Abstract
Detection and characterization of focal lesions in the cirrhotic liver may pose a diagnostic dilemma. Several benign and malignant lesions may be found in a cirrhotic liver along with hepatocellular carcinoma (HCC), and may exhibit typical or atypical imaging features. In this pictorial essay, we illustrate computed tomography and magnetic resonance imaging findings of lesions such as simple bile duct cysts, hemangioma, focal nodular hyperplasia-like nodules, peribiliary cysts, intrahepatic cholangiocarcinoma, lymphoma, and metastases, all of which occur in cirrhotic livers with varying prevalences. Pseudolesions, such as perfusion anomalies, focal confluent fibrosis, and segmental hyperplasia, will also be discussed. Imaging characterization of non-HCC lesions in cirrhosis is important in formulating an accurate diagnosis and triaging the patient towards the most appropriate management.
The detection and characterization of focal lesions in a cirrhotic liver on computed tomography (CT) and magnetic resonance imaging (MRI) is a challenging task due to the marked changes in the organ architecture. Although hepatocellular carcinoma (HCC) is the most frequent primary tumor arising in a cirrhotic liver, several other benign and malignant lesions may be encountered in this setting (1, 2). It is thus not surprising that CT and MRI have limited specificity for the diagnosis of HCC in cirrhosis.
Imaging characterization of focal lesions in cirrhosis is of the utmost importance for appropriate patient management. The radiologist’s primary task is to maximize tumor detection (i.e., minimize false negatives), because missing the diagnosis of HCC may preclude potentially curative therapies, such as hepatic resection, percutaneous ablation procedures, and, in selected patients, liver transplantation. However, it is equally important to avoid the misdiagnosis of benign liver lesions as HCC (i.e., minimize false positives) because this diagnostic interpretation may incorrectly increase the tumor burden. This may also result in the ineligibility of the patient for potentially curative treatments or the inappropriate assignment of increased priority scores for patients on the waiting list for liver transplantation. In this paper, we discuss and illustrate CT and MRI features of both common and uncommon non-HCC liver lesions occurring in cirrhotic patients.
Focal lesions beyond hepatocellular carcinoma in cirrhotic liver
A wide spectrum of benign and malignant lesions other than HCC may be encountered in the cirrhotic liver. Familiarity with the imaging findings of these lesions may be helpful for radiologists for precise diagnosis and appropriate management. The Table summarizes the incidence and frequency of imaging features of focal liver lesions occurring in the cirrhotic and noncirrhotic liver.
Table.
Incidence and imaging features of focal liver lesions in cirrhotic and noncirrhotic liver
| Cirrhotic liver | Noncirrhotic liver | |
|---|---|---|
| Hemangioma | ||
| Nodular peripheral enhancement | Less common | Common |
| Isoattenuation to vessels | Less common | Common |
| Centripetal filling | Less common | Common |
| Moderate/strong hyperintensity on T2-weighted images | Common | Common |
| Incidence | Less common | Common |
| Focal nodular hyperplasia | ||
| Strong homogeneous enhancement on hepatic arterial phase | Common | Common |
| Isoattenuation/intensity to liver parenchyma on nonenhanced, hepatic venous, and delayed phases | Common | Common |
| Incidence | Rare | Common |
| Adenoma | ||
| Incidence | Extremely rare | Rare |
| Peribiliary cysts | ||
| Incidence | Rare | Rare |
| Cholangiocarcinoma | ||
| Ring enhancement | Common | Common |
| Capsular retraction | Less common | Common |
| Delayed enhancement | Less common | Common |
| Incidence | Less common | Common |
| Metastases | ||
| Ring enhancement | Common | Common |
| Incidence | Rare | Common |
| Arteriovenous shunts | ||
| Incidence | Common | Uncommon |
| Focal confluent fibrosis | ||
| Capsular retraction | Common | N/A |
| Enhancement on delayed phase | Common | N/A |
| Incidence | Uncommon | N/A |
N/A, not applicable.
