke wheel’ appearance of a central scar is only visible in FNH (Fig. 2) (2).
Figure 1. a, b.
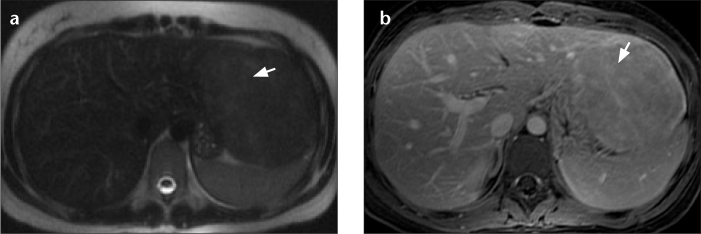
Axial T2-weighted (a) and T1-weighted (b) MR images of the liver after contrast injection. Histologically, β-catenin staining is positive in this patient. The faint scar-like region is T2 hyperintense (a, arrow) with late enhancement after contrast injection of a nonspecific gadolinium-based contrast agent (b, arrow), a finding that is similar to that expected in focal nodular hyperplasia (FNH). In our opinion, a lesion with a scar but lacking a “spoke wheel” aspect is not only typical of FNH, but may also occur in hepatocellular adenoma (HCA) and more typically in β-catenin-mutated HCA.
Figure 2. a–c.
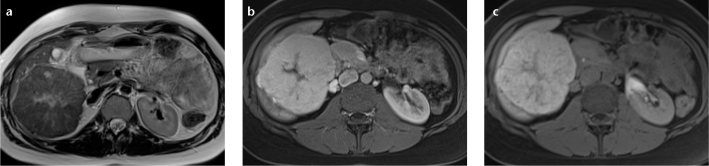
Axial T2-weighted (a), venous phase T1-weighted (b), and 20-min T1-weighted (c) MR images of a patient with the “spoke wheel” aspect that is typical for FNH. A central scar with divergences to the periphery is visible on T2-weighting and is reminiscent of a “spoke wheel” (a). These so-called spokes are normally enhanced in the venous phase using a nonspecific contrast agent or gadobenate dimeglumine. However, when using gadoxetate disodium, the central scar and the spokes are hypointense due to pseudo washout (b). After 20 min, the majority of the lesion (except the scar) becomes hyperintense due to internal bile duct proliferation (c).
The surrounding liver steatosis, intralesional fat accumulation, faint arterial enhancement pattern and presence of multiple lesions are more or less typical for HCA but can also occur in FNH (21–23). A lobular border is often described as a typical sign for the diagnosis of FNH, but our research group and others have found that the occurrence of a lobular border is not uncommon in HCAs (21, 22).
MRI findings based on the new subclassification
Laumonier et al. (23) were the first to publish the typical MRI features of HCA according to the subgroup classification. A homogeneous dropout of signal on the T1-weighted out-of-phase sequence had a sensitivity of 86.7% and a specificity of 100% for HNF1A-mutated HCA (Fig. 3), whereas this dropout was absent or only focal (heterogeneous) in inflammatory HCA. Moreover, marked hyperintensity on T2-weighted sequences was found to be typical for inflammatory HCA, with a sensitivity of 85.2% and a specificity of 87.5%.
Figure 3. a, b.
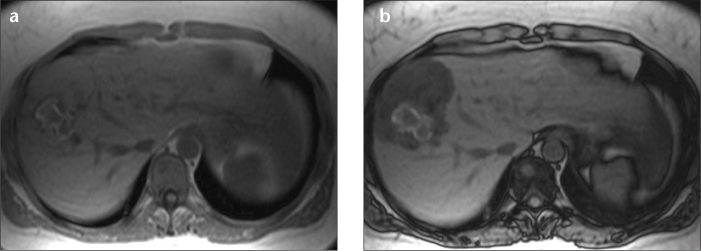
Axial T1-weighted in-phase (a) and opposed-phase (b) MR images of a hepatocyte nuclear factor 1α (HNF1A)-mutated HCA. This lesion shows the typical diffuse and homogeneous suppression of signal in the lesion due to fat accumulation. Also note the blood residue centrally located in the lesion as a T1-weighted hyperintense zone. In addition to steatosis, this lesion showed no liver fatty acid binding protein staining upon histology, which is a very sensitive marker for HNF1A mutation.
We found similar presentations for HNF1A-mutated HCA and inflammatory HCA (21). Additionally, we showed that a hyperintense rim on the T2-weighted sequences was diagnostic for inflammatory HCA. This hyperintense rim sign corresponds to sinusoidal dilatation (Fig. 4) and is also referred to as an “atoll sign”. This characteristic includes a hyperintense rim in the periphery of the lesion on T2-weighted imaging with an isointensity in the center of the lesion reminiscent of the sea within an atoll (24). Small intralesional T2-hyperintense nodules can be found in the center of the lesion (small islands) (21).
