Abstract
PURPOSE
We aimed to evaluate the long-term outcome and efficacy of percutaneous transluminal renal angioplasty (PTRA) for pediatric renal artery stenosis (RAS), which is an important cause of medication-refractory pediatric hypertension.
MATERIALS AND METHODS
We retrospectively evaluated 22 hypertensive children (age range, 3–17 years) who underwent PTRA from February 2000 to July 2012. Sixteen patients had Takayasu arteritis and six fibromuscular dysplasia. Five were not included in the statistical analysis due to loss to follow-up.
RESULTS
Technical success was achieved in 32 of 34 procedures (94.1%). The stenosis rate decreased from 84.5% before PTRA to 20.1% after PTRA. Treatment was effective in 72.7% (16/22) of patients, including complete cure in 27.3% (6/22) and improvement in 45.5% (10/22). Systolic and diastolic blood pressures decreased from 153±19.1 to 131.7±21.4 mmHg and from 97.9±14.2 to 83.6±19.3 mmHg, respectively (P < 0.01). Number of antihypertensive agents decreased from 2.7 to 0.5 per patient. Restenosis was detected in 40.9% (9/22) of patients, with a restenotic interval of 11.8 months (range, 3–47 months). Lesion length was strongly correlated with clinical success (cure and improvement) (independent-sample t test, P < 0.001; binary logistic regression, P = 0.040).
CONCLUSION
Lesion length is an important determination of clinical success with PTRA for pediatric RAS. PTRA is an appropriate treatment option for pediatric renovascular hypertension due to Takayasu arteritis and fibromuscular dysplasia.
Although hypertension affects only 1%–2% of children and adolescents based on reliable assessments (1, 2), it is a major risk factor for cerebrovascular disease, myocardial infarction, and renal failure. Renal artery stenosis (RAS), a vascular disease, leads to 5%–25% of pediatric hypertension. Although a few conditions, including Kawasaki disease, polyarteritis nodosa, Wegener’s granulomatosis, neurofibromatosis, Williams syndrome, and midaortic syndrome, may cause RAS in pediatric patients (3, 4), Takayasu arteritis (TA) is the most common cause of pediatric RAS in the East, especially in China, Korea, and India (5, 6). Fibromuscular dysplasia (FMD) is another important cause of RAS in children, particularly in Western countries. A substantial proportion of patients with RAS suffer from hypertension and poor sequelae. Additionally, pediatric hypertension is strongly associated with hypertension in adults (7). However, blood pressure is not frequently measured in children. Due to the lack of sufficient information on blood pressure, renovascular hypertension is often ignored and/or diagnosed with a considerable time delay by referring physicians (5). Despite many antihypertensive agents, valid control of blood pressure is often impossible. Moreover, normalized blood pressure with multiple drugs may result in underperfusion of the kidney and cerebral blood vessels, thereby aggravating multiple organ dysfunction. Therefore, surgical interventions are considered as an alternative strategy to achieve an adequate control of blood pressure in pediatric patients (8).
Here, we present 13 years of data on the outcomes of 22 children with renovascular hypertension caused by TA or FMD following treatment with a percutaneous transluminal renal angioplasty (PTRA) procedure.
Materials and methods
The study was performed in accordance with to the World Medical Association’s Declaration of Helsinki. Access to the medical records of patients included in this retrospective study was approved by the Ethics Committee of Research of Xuan Wu Hospital, Capital Medical University.
Patients
This was a single-center, retrospective, longitudinal follow-up study. Medical records of hypertensive children who underwent PTRA for treatment of renovascular hypertension caused by FMD or TA between February 2000 and July 2012 were obtained from the Records Department of Xuan Wu Hospital, Capital Medical University. Twenty-seven patients fit these criteria. Five were lost to follow-up and were excluded from our analysis, leaving 22 patients for this study. The age of the 22 patients was between three and 17 years (median, 9 years; 12 girls and 10 boys). The average weight of patients was 31.7±6.3 kg (range, 9.5–70 kg). Hypertension was defined as blood pressure greater than the 95th age/gender–specific percentile (9, 10). Patients with neurofibromatosis type 1 or polyarteritis nodosa, and patients who were lost to follow-up were excluded. Records of patients’ demographic characteristics, clinical presentations, medical history, signs, laboratory test results, and treatment outcomes were all retrieved and analyzed.