Benign lesions
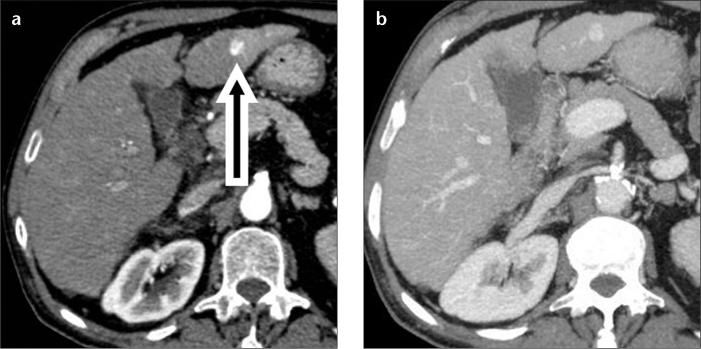
Hemangioma is seen less frequently in cirrhotic patients than in the general population (3). There are two main challenges in the diagnosis of hemangioma in cirrhosis. The first is that of a capillary hemangioma, also commonly referred to as a flash-filling hemangioma. The differential diagnosis with small hypervascular HCC is based on the differing enhancement patterns. Although both lesions will typically show hypervascularity in the hepatic arterial phase, flash-filling hemangiomas demonstrate strong and homogeneous attenuation, typically matching the enhancement of the aorta during the hepatic arterial phase (Fig. 1a), and of the intrahepatic veins during the hepatic venous phase (Fig. 1b). This enhancement pattern differs significantly from that of HCC, where tumor enhancement during the hepatic arterial phase is typically milder and followed by washout during the hepatic venous and delayed phases (i.e., tumor hypoattenuation/intensity compared with the surrounding liver parenchyma). In small HCC, washout may be absent (Fig. 2), likely because of conservation of the portal blood supply, thus making the tumor appear isoattenuating/isointense to the surrounding liver parenchyma (4). This appearance may pose a diagnostic challenge for the differential diagnosis with a small capillary hemangioma.
Figure 1. a, b.
A 67-year-old male with hepatitis C-related cirrhosis and capillary hemangioma. Axial contrast-enhanced CT images show a lesion (a, arrow) in the left hepatic lobe with flash-filling enhancement in the hepatic arterial phase, and isoattenuation to blood vessels in both arterial (a) and hepatic venous (b) phases.
Figure 2. a, b.
Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MR images in a 71-year-old female with hepatitis C-related cirrhosis and small atypical HCC. Hepatic arterial phase fat saturated volumetric T1-weighted image (a) shows a small, hypervascular nodule (arrow) in the left hepatic lobe. Hepatic delayed phase fat-saturated volumetric T1-weighted image (b) at the same level shows isointensity of the lesion to the liver parenchyma. The lack of washout did not allow a confident diagnosis of HCC. In this patient, the diagnosis of HCC was based on lesion hypointensity in the hepatobiliary phase image (not shown). Note the hypointense lesion (arrowheads) in segment VII, representing complete necrosis of the hepatocellular carcinoma after transcatheter arterial chemoembolization.
The second scenario is when the fibrotic and scarring processes distort liver architecture, also causing a fibrotic involution of the lesion. In such cases, hemangiomas are typically hypovascular, losing features commonly observed in noncirrhotic livers, such as globular peripheral enhancement patterns and isointensity to vessels on multiphasic imaging (Fig. 3) (3). It is our anecdotal observation that in these instances, differential diagnosis with those HCC referred to as “hypovascular,” because of the lack of enhancement in the hepatic arterial phase, will be based on lesion shape and attenuation. Hypovascular HCC is a spherical lesion, slightly hypoattenuating, and therefore recognizable, versus the surrounding liver in the venous phase (Fig. 4), while fibrotic hemangiomas commonly have an irregular shape and more pronounced hypoattenuation in comparison with the surrounding liver on unenhanced and postcontrast images (Fig. 3b). Furthermore, strong hyperintensity on T2-weighted MR images remains helpful information for the more confident characterization of atypical hemangiomas in cirrhotic livers.
Figure 3. a, b.
A 70-year-old female with alcoholic cirrhosis and shrinking hemangioma. Hepatic venous phase CT image (a) shows a lesion (arrow) in the right hepatic lobe, which demonstrated nearly complete homogeneous enhancement with isoattenuation to the intrahepatic blood vessels. Hepatic venous phase image (b) through the same level obtained three years later, demonstrating a substantial decrease in the size of the lesion, which showed a lack of contrast enhancement. Note the absence of characteristic CT features of hemangioma in the later CT scan.
Figure 4. a, b.
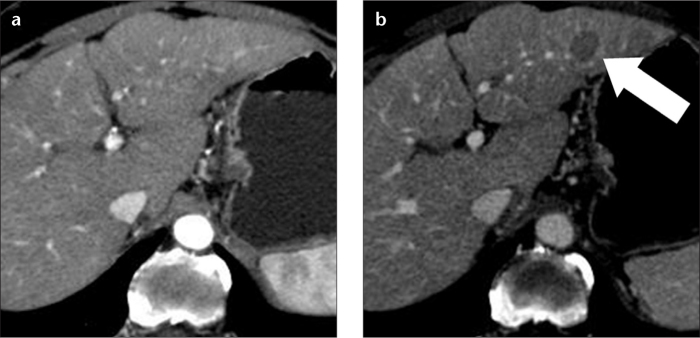
A 73-year-old female with hepatitis C-related cirrhosis and hypovascular HCC in the left hepatic lobe. A hepatic arterial phase CT image (a) does not show any lesion. On a hepatic venous phase CT image (b), the lesion (arrow) is spherical and hypoattenuating to the liver parenchyma. This lesion was new in comparison with an MR examination performed one year earlier and was interpreted as hypovascular HCC, although these imaging features are also compatible with a high-grade dysplastic nodule.