Figure 4. a–c.
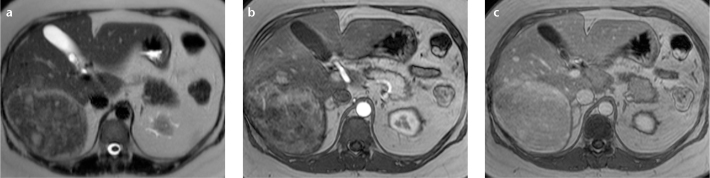
Axial T2-weighted (a), arterial (b), and venous (c) T1-weighted MR images of the liver with a typical presentation of an inflammatory HCA. The lesion presents with an “atoll sign” (a), which appears as a T2-weighted hyperintense rim (peripheral island with central sea) with or without central hyperintense islands as can be found inside an atoll. The lesion is hypervascular in the arterial phase (b), with late enhancement of the peripheral rim and central islands (c). Upon histology, these lesions demonstrate positive immunostaining of inflammatory proteins including C-reactive protein and serum amyloid A. The T2-weighted hyperintense rim is thought to be caused by local peliosis.
Several authors have reported that a faint scar may be a possible sign of β-catenin-mutated HCA (21, 23, 25), but the number of published β-catenin-mutated HCA cases to date is too low to draw any firm conclusions.
MRI findings after using liver-specific contrast agents
With the introduction of hepatobiliary contrast agents, an important tool became available for differentiating HCA from FNH (26).
Two gadolinium-based contrast agents are currently available, gadobenate dimeglumine (Multihance, Milan, Italy) and gadoxetate disodium (Primovist, Berlin, Germany; brand name in the USA, Eovist).
Both agents show hepatocyte uptake and biliary excretion, with a hyperintense liver in the hepatocyte phase on T1-weighted imaging as a consequence. Lesions that involve bile ducts also appear to enhance. This observation is typically the case in FNHs and in some hepatocellular carcinomas (26). The most important finding is that HCAs do not normally show hepatocyte uptake and biliary excretion and are therefore observed as hypointense compared to the liver in the hepatocyte phase.
There are strong arguments for the uptake of gadoxetate disodium in FNHs and some hepatocellular carcinomas by organic anion transporter polypeptide channels (25, 27, 28). However, one study suggested another mechanism of uptake of both gadobenate dimeglumine and gadoxetate disodium in the liver (29).
Yoneda et al. (27) found that organic anion transporter polypeptide-8 is present in the periphery and not in the center of FNH, explaining the peculiar aspect of some FNH cases in the hepatocyte phase (ring-enhancement-type).
Gadobenate dimeglumine is excreted by the liver at a significantly lower percentage (5%) than gadoxetate disodium (50%) (26). As a result, gadoxetate disodium produces a significantly greater signal intensity change in the hepatocyte phase than gadobenate dimeglumine. This greater change can be helpful in cases where liver activity and thus changes in enhancement are low, as is observed in cirrhosis. One cause of this lower liver enhancement is the competitive uptake between these contrast agents and bilirubin, with uptake of the latter increased in patients with liver cirrhosis. Additionally, lesions that have an intrinsic T1-weighted hyperintensity, as frequently observed in HCAs, may often show insufficient liver enhancement when using gadobenate dimeglumine. This insufficient enhancement results in the hyperintense HCA becoming isointense instead of hypointense compared to the surrounding liver (30).
Whether there is a difference in up-take of hepatobiliary contrast agents between the different HCA subtypes is still largely unknown. Our recently reported initial results noted that inflammatory HCAs range from isointense to hyperintense in relation to the liver in the hepatocyte phase (Fig. 5) (30). However, the underlying cause of this finding was not obvious. Of particular interest is the fact that, in contrast to other HCAs, inflammatory HCA often harbors internal bile ducts, which could possibly explain the late isointensity in the hepatocyte phase. However, this phenomenon is difficult to discern from isointensity due to intrinsic hyperintensity prior to contrast.
Figure 5. a–c.