RAS was diagnosed by Doppler renal ultrasonography examination. Diagnostic angiography was performed in suspected RAS patients before the intervention procedure. The diagnosis of TA was primarily based on the presence of angiographic abnormalities plus at least one of the following four conditions: 1) decreased peripheral artery pulse(s) and/or claudication of extremities; 2) bruits over the aorta and/or its major branches; 3) blood pressure difference >10 mmHg; and 4) hypertension as previously described (3, 11). Other clinical manifestations of different phases, such as low-grade fever, malaise, and fatigue, were also taken into account. The diagnosis of FMD was made if a patient presented with hypertension and had the string of beads appearance or focal lesions in the middle or distal segment of the renal artery on angiographic images (12).
All patients were evaluated by an expert team to avoid an individual bias. Patients with a suspected diagnosis or high risk of PTRA were evaluated by a group of pediatricians, anesthetists, cardiologists, immunologists, and vascular and interventional radiologists. The indication for PTRA was RAS >70%, especially with refractory hypertension, impaired renal function, impaired myocardium or hypertensive encephalopathy due to RAS. PTRA was not performed on patients with severe comorbidities such as congenital heart disease or systemic infection, or on patients without appropriate vascular access.
Extended-release nifedipine tablets (initially 0.25–0.5 mg/kg daily, for a maximum of 3 mg/kg up to 120 mg daily, used qd or bid) or metoprolol tartrate sustained-release tablets (initially 1–2 mg/kg daily for a maximum of 6 mg/kg up to 200 mg daily, bid) were given as the first-line antihypertensive agents. Whenever necessary, hydrochlorothiazide, spironolactone, captopril, or propanolol was used. Aspirin (3 mg/kg daily, for a maximum of 100 mg daily) was prescribed for more than three days. Prednisone was initiated with an oral dose of 0.5–1 mg/kg daily as an anti-inflammatory treatment for TA. Immunosuppression with cyclophosphamide (2–6 mg/kg daily) was used for 10–14 days in patients with active TA.
Percutaneous transluminal renal angioplasty technique
PTRA was performed by a professional team including at least one specialist with at least 10 years of experience. For patients with TA, the procedure was performed in the inactive stage with normal acute-phase reactants, such as the erythrocyte sedimentation rate, C-reactive protein, antistreptolysin-o, and γ-globulin. In children younger than seven years, PTRA was performed under general anesthesia. In older children, the procedure was performed under local anesthesia with common femoral artery access using 4 F to 7 F sheaths (Terumo Medical Co., Tokyo, Japan). Heparin (50–100 U/kg) was administered intra-arterially before arteriography. After diagnostic aortography with a 4 F or 5 F pigtail catheter (Terumo Medical Co., Cook Medical, Bloomington, Indiana, USA), a 0.014-inch guidewire (Abbott, Abbott Park, Illinois, USA) was used to cross the RAS over a 4 F or 5 F Cobra catheter (Terumo Medical Co.). Following Cobra catheter withdrawal, a 5 F to 7 F guiding catheter (Cordis/Johnson and Johnson, Miami, Florida, USA) was advanced over the wire to the ostium of the renal artery. The selection of balloon diameter (Terumo Medical Co., Grüntzig, Schneider Medintag, Zurich) depended on patient age and renal arterial lesion, ranging from 2 to 6 mm according to the adjacent nonstenotic, nonaneurysmal portion of the poststenotic renal artery. The lengths of the balloon catheters were 10, 12, and 20 mm. The balloon was inflated to 4–12 atm 1–3 times for 30 to 60 s each time. Intravenous normal saline hydration was maintained during the whole procedure. Technical success was defined as residual stenosis ≤30%, as confirmed by immediate repeated arteriography examinations. Technical failure was defined as residual stenosis >30% or RAS or renal artery occlusion that could not be passed by or dilated with the balloon catheter. Pressure gradients were not routinely measured. Restenosis was presumed when the patient’s blood pressure increased again after a period of improvement post-PTRA, as confirmed by angiography.
Postoperative care and follow-up
Either intravenous unfractionated heparin (10 U/kg every hour for 12–24 hours) or low-molecular-weight heparin was given to patients after angioplasty to minimize the risk of thrombosis. Aspirin was prescribed for 3–6 months. Prednisone for TA was administered continuously. Antihypertensive agents were given if necessary. Blood pressure management was achieved in a clinic or hospital close to the patients’ habitual residence, where renal vascular patency was monitored with Doppler renal ultrasonography, computed tomography angiography or magnetic resonance angiography every 6–12 months, depending on the patient’s compliance. Repeat PTRA procedures were performed for those patients with markedly worsening blood pressure and positive imaging of recurrent RAS (>70%). The final clinical outcome was categorized as a cure if normal blood pressure (<95th percentile for age, gender, and height) was maintained without antihypertensive drugs; improvement, if normal blood pressure was achieved with less antihypertensive drugs or there was at least a 15% reduction in diastolic blood pressure without antihypertensive drugs; failure, if there was no change or worsening in blood pressure despite angiographic success.