While a few case reports have described focal nodular hyperplasia-like nodules in the cirrhotic liver (5–7), in our experience, this coexistence is exceedingly rare. These nodules are usually small (<2 cm) and share several imaging and histological features with focal nodular hyperplasia arising in noncirrhotic patients, including homogeneous hypervascularity in the hepatic arterial phase, and a central stellate scar-like fibrosis. Focal nodular hyperplasia will typically show fading to isoattenuation in the hepatic venous phase, thus showing overlapping features with hypervascular dysplastic nodules and small well-differentiated HCC (Fig. 5). These similarities in imaging create difficulties in the differential diagnosis and may lead to unnecessary treatment. MR contrast agents with combined vascular and delayed hepatobiliary excretion, such as gadobenate dimeglumine (Multihance, Bracco, Milano, Italy) and gadolinium ethoxybenzyl dimeglumine (Primovist, Bayer, Berlin, Germany) suggest a diagnosis of HCC when showing hypointensity in the hepatobiliary phase. However regenerative nodules, dysplastic nodules, focal nodular hyperplasia-like lesions (Fig. 6), and about 10% of HCC can all show hyperintensity in the hepatobiliary phase (8, 9). Thus, hyperintensity alone does not allow a specific diagnosis.
Figure 5.
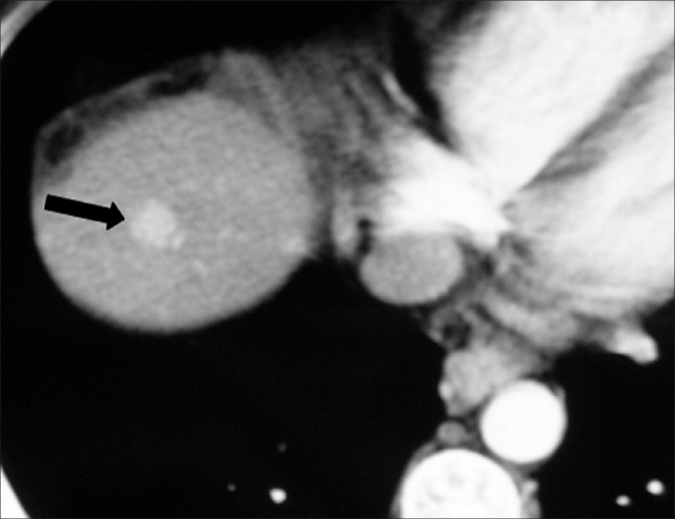
A 38-year-old male with hepatitis C-related cirrhosis and focal nodular hyperplasia. Hepatic arterial phase CT image shows a homogeneously enhancing lesion (arrow) in the right hepatic lobe. This lesion was prospectively misinterpreted as HCC. The patient was transplanted, and the diagnosis of focal nodular hyperplasia was made only at explantation.
Figure 6.
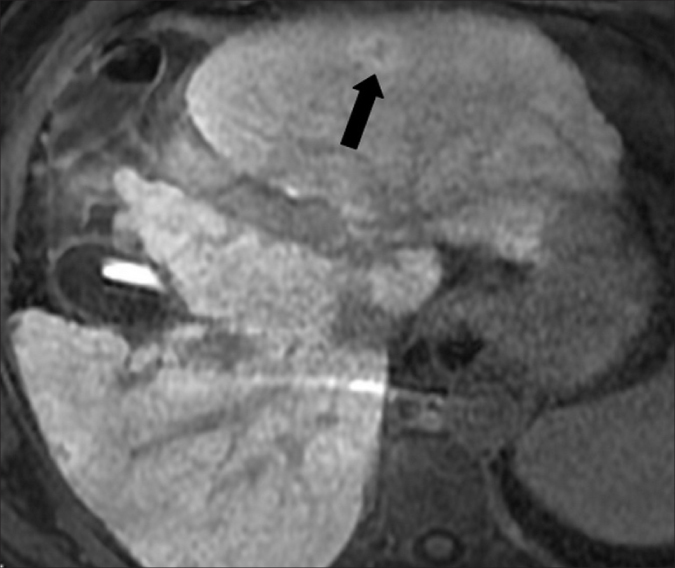
Gd-EOB-DTPA-enhanced MR image in a 57-year-old male with hepatitis C-related cirrhosis and a lesion in the left hepatic lobe. In the hepatobiliary phase, 20 min after Gd-EOB-DTPA administration, the lesion (arrow) was hyperintense. The lesion remained unchanged at follow-up performed two years later (not shown). Due to the stability in size and imaging features, this lesion was interpreted as a focal nodular hyperplasia-like nodule. Note contrast medium accumulation in the nondependent portion of the gallbladder, as typically observed in the hepatobiliary phase.