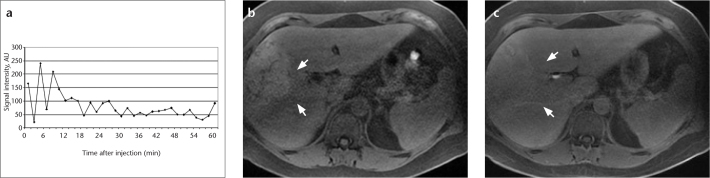
Enhancement curve (a) of a known inflammatory HCA that was scanned every 2.5 min over a period of one hour following the injection of gadobenate dimeglumine. This approach allows an enhancement curve that shows no late enhancement to be reproduced. Comparison of axial fat-saturated T1-weighted sequences prior to contrast injection (b) and one hour after contrast injection (c) reveals that the lesion remains isointense (arrows) due to intrinsic hyperintensity.
Implications for patient care
Though the use of either gadobenate dimeglumine or gadoxetate disodium may often be indicated, it can be difficult for a radiologist to choose between these two contrast agents. The major differences are listed in Table 3.
Table 3.
Comparison of the hepatobiliary contrast agents gadobenate dimeglumine and gadoxetate disodium for liver imaging
| Gadobenate dimeglumine | Gadoxetate disodium | |
|---|---|---|
| T1 effect | +++ | + |
| Cost | + | ++ |
| Shortness of investigation | + | ++(+) |
| Differentiation of HCA from FNH | + | + |
| Differentiation of benign liver lesions (hemangiomas) | ++ | − (+) |
| Hepatobiliary excretion | + | +++ |
FNH, focal nodular hyperplasia; HCA, hepatocellular adenoma.
As previously noted by others, gadoxetate disodium may be preferred over gadobenate dimeglumine when considering the rapid hepatobiliary phase at 20 minutes or earlier, whereas in patients scanned with gadobenate dimeglumine, the hepatobiliary phase is obtained with a second MRI performed a minimum of one hour after the initial MRI and injection of contrast agent (24). However, it is important to note that the early hepatobiliary excretion of gadoxetate disodium prevents the dynamic phase from being separated from the hepatobiliary phase, which results in the disturbing interpretation of the dynamic phase in cases of suspected hemangiomas. Additionally, when using gadoxetate disodium, a large number of liver lesions become hypointense in the late dynamic phase due to pseudo washout rather than actual washout (due to enhancement of the surrounding liver). Finally, radiologists should be aware that because of this pseudo washout, the central scar in FNH may become hypointense instead of hyperintense in the late venous phase when using gadoxetate disodium.
Another argument in favor of gadobenate dimeglumine over gadoxetate disodium is the considerably lower cost of the former. This difference is most likely due to the wider range of possible indications for the use of gadobenate dimeglumine, which appears to be a useful, nonspecific gadolinium-based contrast agent. With the less pronounced T1-effect of gadoxetate disodium, this agent is indicated for specific liver imaging only.
In the case of an atypical liver lesion where the differential diagnosis is broad and includes hemangioma and malignant tumors, a nonspecific contrast agent or gadobenate dimeglumine is generally preferred over gadoxetate disodium. In cases where MRI is prescribed solely for the differentiation between FNH and HCA, gadoxetate disodium should be sufficient, which is often the case when preliminary external computed tomography or MRI data are present.
Although contrast-enhanced ultra-sonography is beyond the scope of this review, it may be speculated that in the near future differentiation between the different HCA subtypes may also be possible with the use of contrast sonography (31).
Additionally, other markers may come to play a more prominent role in the differentiation of HCA from FNH or hepatocellular carcinomas (32, 33).
Malignant degeneration of hepatocellular adenoma
Malignant degeneration of HCA has been reported but seems to occur very rarely. In a recent meta-analysis, a total of 1635 HCA cases were examined and yielded an overall malignant transformation frequency of 4.2%. Most of those lesions were larger than 5 cm in diameter. However, the described overall frequency is most likely an overestimation due to the limited sample sizes of the studies and the fact that most studies only described resected HCA (29).
Furthermore, it is interesting that these so-called malignant degenerated HCAs show a pattern of a nodule within a nodule or two tumors lying adjacent to each other (28, 30). In this situation, it might be questioned whether these nodules have undergone the same cellular changes.
The malignant potential of HCA seems stronger in β-catenin-mutated HCA, which is also more prevalent in men (15). Bioulac-Sage et al. (15) reported the occurrence of six hepatocellular carcinomas in 128 proven cases of HCA, all of which were β-catenin-mutated HCAs, whether they were inflammatory or not.
Management of these β-catenin-mutated HCAs can vary between hospitals from conservative to aggressive (surgical). Additional experiences from more hospitals are needed to correlate the classification system with clinical management (28).