Statistical analysis
Statistical analysis was performed using SPSS version 19.01 (SPSS Inc., Chicago, Illinois, USA). Quantitative data with a normal distribution were expressed as the mean±standard deviation (SD) and were analyzed by an independent-sample or paired t test. Categorical data were analyzed by Fisher’s exact test. The relationship between clinical data and clinical success was analyzed using binary logistic regression. Differences were considered significant when P < 0.05.
Results
Clinical features
Follow-up intervals ranged from six months to 13 years 10 months (mean, 7 years 2 months). All children had unstable or sustained hypertension (Table 1). Claudication of upper limbs was found in four patients with TA, in three patients with occlusion of the left subclavian artery (SCA), and in one patient with severe stenosis of the right brachial artery. Syncope was found in three patients with TA. A boy (aged 3 years 2 months) with TA had a stroke with right hemiplegia and distortion of the commissure for a month. Left ventricular hypertrophy was confirmed by electrocardiography and/or echocardiography in this patient. Eleven patients had renal atrophy with normal serum creatinine at presentation. The split-renal function was not routinely tested. The mean systolic and diastolic blood pressure was 153±19.1 and 97.9±14.2 mmHg at referral. The children took between one and five anti-hypertensive medications as suggested by their pediatricians and cardiologists in our hospital. All patients received steroid therapy for TA, and three patients received cyclophosphamide for immunosuppression prior to intervention.
Table 1.
Symptoms on the first hospital admission
| Symptoms | TA (n=16) | FMD (n=6) | Total (n=22) |
|---|---|---|---|
| Hypertension | 16 | 6 | 22 (100%) |
| Dizziness | 5 | 4 | 9 (40.9%) |
| Headache | 4 | 3 | 7 (31.8%) |
| Nausea and vomiting | 4 | 1 | 5 (22.7%) |
| Syncope | 3 | 0 | 3 (13.6%) |
| Stroke | 1 | 0 | 1 (4.5%) |
| Ischemia/claudication | 4 | 0 | 4 (18.2%) |
| Visual complaints | 1 | 0 | 1 (4.5%) |
| Left ventricular hypertrophy | 10 | 3 | 13 (59%) |
| Frequent micturition | 0 | 2 | 2 (9%) |
| Renal atrophy | 8 | 3 | 11 (50%) |
FMD, fibromuscular disease; TA, Takayasu arteritis
Data are given as n or n (%)
Angiographic findings
Angiography showed that seven patients (31.8%) had left RAS and 12 (54.5%) had right RAS. Three patients (13.6%) had bilateral RAS. Multiple intrarenal aneurysmal dilations were present in three patients (13.6%). The mean length of the lesion was 10.6±5.3 mm (range, 1.0–25 mm). Long-segment stenosis (>10 mm) was observed in 11 patients (50%). The main angiographic features are listed in Table 2. Some complex cases were observed, as follows. A 15-year-old girl with TA had left RAS, left SCA occlusion, left common carotid artery stenosis and abdominal aorta stenosis complicated by nephrolithiasis (Fig. 1). Another 15-year-old girl with TA had severe left RAS (>90%), occlusion of the left SCA and axillary artery, severe stenosis of the bilateral carotid arteries and right RAS complicated by stenosis of the superior mesenteric artery and thoracoabdominal aorta (50%) (Fig. 2). She presented with syncope and impaired vision. A 16-year-old girl with TA had left renal artery occlusion, left SCA occlusion, stenosis of the abdominal aorta, and left ventricular hypertrophy. She presented with syncope and symptomatic epilepsy. The three-year-old boy with stroke, mentioned above, had occlusion of the left internal carotid artery, severe stenosis of the left middle cerebral artery, multiple cerebrovascular anomalies, and old cerebral infarction, as confirmed by magnetic resonance imaging and ultrasonography. A 16-year-old girl with FMD had right RAS complicated by left SCA occlusion and left carotid artery stenosis (Fig. 3).
Table 2.