We have never observed hepatocellular adenoma in cirrhosis. In the literature, few reports describe the association (10, 11).
Simple biliary cysts have similar features in cirrhotic and noncirrhotic livers: low attenuation on CT, low signal intensity on T1-weighted MR sequences, and high signal on heavily T2-weighted MR sequences, with no contrast enhancement (Fig. 7).
Figure 7. a–c.
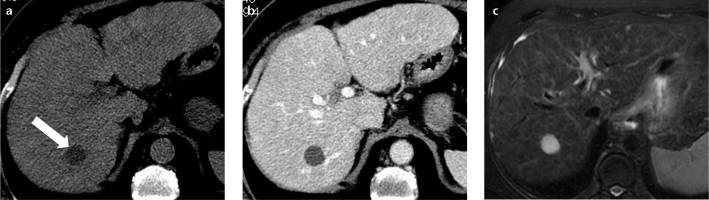
A 72-year-old female with hepatitis C-related cirrhosis. Unenhanced axial CT image (a) shows a homogeneous, rounded, well-defined lesion (arrow) in the right hepatic lobe that is hypoattenuating versus the blood vessels. On hepatic venous-phase CT image (b), the lesion does not enhance and is consistent with a simple bile duct cyst. Axial fat-saturated T2-weighted fast spin-echo MR image (c) is consistent with the diagnosis of simple cyst due to the homogeneous high signal intensity.
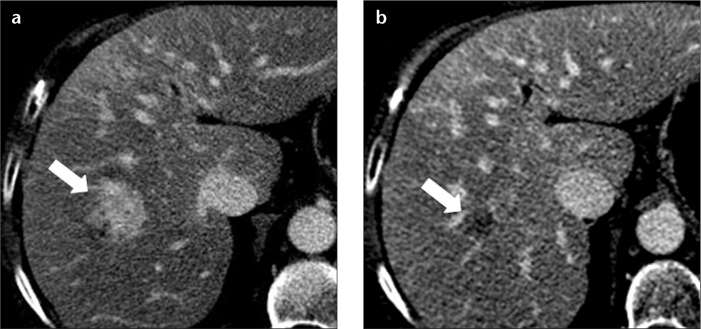
Peribiliary cysts are cystic lesions typically found on both sides of the intrahepatic portal venous branches, as opposed to intrahepatic biliary dilatation, which is located on one side only of portal venous branches. These lesions may have variable sizes and morphologies. They represent cystic dilatation of the extramural glands in the periductal connective tissue and show the same imaging findings as simple cysts (Fig. 8) (12).
Figure 8.
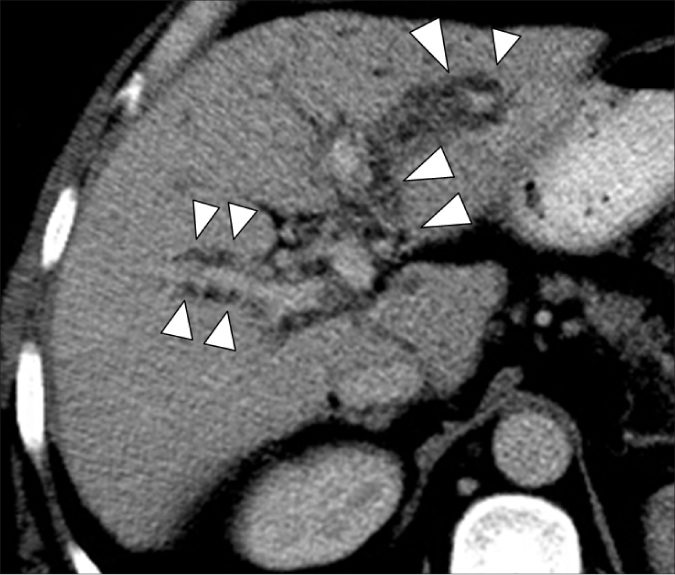
A 70-year-old female with hepatitis-C-related cirrhosis. Contrast-enhanced axial CT image in the hepatic venous phase shows numerous hypoattenuating peribiliary cysts (arrowheads) surrounding the intrahepatic portal vein branches.
