Abstract
PURPOSE
We aimed to evaluate the accuracy of multidetector computed tomography (MDCT) venous mapping for the localization of the right adrenal veins (RAV) in patients suffering from primary aldosteronism.
METHODS
MDCT scans of 75 patients with primary aldosteronism between March 2008 and November 2011 were evaluated by two readers (a junior [R1] and a senior [R2] radiologist) according to the following criteria: quality of RAV depiction (scale, 1–5), localization of the RAV confluence with regard to the inferior vena cava, and depiction of anatomical variants. Results were compared with RAV venograms obtained during adrenal vein sampling and corroborated by laboratory testing of cortisol in selective RAV blood samples. Kappa statistics were calculated for interobserver agreement and for concordance of MDCT mapping with the gold standard.
RESULTS
Successful RAV sampling was achieved in 69 of 75 patients (92%). Using MDCT mapping, adrenal veins could be visualized in 78% (R1, 54/69) and 77% (R2, 53/69) of patients. MDCT mapping led to correct identification of RAV in 70% (R1, 48/69) and 88% (R2, 61/69) of patients. Venograms revealed five cases of anatomical variants, which were correctly identified in 60% (R1, R2). MDCT-based localizations were false or misleading in 16% (R1, 11/69) and 7% (R2, 5/69) of cases.
CONCLUSION
Preinterventional MDCT mapping may facilitate successful catheterization in adrenal vein sampling.
Primary aldosteronism (PA) has lately been claimed to be one of the most common causes of secondary hypertension, with reports indicating a prevalence of more than 10% (1, 2), especially in patients with resistant hypertension (3). While PA is more common than previously thought, the majority of cases is not accompanied by the full clinical picture of Conn’s syndrome (triad of hypertension, hypokalemia, and metabolic alkalosis), and many patients are in fact normokalemic. In addition to its role in causing hypertension, PA may also be an independent cardiovascular risk factor, as demonstrated by higher cardiovascular and renal morbidity in patients suffering from PA in comparison to matched controls with essential hypertension (2, 4, 5). PA is caused either by an aldosterone-producing adenoma (65%–70% of cases) or bilateral adrenal hyperplasia (30%–35% of cases), whereas unilateral adrenal hyperplasia, aldosterone-producing carcinomas, or an ectopic secretion of aldosterone are rare (6). Differentiation of the underlying condition is crucial for the treatment of patients with PA: while unilateral disease can be cured by laparoscopic adrenalectomy, cases of bilateral aldosterone secretion will be medically treated with mineralocorticoid receptor antagonists.
The 2008 Endocrine Society Clinical Practice Guidelines recommend computed tomography (CT) of the adrenal region in all patients with biochemically confirmed PA, to rule out malignancy (2). The primary indication does not involve the subtype differentiation of PA, because multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) have both been proven to be scarcely sensitive and specific in the detection of aldosterone-producing adenomas (7). Hence, adrenal vein sampling (AVS) continues to represent the gold standard in the subtype differentiation of PA. However, AVS is a technically demanding interventional procedure even in experienced institutions. While the catheterization of the left adrenal vein is usually uncomplicated, sampling of the right adrenal vein (RAV) is often more challenging. Therefore, in the majority of cases successful bilateral AVS fails because of the missing catheterization on the right side (8–10). Published success rates for this procedure range from 42% up to 98% in experienced hands (11).
Few authors have mentioned the possible advantage of reading CT-scans prior to AVS to identify the RAV (8, 12). To our knowledge, this is the first report on venous MDCT mapping for AVS. The purpose of this study was to evaluate the usefulness of newly introduced MDCT venous mapping for the localization of the RAV prior to selective catheterization in patients suffering from PA.
Methods
Internal review board of the Ludwig-Maximilians-University (LMU) Munich approved this chart review study. Patient data was extracted from the German Conn Registry (13). Patients provided formal written informed consent for taking part in the prospective German Conn Registry.