Summary of angiographic findings
| TA (n=16) | FMD (n=6) | Total (n=22) | |
|---|---|---|---|
| Bilateral disease | 1 | 2 | 3 (13.6%) |
| Ostial stenosis | 13 | 0 | 13 (59.1%) |
| Mid and distal stenosis | 3 | 6 | 9 (40.9%) |
| Length of lesion (mm), mean±SD | 10.1±4.3 | 11±7.9 | 10.6±5.3 |
| Pre-PTRA stenosis rate (%), mean | 85.3 | 82.5 | 84.5 |
| Beading | 0 | 6 | 6 (27.2%) |
| Aneurysms | 1 | 2 | 3 (13.6%) |
| Collaterals | 3 | 0 | 3 (13.6%) |
| Intraparenchymal disease | 2 | 0 | 2 (9%) |
| Concomitant arterial disease | |||
| Midaortic stenosis | 4 | 1 | 5 (22.7%) |
| Left SCA occlusion | 3 | 1 | 4 (18.2%) |
| Left carotid artery stenosis/occlusion | 3/1 | 1/0 | 5 (22.7%) |
| Left middle cerebral artery stenosis | 1 | 0 | 1 (4.5%) |
| Right brachial artery stenosis | 1 | 0 | 1 (4.5%) |
| SMA stenosis/occlusion | 1/1 | 0/0 | 2 (22.7%) |
| Retinal arteriosclerosis | 1 | 1 | 2 (22.7%) |
FMD, fibromuscular disease; PTRA, percutaneous transluminal renal artery angioplasty; SCA, subclavian artery; SD, standard deviation; SMA, superior mesenteric artery; TA, Takayasu arteritis.
Unless otherwise specified, data are given as n or n (%).
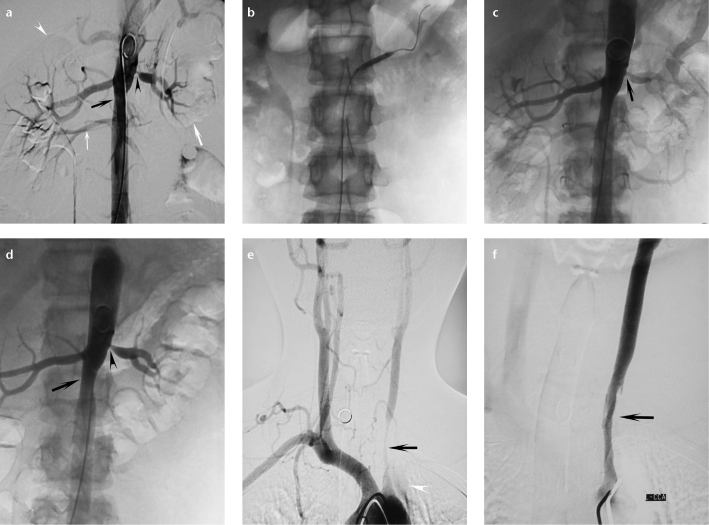
Figure 1. a–f.
Angiographic findings of a 15-year-old girl with Takayasu arteritis. Image before percutaneous transluminal renal angioplasty (a) shows severe ostial stenosis of the left renal artery (black arrowhead), midaortic stenosis (black arrow), left renal atrophy (thick white arrow), compensatory hypertrophy of the right renal artery (white arrowhead), and an aberrant renal artery below the main renal artery on the right (thin white arrow). After balloon angioplasty (b), left renal artery is visibly dilated (c, arrow). Stenosis of the left renal artery becomes severe four months later (d, arrowhead). The patient also has severe stenosis of the left common carotid artery (d, e, arrows) and occlusion of the left subclavian artery (e, arrowhead). Angioplasty performed on the left carotid artery (f, arrow).
Figure 2. a–c.
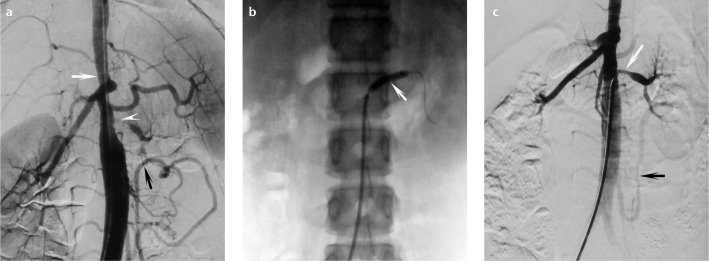
Angiographic findings for a 15-year-old Takayasu arteritis girl with midaortic stenosis. Image before percutaneous transluminal renal angioplasty (a) shows severe stenosis of the left renal artery (arrowhead), midaortic stenosis (50%) (white arrow), and enlarged collaterals of the inferior mesenteric artery due to stenosis of the superior mesenteric artery and midaorta (black arrow). The patient also has left renal atrophy. After balloon angioplasty (b, arrow), the left renal artery is dilated with residual stenosis (c, white arrow). The left kidney is partially supplied by an enlarged collateral of the inferior mesenteric artery (c, black arrow).