Malignant lesions
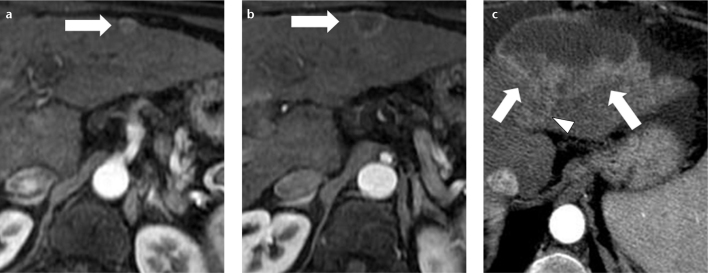
Intrahepatic cholangiocarcinoma is the second most common primary malignancy, after HCC, in both cirrhotic and noncirrhotic liver (13). Liver cirrhosis is considered a risk factor not only for HCC development, but also for cholangiocarcinoma. In patients with cirrhosis, cholangiocarcinoma will typically be hypervascular when smaller than 3 cm, and this enhancement pattern is more common in patients with chronic liver disease versus patients with noncirrhotic livers (14, 15). Thus, the differential diagnosis between small cholangiocarcinoma and HCC is difficult in the cirrhotic liver (Fig. 9a). When larger than 3 cm, a peripheral enhancing rim, centripetal pattern, capsular retraction, and segmental biliary ductal dilatation will be major imaging features, resembling the classic CT findings of cholangiocarcinoma in the noncirrhotic liver (Fig. 9b, 9c) (16, 17).
Figure 9. a–c.
A 62-year-old female with hepatitis-C-related cirrhosis and cholangiocarcinoma. Hepatic arterial phase contrast-enhanced MR image (a) shows a small heterogeneous hypervascular lesion (arrow) in the left hepatic lobe, prospectively interpreted as atypical HCC (due to the lack of washout on later phases). On hepatic arterial phase contrast-enhanced MR image (b) at the same level, obtained six months later, the lesion (arrow) has developed a ring pattern of enhancement. At 12 months from the baseline examination (c), the lesion is enlarged (arrows), has a central necrotic component and has invaded the left hepatic vein (c, arrowhead). In the interval between (a) and (b), the patient was not treated because the lesion did not have typical features of HCC in (a), and thus a follow-up (shown in b) was performed to clarify the nature of the lesion. Because in (b) the lesion did not show typical features of HCC, a percutaneous biopsy was performed, which showed a cholangiocarcinoma.
In our experience, mixed hepatocellular-cholangiocarcinomas can occur in patients with chronic liver disease (18). These tumors may show imaging features of both hepatocellular carcinoma and cholangiocarcinoma, so a confident diagnosis may not be possible on the basis of imaging alone.
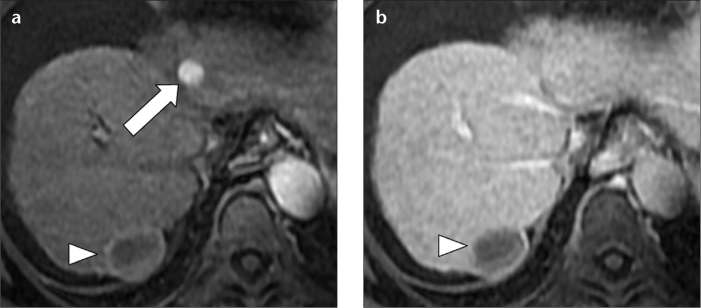
An association between hepatitis C virus and non-Hodgkin B-cell lymphoma has been described by several authors (19). Primary liver involvement is rare, and causes diffuse hepatic involvement, while secondary hepatic lymphoma is more common, and typically consists of multiple small nodules. On MRI, lymphomatous nodules are commonly hypointense on T1, hyperintense on T2, and have variable vascularity after gadolinium injection (Fig. 10a). Because they do not contain functioning hepatocytes, they are hypointense in the hepatobiliary phase (Fig. 10b), and due to high cellularity, they show signal restriction on high-B diffusion-weighted imaging (Fig. 10c).
Figure 10. a–c.
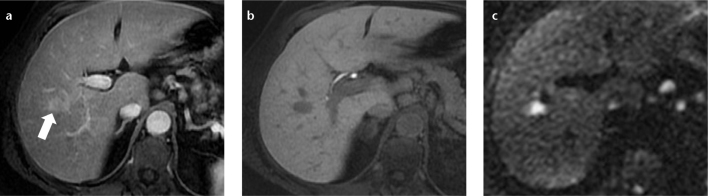
A 67-year-old female with hepatitis C-related cirrhosis and secondary non-Hodgkin B-cell lymphoma. Hepatic arterial phase fat-saturated volumetric T1-weighted image (a) demonstrated one of several small, well-defined hepatic lymphomatous nodules (arrow) that appeared hypervascular. No washout was noted in the delayed phase (not shown). In the hepatobiliary phase, 20 min after Gd EOB-DTPA administration, the lesion was hypointense (b). On high-B-value diffusion-weighted axial imaging (c), the lesion showed restriction. Although in the setting of cirrhosis these findings were interpreted as hepatocellular carcinoma, due to the number and size stability in comparison to a CT performed three years earlier (not shown), a biopsy was performed, which showed non-Hodgkin B-cell lymphoma.