Patients
MDCT scans of 75 consecutive patients with PA (52 men, 23 women; mean age, 52±11 years; range, 15–78 years), obtained between March 2008 and November 2011 were analyzed by two blinded readers independently and retrospectively. All patients had arterial hypertension, elevated aldosterone-to-renin ratio and abnormal saline suppression (2 L of normal saline infused over four hours). For the diagnosis of unilateral aldosterone secretion the aldosterone-to-cortisol ratio from one adrenal vein had to be ≥4 times that of the contralateral side (14). Interpretation of laboratory results confirmed 45 cases of unilateral excessive aldosterone secretion (27 cases left, 18 cases right) and 24 cases of bilateral disease.
CT examinations
Patients with diagnosed PA received adrenal CT-imaging according to the Endocrine Society Clinical Practice Guidelines (2, 6). These preinterventional CT scans were used for MDCT mapping. Therefore, no additional radiation exposure occurred in this retrospective study. A total of 70 patients had both unenhanced and contrast-enhanced MDCT scans, while five patients were examined without intravenous contrast due to impaired renal function. A 64-slice MDCT scanner (Brilliance; Philips Healthcare, Best, the Netherlands) was used and the following scanning parameters were applied. For unenhanced scanning, collimation was 64×0.625 mm, pitch 0.891, rotation time 0.5 s, tube voltage 120 kV, tube current 200 mAs. The dose modulation was not adapted to thin slices but to the reconstruction slice thickness of 3 mm. As aldosterone-producing adenomas of the adrenal glands are typically very small in size and some patients present with diffuse micronodular changes, contrast-enhanced CT for patients without contraindications is a standard at our institution. Contrast-enhanced scans were obtained in a late venous phase after the administration of 2 mL/kg bodyweight of Iomeprol 350 mg I/mL (Imeron, Bracco Imaging Deutschland GmbH, Konstanz, Germany) injected at a flow rate of 2.5 mL/s via an antecubitally positioned peripheral venous line. Scanning was performed in breath-hold expiration 90 s after contrast injection in order to obtain optimal visibility of venous vessels in the venous phase. Adjustments were as follows: collimation 64×0.625 mm, pitch 0.951, rotation time 0.75 s, tube voltage 120 kV, max tube current 250 mAs. Reconstructed slice thickness was 3 mm including multiplanar reconstructions in three standard planes.
CT interpretation
MDCT images were evaluated using the hospital PACS (Syngo Imaging V35A, Siemens Healthcare, Erlangen, Germany). CT images were analyzed retrospectively and independently by two radiologists: reader 1 (R1) had two years of experience in radiology (no adrenal venous sampling), reader 2 (R2) had six years of experience including adrenal venous sampling. The location and level of the right adrenal veins orifice were evaluated in axial, coronal and sagittal reformats using slices of 3 mm thickness. The position of the RAV, as assessed this way, was then displayed on the CT-scout using the slice tool as performed in our clinical routine by the interventional radiologist planning the AVS (Fig. 1).
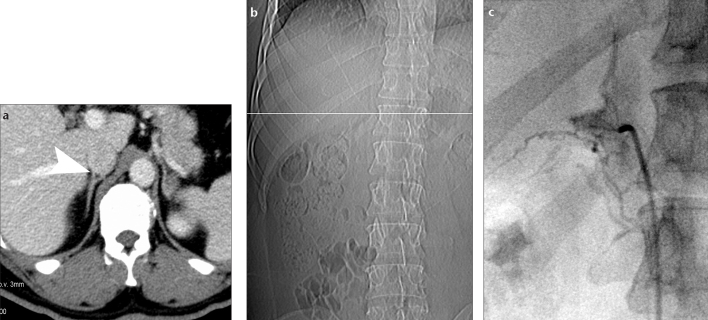
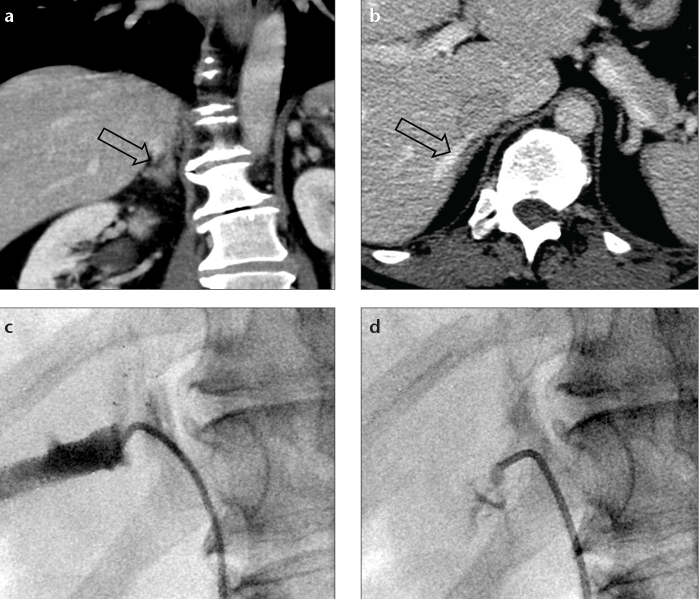
Figure 1.