Figure 3. a–c.
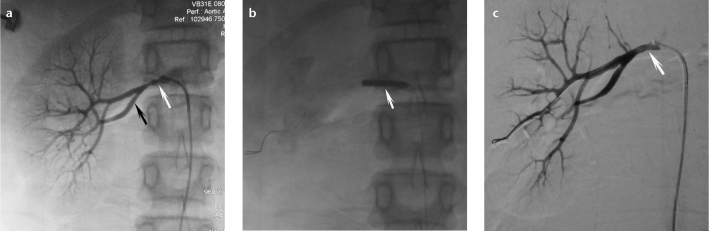
Typical angiographic findings of a 16-year-old girl with fibromuscular dysplasia. Image before percutaneous transluminal renal angioplasty (a) shows the string of beads appearance of the right renal artery (white arrow) and early division in the main renal artery on the right (black arrow). After angioplasty (b, arrow), the right renal artery is fully dilated (c, arrow).
Success rate of percutaneous transluminal renal angioplasty
A total of 34 PTRA procedures were performed in the 22 cases analyzed in this study (Table 3): two procedures in six patients, three procedures in three TA patients, and one procedure in each of the remaining patients. No stents were implanted in renal arteries. An overall technical success rate of 94.1% (32/34) was achieved. The PTRA procedure was unsuccessful due to the lack of response to balloon dilation in a three-year-old boy with TA, who presented with hypertension, and left ventricular hypertrophy, and 1 mm membranous stenosis (>90%) at the ostial segment of renal artery. He underwent autotransplantation of kidney tissue one week later. His blood pressure remained 110/60 mmHg without any medication. The other case of PTRA failure occurred in a 15-year-old girl with TA due to residual stenosis (50%) of the left renal artery (Fig. 2). The overall effectiveness rate was 72.7% (cured, 27.3%; improved, 45.5%) at the end of follow-up. Restenosis was detected in nine of 22 (40.9%) patients, including seven TA patients and two FMD patients. In cases where repeated PTRA procedures were performed, the interval of restenosis ranged from three to 47 months (mean, 11.8 months). Mean systolic and diastolic blood pressures post-PTRA were 131.7±21.4 mmHg and 83.6±19.3 mmHg, respectively; both were significantly lower than those before the PTRA treatment (P < 0.01). Six patients (27.3%) failed to respond to the procedure in terms of blood pressure control. Nephrectomy was performed on two patients. One of them presented with FMD and had severe restenosis of the left renal artery 19 months after successful PTRA. The other presented with TA together with left renal artery occlusion and had rest-enosis three months after PTRA. Their families refused another PTRA. No small-diameter tissue-engineered graft was appropriate to reconstruct their renal arteries. The postoperative blood pressure in these two patients was normal without any medication.
Table 3.
Percutaneous transluminal renal angioplasty outcome
| TA (n=16) | FMD (n=6) | Total (n=22) | |
|---|---|---|---|
| Number of PTRA | |||
| 1 | 9 | 4 | 13 |
| 2 | 4 | 2 | 6 |
| 3 | 3 | 0 | 3 |
| Technical successa, n/N (%) | 24/26 (92.3%) | 8/8 (100%) | 32/34 (94.1%) |
| Post-PTRA stenosis rate (%), mean | 20.6 | 18.7 | 20.1 |
| Interval of restenosis (months), mean±SD | 11.5±10.4 | 13±9.7 | 11.8±10.0 |
| Decrease in blood pressure (mmHg), mean±SD | |||
| Systolic | 21.12±20.6 | 21.8±13.9 | 21.4±18.7 |
| Diastolic | 26.9±19.0 | 13.3±10.4 | 14.4±10.0 |
| Number of antihypertensive drugs, mean | |||
| Pre-PTRA | 2.8 | 2.6 | 2.7 |
| Post-PTRA | 0.6 | 0.4 | 0.5 |
| Clinical success, n (%) | |||
| Cured | 5 | 1 | 6 (27.3%) |
| Improved | 6 | 4 | 10 (45.5%) |
| Failed | 5 | 1 | 6 (27.3%) |
Technical success was based on the total number of procedures.