The rare occurrence of metastases in cirrhosis may be due to hepatofugal portal venous flow that prevents neoplastic cells from seeding and flourishing in the liver (20). However, some primary neoplasms, such as colorectal adenocarcinoma, may infrequently spread to the liver in cirrhotic patients (21). Typical imaging features of metastases occurring in cirrhosis include mild hyperintensity on T2-weighted images and ring enhancement in the hepatic arterial phase (Fig. 11), although these findings may also be encountered in cholangiocarcinoma.
Figure 11. a, b.
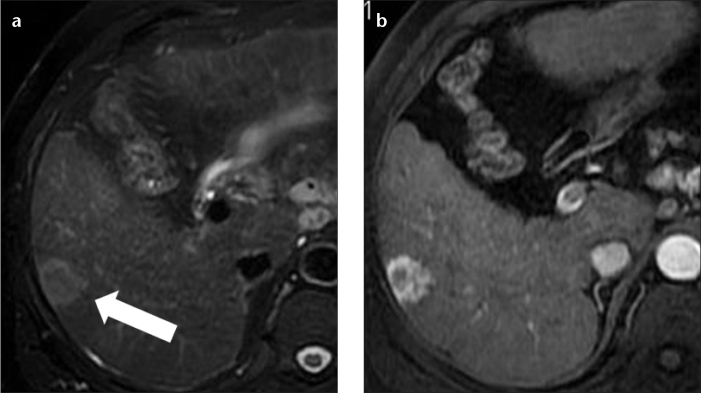
A 62-year-old male with hepatitis C-related cirrhosis and metastasis from an unknown primary malignancy. Axial fat-saturated T2-weighted fast spin-echo MR image (a) shows a round, heterogeneous, moderately high signal intensity lesion (arrow) in the right hepatic lobe. Hepatic arterial phase fat-saturated volumetric T1-weighted image (b) shows intense heterogeneous rim hyperenhancement of the lesion that persisted in the hepatic venous phase (not shown). The differential diagnosis in this case included both intrahepatic peripheral cholangiocarcinoma and secondary lesion. A percutaneous biopsy showed metastasis from an unknown primary malignancy.
Pseudolesions: perfusion abnormalities
The distorted architecture of the hepatic parenchyma in advanced cirrhosis is a predisposing condition to the development of arterioportal shunting. These vascular derangements are commonly referred to as “pseudolesions”. They are typically visible as focal areas of hyperenhancement on images acquired during the hepatic arterial phase, with no washout in the hepatic venous or delayed phases. Arterioportal shunts are usually wedge-shaped and subcapsular, they have no mass effect on adjacent vessels or bile ducts, and they do not grow at imaging follow-up. In MRI, these “pseudolesions” are isointense to the surrounding liver parenchyma on precontrast T1- and T2-weighted images, as well as on images acquired during the hepatobiliary phase after administration of gadobenate dimeglumine or gadolinium ethoxibenzyl diethylenetriamine pentaacetic acid (Fig. 12). However, the specificity of these imaging findings does not allow reliable exclusion of small, well-differentiated HCC (Fig. 2), especially when the hyperenhancing focus is round in shape (22). The latest guidelines of the American Association for the Study of Liver Diseases (AASLD) recommend imaging follow-up for small indeterminate hyperenhancing nodules in a cirrhotic liver, to assess growth or change in imaging pattern (23). At our institution, when we detect subcentimetric round enhancing indeterminate lesions, we perform ultrasonography follow-up at six months and CT or MRI follow-up at 12 months (24).
Figure 12. a–c.
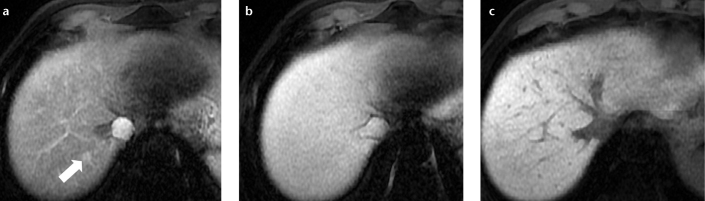
A 63-year-old male with hepatitis C-related cirrhosis and a pseudolesion in segment VII. Hepatic arterial phase fat-saturated volumetric T1-weighted image (a) shows nodular, arterial hyperenhancement (arrow) in segment VII. On hepatic venous (b) and hepatobiliary (c) fat-saturated volumetric T1-weighted images, the lesion was not visible. Due to its stability over one year and the lack of hypointensity in the hepatobiliary phase, it was interpreted as a pseudolesion, although these imaging features are also compatible with a dysplastic nodule.
Fibrosis is always present to some degree in cirrhosis. In more advanced disease, fibrosis can appear as a focal, wedge-shaped area, radiating from the porta hepatis, widest at the capsular surface, with associated capsular retraction more frequently located in segments IV, VII, or VIII of the liver. This entity is known as focal confluent fibrosis. On contrast-enhanced CT or T1-weighted MRI, it usually appears hypoattenuating/hypointense compared with the surrounding liver parenchyma, and slightly hyperintense on delayed-phase images, because of contrast accumulation in the fibrotic tissue (Fig. 13). Less frequently, focal confluent fibrosis may appear hypervascular in the arterial phase without a clear wedge shape or associated capsular retraction, thus potentially resembling HCC (25, 26).