a–c. Example of an adrenal vein mapping. Axial contrast-enhanced CT scan (a) gives excellent visualization of the right adrenal vein (RAV, arrowhead) located in Level 10. CT scout image (b) displays the slice position (white line) corresponding to (a). The angiogram (c) shows excellent correlation between the location of the RAV during adrenal vein sampling and the position that was found in adrenal vein mapping.
The extra-glandular part of the RAV was identified according to the criteria described by Matsuura et al. (12), as follows: It was assumed that the RAV was depicted when a tubular or linear structure was seen originating from the right adrenal gland and entering the inferior vena cava (IVC) either directly or indirectly. Contrast-enhancement of the structure was not a requirement; it could also be in contrast to the surrounding fat tissue or to other surrounding structures.
Adrenal vein sampling
AVS was performed by the senior radiologist (R2). He performed AVS after evaluation of MDCT images regarding the RAVs position and anatomical variants (MDCT mapping), without making any records. At the time of intervention, he was unaware of the results of CT-mapping reported in this study. This data was collected retrospectively, at least 12 months after AVS to avoid bias. Patients were placed in supine position with arms besides their body. A 5 F sheath was introduced via the right common femoral vein, and 4 F hydrophilic angiography catheters were used for the selective catheterization of the adrenal veins. In particular, the cannulation of the left adrenal vein was performed using a catheter with Bentson-Hanafee-Wilson-1 (Radiofocus Glidecath, Terumo Europe N.V., Leuven, Belgium) configuration and the RAV was catheterized either with Simmons-Sidewinder 1 (65 cm) or Cobra 2 (Radiofocus Optitorque, Terumo Europe N.V.) configuration. Positioning of the catheter was assessed by pulsed fluoroscopy (15–30 p/s, Polytron TOP, Siemens Healthcare) under free breathing and by selective retrograde venograms of the adrenal veins. A sample from the sheath was collected at the same time as the selective adrenal vein sample to allow for gradient calculations of selective and peripheral cortisol and aldosterone serum levels. Selective AVS was assumed when the concentration of cortisol in the selectively taken sample was at least twice the concentration of the simultaneously drawn sample from the catheter sheath in the femoral vein (selectivity index: cortisol in adrenal vein/cortisol in right femoral vein ≥2.0) (15). Selective blood samples of the right adrenal vein were collected in 69 of 75 patients, with the selectivity index ranging from 2.0 to 151.7 (mean, 17.9; median, 6.1).