FMD, fibromuscular disease; PTRA, percutaneous transluminal renal angioplasty; SD, standard deviation; TA, Takayasu arteritis.
Statistical analysis showed that the stenosis length was strongly correlated with clinical success in terms of both cure and improvement rates (Table 4). Binary logistic regression analysis with the stenosis length and renal atrophy as predictors of outcome showed that the stenosis length was strongly correlated with clinical success (Hosmer-Lemeshow test for goodness of fit, 0. 839; P = 0.040) (Table 5).
Table 4.
Relationship between clinical success and clinical features
| Cure or improvement | Failure | P | |
|---|---|---|---|
| Gender (male/female) | 6/10 | 4/2 | 0.348 |
| Disease (TA/FMD) | 11/5 | 5/1 | 0.634 |
| Side (left/right) | 5/8 | 2/4 | 1.000 |
| Ostial/mid-distal segment location | 11/5 | 2/4 | 0.178 |
| Renal atrophy (yes/no) | 6/10 | 5/1 | 0.149 |
| Age (years), mean | 10.3 | 9 | 0.589 |
| Stenosis length (mm) | 8.06±3.10 | 16.6±5.16 | <0.001 |
| Systolic blood pressure (mmHg) | 153.2±17.1 | 152.6±25.6 | 0.951 |
| Diastolic blood pressure (mmHg) | 99.1±15.5 | 95.0±10.5 | 0.563 |
| Stenosis rate | 0.86±0.1 | 0.80±0.1 | 0.157 |
FMD, fibromuscular disease; TA, Takayasu arteritis.
Fisher’s exact test was used for categorical data, and independent-sample t test was used for numeric data.
Unless otherwise specified, data are given as n or mean±standard deviation.
Table 5.
Logistic regression analysis
| Clinical features | Beta | Odds ratio (95% CI) | P |
|---|---|---|---|
| Stenosis length | 0.88 | 2.41 (1.02–5.71) | 0.040 |
| Renal atrophy | 3.35 | 28.75 (0.01–13306) | 0.435 |
CI, confidence interval.
In binary logistic regression, Hosmer-Lemeshow test for goodness of fit was 0.839.
Complications
One patient developed a small groin hematoma. He was managed conservatively, and his hematoma disappeared two weeks later. Serum creatinine was measured after the PTRA procedure in all patients. A slight elevation in this parameter was detected in two patients who developed contrast-induced nephropathy. These two patients recovered from this complication after three and seven days, respectively, after postcontrast hydration with 0.9% saline solution infusion. No dissection, perforation or thrombosis was found.
Discussion
Previous multiple longitudinal studies have shown that children with hypertension are two to three times more likely to develop adult hypertension than the general population (13, 14). Early identification of abnormal blood pressure and appropriate management in childhood are helpful to reduce the risk of cardio- and cerebrovascular events and renal damage in the future (15). The management of pediatric renovascular hypertension poses a significant challenge to pediatrician, and the current strategies remain controversial. In our series, PTRA achieved a cure rate of 27.3% (6/22) and an improvement rate of 45.5% (10/22) in pediatric patients with renovascular hypertension. However, 27.3% (6/22) of children with renovascular hypertension failed to respond to PTRA. The success rate of PTRA for pediatric renovascular hypertension varies from 48.5% to 100% (8, 16, 17). The outcome of PTRA may be influenced by many factors, such as patient age, etiology, distribution of lesions, length and severity of lesions, and impairment degree of the target organ. Our study showed that stenosis length was the most important factor, which was inversely correlated with the effectiveness rate of PTRA. Srinivasan et al. (1) found that most pediatric patients with RAS who were cured or improved after a PTRA procedure(s) had lesions less than 10 mm. Tyagi et al. (17) found that patients with discrete stenosis (≤10 mm) or residual stenosis (≤20%) had a better blood pressure response. Similar results were also reported in another study (18). Supporting the notion that stenosis length is the main determinant of clinical success with PTRA (19), we found that the length of stenosis was significantly shorter in patients who were cured or improved after PTRA treatment than in those who failed to respond to the treatment (8.06±3.10 mm vs. 16.6±5.16 mm, P < 0.01), and our logistic regression analysis showed that the length of stenosis was inversely correlated with the effectiveness rate of PTRA.