Internal bleeding in hepatocellular adenoma
Rupture and bleeding have both been described in HCA; the hypervascular nature of these lesions may make them more prone to bleeding. In contrast, FNHs do not demonstrate rupture or bleeding despite their hypervascularity. In a meta-analysis, we determined a HCA rupture prevalence of 16% (3). However, the papers studied did show some evidence of selection bias, as patients with silent HCAs were underreported due to the lack of any indication for imaging in most of these patients. The meta-analysis showed that larger lesions (larger than 5 cm) are more often involved. There also seems to be no difference in the occurrence of internal bleeding depending on HCA subtype. After analyzing their own database, Bioulac-Sage et al. (15) also found no difference in the chance of macroscopic bleeding between HNF1A-mutated HCA and inflammatory HCA, the two most prevalent subtypes (Fig. 4).
As the actual risk of bleeding is reported to be higher in lesions larger than 5 cm, resection of these lesions may be warranted. Decisions related to the treatment of smaller HCAs are still complex and other characteristics, such as male gender, β-catenin positivity, and the wish to become pregnant, can also play an important role. Larger studies will need to be performed to evaluate whether there is a correlation between the subtype and bleeding and rupture. Pregnant patients with HCA deserve special attention, as maternal and fetal mortality rates are not negligible (34–36). We advocate that patients with a HCA larger than 5 cm be treated before becoming pregnant. In cases where a HCA larger than 5 cm is found during pregnancy, the data are currently too sparse to draw firm conclusions, and we believe that the management in these patients should be individualized (36, 37).
Hepatocellular adenoma in males
HCA mainly occurs in women in their second and third decades of life (5), and the incidence in men is very low. Most described cases of HCA in men occurred after the chronic intake of exogenous hormones (38) or in men with glycogen storage disease (39). Because of its low incidence, its diagnosis should be questioned, and we advise that a biopsy be performed more often when these tumors are found in men to exclude the presence of a premalignant or malignant liver tumor.
Conclusion
In summary, advances in recent years have greatly improved our understanding of HCA, leading to subtype recognition and better differentiation from FNH. Reports and conclusions drawn from MRI data in older studies, particularly prior to the introduction of glutamine synthetase staining, should therefore be evaluated with caution.
HCA diagnosis has been improved by additional experiences with atypical findings and potential pitfalls that may be encountered with MRI. The introduction of hepatobiliary contrast agents has helped even more, particularly in differentiating between HCA and FNA, and these agents have now become valuable tools in daily clinical practice. However, the radiologist must always be aware of possible errors in diagnosis due to differences between hepatobiliary and nonspecific gadolinium-based contrast agents. With respect to the treatment of HCA, a number of recommendations can be made; however, other questions require further studies.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hussain SM, van den Bos IC, Dwarkasing RS, Kuiper JW, den Hollander J. Hepatocellular adenoma: findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16:1873–1886. doi: 10.1007/s00330-006-0292-4. [DOI] [PubMed] [Google Scholar]
- 2.Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics. 2004;24:3–19. doi: 10.1148/rg.241035050. [DOI] [PubMed] [Google Scholar]
- 3.van Aalten SM, de Man RA, IJzermans, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911–916. doi: 10.1002/bjs.8762. [DOI] [PubMed] [Google Scholar]
- 4.Buhler H, Pirovino M, Akobiantz A, et al. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775–782. [PubMed] [Google Scholar]
- 5.Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423–435. doi: 10.1055/s-2007-1007370. [DOI] [PubMed] [Google Scholar]
- 6.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- 7.Steinbrecher UP, Lisbona R, Huang SN, Mishkin S. Complete regression of hepatocellular adenoma after withdrawal of oral contraceptives. Dig Dis Sci. 1981;26:1045–1050. doi: 10.1007/BF01314771. [DOI] [PubMed] [Google Scholar]
- 8.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 9.Bioulac-Sage P, Balabaud C, Bedossa P, et al. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol. 2007;46:521–527. doi: 10.1016/j.jhep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Bluteau O, Jeannot E, Bioulac-Sage P, et al. Biallelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama TE, Ward JM, Gonzalez FJ. Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1alpha (HNF1alpha). Alterations in fatty acid homeostasis in HNF1alpha-deficient mice. J Biol Chem. 2000;275:27117–27122. doi: 10.1074/jbc.M004388200. [DOI] [PubMed] [Google Scholar]