Evaluation of MDCT mapping
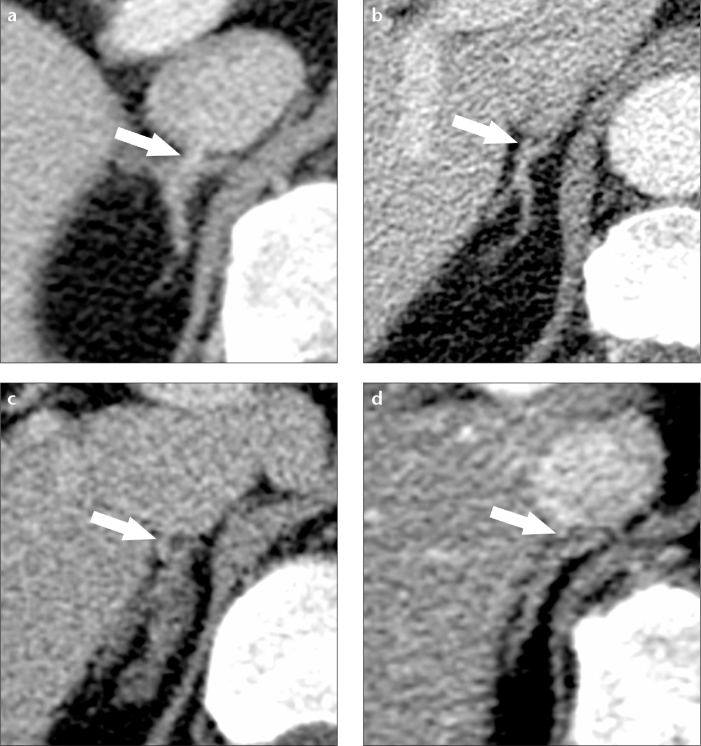
RAV visualization was evaluated on contrast-enhanced scans, except for cases having only unenhanced scans. The quality and reliability of imaging for visualization of the RAV was scored using a five-point semiquantitative confidence scale (5, excellent; 4, good; 3, moderate; 2, poor; 1, not visible; Fig. 2). Scores 3–5 represented adequate visualization.
Figure 2.
a–d. Different degrees of right adrenal vein (RAV) visualization. Examples of axial contrast-enhanced CT scans with identical ratings from both readers for excellent (a), good (b), moderate (c) and poor (d) visualization. The RAV is marked with arrows.
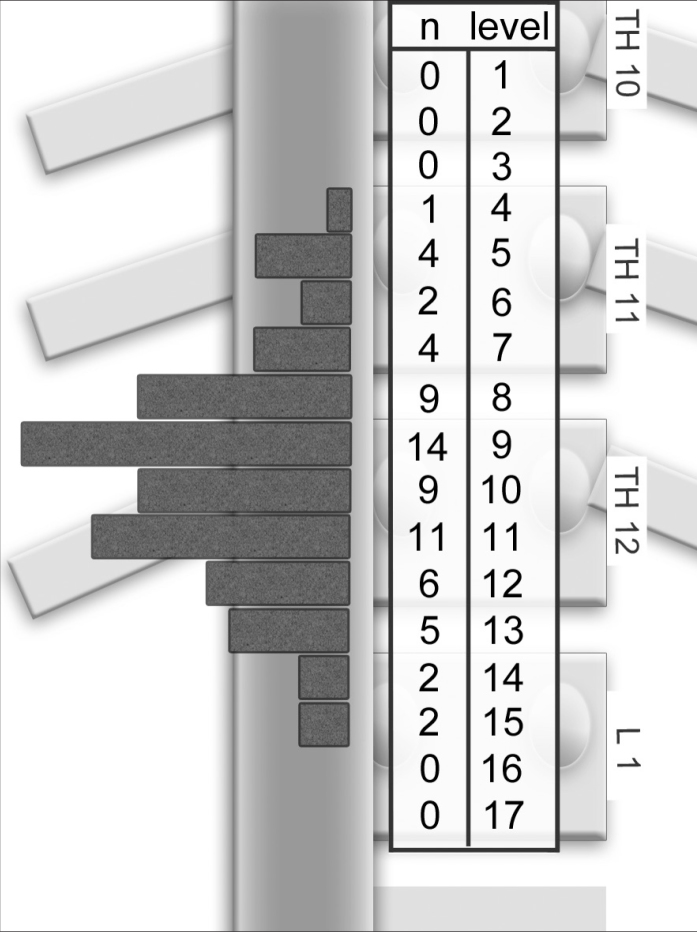
Localization of the RAV orifice was determined and labeled from cranial to caudal in 17 anatomical levels from the middle of the 10th thoracic vertebra to the bottom of the 1st lumbar vertebra (Fig. 3). Each vertebral body was subdivided into four equal levels from cranial to caudal and one additional section representing the vertebral disc. Segmentation was done on the basis of the anatomical study by Monkhouse and Khalique (16). According to his data, Levels 8–12 were defined as the center, whereas Levels 1–7 and Levels 13–17 were named off-center regions. In cases with no visible adrenal vein, the RAV orifice was estimated using the level of the adrenal gland center, as suggested by Daunt (8). The exact metric extent of one level is not important for the study or the AVS procedure. However, to provide an estimation of segment sizes, the vertebral bodies and the intervertebral discs were measured in a randomly chosen exemplary subcohort of 12 patients (six males and six females; mean age, 49.8 years).