Midaortic syndrome, which is characterized by abdominal aorta stenosis, is an important cause of pediatric renovascular hypertension. A previous study reported that 91% of patients with midaortic syndrome had RAS (20). Renovascular hypertension patients with midaortic syndrome are often refractory to antihypertensive medications. Konig et al. (21) reported that maximum doses of six antihypertensive drugs failed to achieve successful blood pressure control in two children with midaortic syndrome, who eventually underwent PTRA with 1–3 stents. In our series, five of 22 (22.7%) patients with renovascular hypertension had mid-aortic syndrome; this prevalence rate of midaortic syndrome is within the previously reported range (20%–48%) (5, 21). It has been suggested that renovascular hypertension patients with midaortic syndrome may be appropriate for surgical revascularization (19, 22). This might be one explanation for the PTRA procedure failure in some patients in this study.
Four of five patients with midaortic syndrome had TA, characterized by a chronic inflammation affecting the aorta and its primary branches. Sustained inflammatory activity may lead to neointima formation in renal arteries post-PTRA or inside the stent, which aggravates RAS and often requires redilatations. Constant immunosuppressive therapies are believed to be beneficial to TA patients. In our series, a nine-year-old girl with TA underwent three PTRA procedures during four years. She discontinued anti-inflammatory drugs after the first two procedures. After the third PTRA, she followed her doctor’s advice to take anti-inflammatory drugs. Her blood pressure was maintained at 115/60 mmHg without any antihypertensive medication until the last follow-up. One patient discontinued prednisone therapy one year post-PTRA. She underwent another PTRA for recurrent RAS two and a half years later. Then, she received prednisone for TA continuously and had no recurrent RAS. Her blood pressure was maintained at 120–130/60–70 mmHg with one antihypertensive drug until the last follow-up. One patient discontinued prednisone and antihypertensive medication two months post-PTRA. His blood pressure was maintained at 110/60 mmHg without recurrent RAS until the last follow-up. Another patient discontinued medical therapy after three procedures. His blood pressure was maintained at approximately 120/65 mmHg without recurrent RAS. So it is uncertain whether our patients benefitted from long-term administration of steroid and immunosuppressant drugs for TA. This concern was also expressed in other study (23).
Restenosis, accounting for poor blood pressure control, was detected in nine of 22 patients (40.9%) in this study, giving a prevalence similar to that reported in a previous study (35%, n=20) (16). Neointima formation and recoil of immature renal arteries are responsible for restenosis (21). A successful cutting balloon angioplasty has been reported in children with restenosis, but a wider application of this procedure is substantially limited (4). Although stents may be inserted in stenotic renal arteries without much difficulty, there is a high restenosis rate, which halts the use of the technique in children (8). For this reason, we decided not to use stents for RAS. Surgical revascularization is associated with satisfactory outcomes in patients with TA-induced RAS in terms of five-year patency (primary patency, 79%; primary assisted patency, 89%; secondary patency, 89%) and blood pressure control in adult patients (23). A previous retrospective study demonstrated that surgery treatment was associated with more complications and higher costs than PTRA (24). Similarly, a meta-analysis showed that the major complications of surgery were two and a half times as prevalent as major complications of angioplasty (6% vs. 15%) and that the cure rate of surgery was slightly higher (54%) than that of angioplasty (36%) (12). Although work on small-diameter tissue-engineered grafts is progressing, the long-term patency rate is poor. A small-diameter tissue-engineered graft with long-term patency is urgently needed to reconstruct the pediatric renal artery. All these facts suggest that surgical revascularization may be a good choice for pediatric renovascular hypertension refractory to other therapies.