Figure 13. a–c.
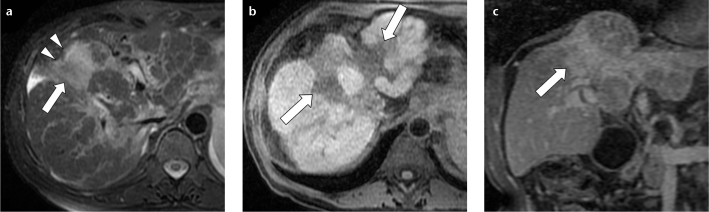
A 65-year-old female with cirrhosis and a focal confluent fibrosis lesion. Axial fat-saturated T2-weighted fast spin-echo MR image (a) shows wedge-shaped region of slightly increased signal intensity (arrow) involving segment VIII with associated retraction of liver capsule (arrowheads). On unenhanced fat-saturated volumetric T1-weighted axial image (b), confluent fibrosis was hypointense (arrows) and enhanced progressively after contrast agent administration on a contrast-enhanced delayed-phase fat-saturated volumetric T1-weighted coronal image (c, arrow).
Segmental or lobar hyperplasia is observed in end-stage cirrhosis, typically secondary to sclerosing cholangitis, although it can also be observed in the setting of alcoholic cirrhosis. It is due to the pseudotumoral enlargement of one or more segments, along with atrophy of the peripheral hepatic segments, and results in a lobulated liver contour (Fig. 14) (27).
Figure 14.
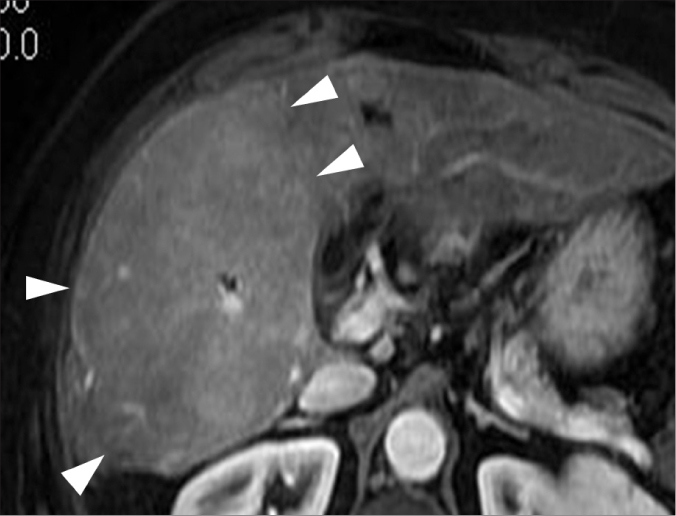
A 67-year-old female with alcoholic cirrhosis and lobar hyperplasia. Contrast-enhanced MR image shows severe hypertrophy of the posterior segment of the right liver lobe (arrowheads), demonstrating a pseudotumor appearance.
Conclusion
A wide spectrum of benign and malignant lesions other than HCC may be found in the cirrhotic liver. MRI should be preferred over CT as the primary imaging modality for the evaluation of cirrhotic patients due to its greater ability to detect and characterize focal lesions. When a lesion lacks the typical features of HCC (hyperenhancement in the arterial phase and hypoenhancement-washout-in the venous and/or delayed phase), the radiologist should consider performing follow-up or a biopsy to pursue the correct diagnosis.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Brancatelli G, Baron RL, Peterson MS, Marsh W. Helical CT screening for hepatocellular carcinoma in patients with cirrhosis: frequency and causes of false-positive interpretation. AJR Am J Roentgenol. 2003;180:1007–1014. doi: 10.2214/ajr.180.4.1801007. [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, Lee JM, Yu NC, et al. Hepatocellular carcinoma in liver transplantation candidates: detection with gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2008;191:529–536. doi: 10.2214/AJR.07.2565. [DOI] [PubMed] [Google Scholar]
- 3.Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology. 2001;219:69–74. doi: 10.1148/radiology.219.1.r01ap3269. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SH, Lee JM, So YH, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:482–489. doi: 10.2214/AJR.08.1818. [DOI] [PubMed] [Google Scholar]
- 5.Quaglia A, Tibballs J, Grasso A, et al. Focal nodular hyperplasia-like areas in cirrhosis. Histopathology. 2003;42:14–21. doi: 10.1046/j.1365-2559.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima O, Kurogi M, Yamaguchi R, et al. Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules? J Hepatol. 2004;41:992–998. doi: 10.1016/j.jhep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Kim SH, Cho MY, Shim KY, Kim MS. Focal nodular hyperplasia-like nodules in alcoholic liver cirrhosis: radiologic-pathologic correlation. AJR Am J Roentgenol. 2007;188:459–463. doi: 10.2214/AJR.05.1998. [DOI] [PubMed] [Google Scholar]
- 8.Golfieri R, Grazioli L, Orlando E, et al. Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging. 2012;36:648–657. doi: 10.1002/jmri.23685. [DOI] [PubMed] [Google Scholar]
- 9.Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to dynamic MRI in the detection of hypovascular small (≤2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233–1242. doi: 10.1007/s00330-010-2030-1. [DOI] [PubMed] [Google Scholar]