Figure 3.
Number of angiographically localized right adrenal vein (RAV)/inferior vena cava confluences per anatomical Levels 1–12 (n=49). RAV orifices were located in the center between the thoracic vertebra (Th11/12) and the lower end plate of Th 12 (Levels 8–12).
Anatomical variants were registered descriptively. The normal finding was defined as one central adrenal vein draining directly into the IVC from dorsal or right-sided lateral direction, in caudocranial or horizontal direction.
Statistical analysis
For statistical analysis, R Data software (http://cran.r-project.org) was used. Data was imported from a Microsoft Excel 2010 spreadsheet. Weighted Cohen’s kappa (square kappa) statistics were used for the calculation of interobserver agreement regarding the predicted anatomical position (Levels 1–17) and the quality of visualization (five-point scale) of the RAV in MDCT. For determination of concordance between the predicted positions of the RAVs in MDCT mapping (Levels 1–17) and the angiographic findings, weighted square kappa statistics were calculated. The kappa values were interpreted as follows: <0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement. The significance level was set to 0.05 and Bonferroni correction was performed to compensate for multiple testing. In this setting, all kappa values were significant.
MDCT-based localization was assumed correct, when it did not vary more than ±1 level in comparison to the gold standard; unconfident, when there was a difference of ±2 levels; and false, when there was ≥3 levels of difference. The calculation of interobserver agreement regarding the semiquantitative rating of visualization was only conducted if the RAV localizations given by both readers were in accordance, i.e., not differing more than ±1 level.
Results
Successful bilateral AVS was obtained in 69 of 75 patients (92.0%). AVS failed on the right side in five patients (6.7%) and bilaterally in one patient (1.3%). Correspondingly, 69 patients met the criteria of the gold standard.
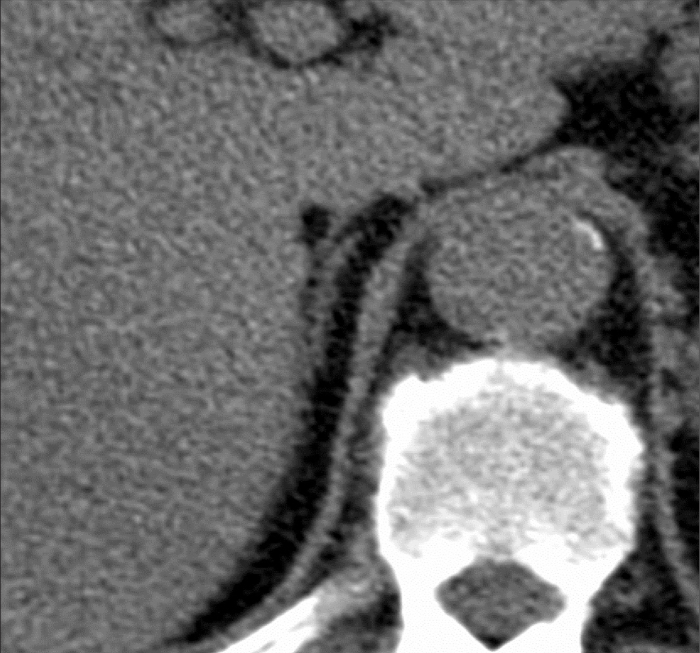
Adequate (i.e., excellent, good, or moderate; scores 5–3) visualization of the RAV in MDCT was achieved in 78% (54/69, R1), and 77% of patients (53/69, R2). In detail, the degree of visualization was rated as excellent in 15 (R1) and 25 patients (R2); good in 20 (R1) and 13 patients (R2); moderate in 19 (R1) and 15 patients (R2); poor in 13 (R1) and 14 patients (R2); and not visible in two patients (both R1 and R2). Readers R1 and R2 agreed on visualization rating in 55 patients; the resulting kappa value of 0.75 was consistent with good agreement between the two observers. In the subgroup of unenhanced examinations, three of five patients had a layer of fat tissue adjacent to their adrenal veins over a distance of 6–8 mm, providing excellent contrast and visualization (Fig. 4). In the remaining two patients, both readers found poor visualization of the vein.