In conclusion, we found that PTRA benefited (either cured or improved) a satisfactorily high proportion (72.7%) of the pediatric patients with renovascular hypertension and that the length of the stenosis lesion is an important determinant of clinical success with PTRA for pediatric renovascular hyper-tension. These findings suggest that PTRA may be an appropriate option for managing pediatric renovascular hypertension caused by TA or FMD. Nevertheless, this is a retrospective study with a limited sample size, and the clinical benefits of PTRA need to be further validated in prospective studies involving more subjects.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Srinivasan A, Krishnamurthy G, Fontalvo-Herazo L, et al. Angioplasty for renal artery stenosis in pediatric patients: an 11-year retrospective experience. J Vasc Interv Radiol. 2010;21:1672–1680. doi: 10.1016/j.jvir.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Pappadis SL, Somers MJ. Hypertension in adolescents: a review of diagnosis and management. Curr Opin Pediatr. 2003;15:370–378. doi: 10.1097/00008480-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumus B, Cevik H, Vuran C, Omay O, Kocyigit OI, Turkoz R. Cutting balloon angioplasty of bilateral renal artery stenosis due to Takayasu arteritis in a 5-year-old child with midterm follow-up. Cardiovasc Intervent Radiol. 2010;33:394–397. doi: 10.1007/s00270-009-9623-6. [DOI] [PubMed] [Google Scholar]
- 5.Tullus K, Brennan E, Hamilton G, et al. Renovascular hypertension in children. Lancet. 2008;371:1453–1463. doi: 10.1016/S0140-6736(08)60626-1. [DOI] [PubMed] [Google Scholar]
- 6.Hari P, Bagga A, Srivastava RN. Sustained hypertension in children. Indian Pediatr. 2000;37:268–274. [PubMed] [Google Scholar]
- 7.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 8.Shroff R, Roebuck DJ, Gordon I, et al. Angioplasty for renovascular hypertension in children: 20-year experience. Pediatrics. 2006;118:268–275. doi: 10.1542/peds.2005-2642. [DOI] [PubMed] [Google Scholar]
- 9.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 10.Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension. 2004;44:387–388. doi: 10.1161/01.HYP.0000143545.54637.af. [DOI] [PubMed] [Google Scholar]
- 11.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 12.Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension. 2010;56:525–532. doi: 10.1161/HYPERTENSIONAHA.110.152918. [DOI] [PubMed] [Google Scholar]
- 13.Redwine KM, Daniels SR. Prehypertension in adolescents: risk and progression. J Clin Hypertens. 2012;14:360–364. doi: 10.1111/j.1751-7176.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartosh SM, Aronson AJ. Childhood hypertension. An update on etiology, diagnosis, and treatment. Pediatr Clin North Am. 1999;46:235–252. doi: 10.1016/s0031-3955(05)70115-2. [DOI] [PubMed] [Google Scholar]
- 16.Bayrak AH, Numan F, Cantasdemir M, Bas A. Percutaneous balloon angioplasty of renovascular hypertension in pediatric cases. Acta Chir Belg. 2008;108:708–714. doi: 10.1080/00015458.2008.11680321. [DOI] [PubMed] [Google Scholar]
- 17.Tyagi S, Kaul UA, Satsangi DK, Arora R. Percutaneous transluminal angioplasty for renovascular hypertension in children: initial and long-term results. Pediatrics. 1997;99:44–49. doi: 10.1542/peds.99.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Mali WP, Puijlaert CB, Kouwenberg HJ, et al. Percutaneous transluminal renal angioplasty in children and adolescents. Radiology. 1987;165:391–394. doi: 10.1148/radiology.165.2.2958896. [DOI] [PubMed] [Google Scholar]
- 19.Hijazi R, Chandar J, Nwobi O, Muneeruddin S, Zilleruelo G, Abitbol CL. Renal manifestations in toddlers with Takayasu’s arteritis and malignant hypertension. Pediatr Nephrol. 2009;24:1227–1230. doi: 10.1007/s00467-008-1088-3. [DOI] [PubMed] [Google Scholar]
- 20.Sethna CB, Kaplan BS, Cahill AM, Velazquez OC, Meyers KE. Idiopathic mid-aortic syndrome in children. Pediatr Nephrol. 2008;23:1135–1142. doi: 10.1007/s00467-008-0767-4. [DOI] [PubMed] [Google Scholar]
- 21.Konig K, Gellermann J, Querfeld U, Schneider MB. Treatment of severe renal artery stenosis by percutaneous transluminal renal angioplasty and stent implantation: review of the pediatric experience: apropos of two cases. Pediatr Nephrol. 2006;21:663–671. doi: 10.1007/s00467-006-0010-0. [DOI] [PubMed] [Google Scholar]
- 22.McTaggart SJ, Gulati S, Walker RG, Powell HR, Jones CL. Evaluation and long-term outcome of pediatric renovascular hypertension. Pediatr Nephrol. 2000;14:1022–1029. doi: 10.1007/s004670050066. [DOI] [PubMed] [Google Scholar]
- 23.Weaver FA, Kumar SR, Yellin AE, et al. Renal revascularization in Takayasu arteritis-induced renal artery stenosis. J Vasc Surg. 2004;39:749–757. doi: 10.1016/j.jvs.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Alhadad A, Ahle M, Ivancev K, Gottsater A, Lindblad B. Percutaneous transluminal renal angioplasty (PTRA) and surgical revascularisation in renovascular disease--a retrospective comparison of results, complications, and mortality. Eur J Vasc Endovasc Surg. 2004;27:151–156. doi: 10.1016/j.ejvs.2003.10.009. [DOI] [PubMed] [Google Scholar]