- 10.Seo JM, Lee SJ, Kim SH, et al. Hepatocellular carcinoma arising from hepatocellular adenoma in a hepatitis B virus-associated cirrhotic liver. Clin Radiol. 2012;67:329–333. doi: 10.1016/j.crad.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki M, Yoneda N, Kitamura S, et al. Characterization of hepatocellular adenoma based on the phenotypic classification: The Kanazawa experience. Hepatol Res. 2011;41:982–988. doi: 10.1111/j.1872-034X.2011.00851.x. [DOI] [PubMed] [Google Scholar]
- 12.Baron RL, Campbell WL, Dodd GD. Peribiliary cysts associated with severe liver disease: imaging-pathologic correlation. AJR Am J Roentgenol. 1994;162:631–636. doi: 10.2214/ajr.162.3.8109511. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Kim SA, Lee JM, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern-correlation with clinicopathologic findings. Radiology. 2011;260:148–157. doi: 10.1148/radiol.11101777. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Igarashi S, Sasaki M, et al. Intrahepatic cholangiocarcinomas in cirrhosis are hypervascular in comparison with those in normal livers. Liver Int. 2012;32:1156–1164. doi: 10.1111/j.1478-3231.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Lee JM, Han JK, et al. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am J Roentgenol. 2007;189:1428–1434. doi: 10.2214/AJR.07.2484. [DOI] [PubMed] [Google Scholar]
- 17.Iavarone M, Piscaglia F, Vavassori S, et al. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol. 2013;13:135–139. doi: 10.1016/j.jhep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Ebied O, Federle MP, Blachar A, et al. Hepatocellular-cholangiocarcinoma: helical computed tomography findings in 30 patients. J Comput Assist Tomogr. 2003;27:117–124. doi: 10.1097/00004728-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Beaty SD, Silva AC, Depetris G. AJR teaching file: incidental hepatic mass. AJR Am J Roentgenol. 2008;190:S62–S64. doi: 10.2214/AJR.07.7075. [DOI] [PubMed] [Google Scholar]
- 20.Seymour K, Charnley RM. Evidence that metastasis is less common in cirrhotic than normal liver: a systematic review of post-mortem case-control studies. Br J Surg. 1999;86:1237–1243. doi: 10.1046/j.1365-2168.1999.01228.x. [DOI] [PubMed] [Google Scholar]
- 21.Gervaz P, Pak-art R, Nivatvongs S, Wolff BG, Larson D, Ringel S. Colorectal adenocarcinoma in cirrhotic patients. J Am Coll Surg Vol. 2003;196:874–879. doi: 10.1016/S1072-7515(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 22.Holland AE, Hecht EM, Hahn WY, et al. Importance of small (<20 mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology. 2005;237:938–944. doi: 10.1148/radiol.2373041364. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. American Association for the Study of Liver Diseases. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furlan A, Marin D, Agnello F, et al. Hepatocellular carcinoma presenting at contrast-enhanced multi-detector-row computed tomography or gadolinium-enhanced magnetic resonance imaging as a small (≤2 cm), indeterminate nodule: growth rate and optimal interval time for imaging follow-up. J Comput Assist Tomogr. 2012;36:20–25. doi: 10.1097/RCT.0b013e31823ed462. [DOI] [PubMed] [Google Scholar]
- 25.Brancatelli G, Baron RL, Federle MP, Sparacia G, Pealer K. Focal confluent fibrosis in cirrhotic liver: natural history assessed with computed tomography. AJR Am J Roentgenol. 2009;192:1341–1347. doi: 10.2214/AJR.07.2782. [DOI] [PubMed] [Google Scholar]
- 26.Baron RL, Peterson MS. Screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics. 2001;21:117–132. doi: 10.1148/radiographics.21.suppl_1.g01oc14s117. [DOI] [PubMed] [Google Scholar]
- 27.Dodd GD, III, Baron RL, Oliver JH, III, Federle MP. End-stage primary sclerosing cholangitis: CT findings of hepatic morphology in 36 patients. Radiology. 1999;211:357–362. doi: 10.1148/radiology.211.2.r99ma49357. [DOI] [PubMed] [Google Scholar]