- 12.van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, de Man RA, IJzermans JN. Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J Hepatol. 2011;55:120–125. doi: 10.1016/j.jhep.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182–1191. doi: 10.1002/hep.24147. [DOI] [PubMed] [Google Scholar]
- 14.Jeannot E, Mellottee L, Bioulac-Sage P, et al. Spectrum of HNF1A somatic mutations in hepatocellular adenoma differs from that in patients with MODY3 and suggests genotoxic damage. Diabetes. 2010;59:1836–1844. doi: 10.2337/db09-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 16.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Cadoret A, Ovejero C, Saadi-Kheddouci S, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 18.Bioulac-Sage P, Blanc JF, Rebouissou S, Balabaud C, Zucman-Rossi J. Genotype phenotype classification of hepatocellular adenoma. World J Gastroenterol. 2007;13:2649–2654. doi: 10.3748/wjg.v13.i19.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bioulac-Sage P, Rebouissou S, Sa Cunha A, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211–1218. doi: 10.1053/j.gastro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Bioulac-Sage P, Cubel G, Taouji S, et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol. 2012;36:1691–1699. doi: 10.1097/PAS.0b013e3182653ece. [DOI] [PubMed] [Google Scholar]
- 21.van Aalten SM, Thomeer MG, Terkivatan T, et al. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172–181. doi: 10.1148/radiol.11110023. [DOI] [PubMed] [Google Scholar]
- 22.Bieze M, van den Esschert JW, Nio CY, et al. Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol. 2012;199:26–34. doi: 10.2214/AJR.11.7750. [DOI] [PubMed] [Google Scholar]
- 23.Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808–818. doi: 10.1002/hep.22417. [DOI] [PubMed] [Google Scholar]
- 24.Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005;236:166–177. doi: 10.1148/radiol.2361040338. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda N, Matsui O, Kitao A, et al. Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol. 2012;30:777–782. doi: 10.1007/s11604-012-0115-2. [DOI] [PubMed] [Google Scholar]
- 26.Fidler J, Hough D. Hepatocyte-specific magnetic resonance imaging contrast agents. Hepatology. 2011;53:678–682. doi: 10.1002/hep.24158. [DOI] [PubMed] [Google Scholar]
- 27.Yoneda N, Matsui O, Kitao A, et al. Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol. 2012;30:499–508. doi: 10.1007/s11604-012-0085-4. [DOI] [PubMed] [Google Scholar]
- 28.Narita M, Hatano E, Arizono S, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793–798. doi: 10.1007/s00535-009-0056-4. [DOI] [PubMed] [Google Scholar]
- 29.Pascolo L, Cupelli F, Anelli PL, et al. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun. 1999;257:746–752. doi: 10.1006/bbrc.1999.0454. [DOI] [PubMed] [Google Scholar]
- 30.Thomeer MG, Willemssen FE, Biermann KK, et al. MRI features of inflammatory hepatocellular adenomas on hepatocyte phase imaging with liver-specific contrast agents. J Magn Reson Imaging. 2014;39:1259–1264. doi: 10.1002/jmri.24281. [DOI] [PubMed] [Google Scholar]
- 31.Laumonier H, Cailliez H, Balabaud C, et al. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol. 2012;199:341–348. doi: 10.2214/AJR.11.7046. [DOI] [PubMed] [Google Scholar]
- 32.Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723–1728. doi: 10.5858/132.11.1723. [DOI] [PubMed] [Google Scholar]
- 33.Palmer PE, Christopherson WM, Wolfe HJ. Alpha1-antitrypsin, protein marker in oral contraceptive-associated hepatic tumors. Am J Clin Pathol. 1977;68:736–739. doi: 10.1093/ajcp/68.6.736. [DOI] [PubMed] [Google Scholar]
- 34.van der Windt DJ, Kok NF, Hussain SM, et al. Case-orientated approach to the management of hepatocellular adenoma. Br J Surg. 2006;93:1495–1502. doi: 10.1002/bjs.5511. [DOI] [PubMed] [Google Scholar]
- 35.Noels JE, van Aalten SM, van der Windt DJ, et al. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54:553–558. doi: 10.1016/j.jhep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 36.van Aalten SM, Broker ME, Busschbach JJ, et al. Pregnancy and liver adenoma management: PALM-study. BMC Gastroenterol. 2012;12:82. doi: 10.1186/1471-230X-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broker ME, IJzermans JN, van Aalten SM, de Man RA, Terkivatan T. The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol. 2012;2012:725–735. doi: 10.1155/2012/725735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psatha EA, Semelka RC, Armao D, Woosley JT, Firat Z, Schneider G. Hepatocellular adenomas in men: MRI findings in four patients. J Magn Reson Imaging. 2005;22:258–264. doi: 10.1002/jmri.20375. [DOI] [PubMed] [Google Scholar]
- 39.Howell RR, Stevenson RE, Ben-Menachem Y, Phyliky RL, Berry DH. Hepatic adenomata with type 1 glycogen storage disease. JAMA. 1976;236:1481–1484. [PubMed] [Google Scholar]