Figure 4.
Unenhanced axial CT scan shows excellent visualization of the right adrenal vein due to high contrast versus the surrounding fat tissue.
Among six patients with unsuccessful catheterization, visualization was rated as excellent or good in two patients (R1 and R2), moderate in four (R1) and two patients (R2), and poor in two patients (R2).
Correlation of venograms and MDCT mapping was possible in 69 of 75 patients meeting the criteria of the gold standard (i.e., true angiographic position of the RAV, confirmed by the laboratory results of AVS). MDCT mapping identified the correct position of the RAV orifice in 70% (48/69, R1) and 88% of patients (61/69, R2), respectively. Unconfident localization was found in 14% (10/69, R1) and 4% (3/69, R2) of patients. False localizations as compared to the angiographic finding were determined in 16% (11/69, R1) and 7% of patients (5/69, R2) (Table). The calculated kappa values for the concordance of localized RAV/VCI confluences in MDCT and gold standard were 0.679 (R1) and 0.864 (R2), equivalent to good and excellent agreement of predicted position and angiographic finding, respectively.
Table.
Comparison of gold standard (angiography and laboratory testing) and MDCT findings
| R1 | R2 | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Level | Gold standard | Correct | Unconfident | False | Correct | Unconfident | False |
| 1 | 0 | - | - | - | - | - | - |
| 2 | 0 | - | - | - | - | - | - |
| 3 | 0 | - | - | - | - | - | - |
| 4 | 1 | 1 | - | - | 1 | - | - |
| 5 | 4 | 2 | - | 2 | 2 | - | 2 |
| 6 | 2 | 2 | - | - | 2 | - | - |
| 7 | 4 | 2 | 1 | 1 | 4 | - | - |
| 8 | 9 | 6 | 2 | 1 | 9 | - | - |
| 9 | 14 | 10 | 3 | 1 | 12 | 2 | - |
| 10 | 9 | 6 | 2 | 1 | 9 | - | - |
| 11 | 11 | 7 | 2 | 2 | 7 | 1 | 3 |
| 12 | 6 | 6 | - | - | 6 | - | - |
| 13 | 5 | 2 | - | 3 | 5 | - | - |
| 14 | 2 | 2 | - | - | 2 | - | - |
| 15 | 2 | 2 | - | - | 2 | - | - |
| 16 | 0 | - | - | - | - | - | - |
| 17 | 0 | - | - | - | - | - | - |
| Total | 69 | 48 | 10 | 11 | 61 | 3 | 5 |
Data are given as the number of patients. Results are classified according to anatomic locations (Levels 1–17). MDCT-based localization was assumed correct, when it did not vary more than ±1 level in comparison to the gold standard; unconfident, when there was a difference of ±2 levels; and false, when there was ≥3 levels of difference.
R1, reader 1; R2, reader 2.
For a detailed evaluation, the localization of the RAV orifice was subdivided into center and off-center regions. The evaluation of the angiograms revealed that 49 of 69 orifices to the IVC were located at Levels 8–12. In other words, 71% of RAV confluences were identified angiographically in a central region that extended between the intervertebral disc Th11/12 and the lower end plate of the 12th vertebral body (Fig. 3).
In the center, R1 detected 71% of orifices (35/49) correctly, while R2 localized 88% (43/49) correctly. Unconfident concordance was found in nine (R1) and three (R2) cases, and inaccurate localizations were found in five (R1) and three cases (R2), respectively.
Of RAV orifices, 29% (n=20) were positioned above or below the center. Using MDCT mapping R1 identified 13 off-center locations and R2 found 18. Incorrect results were found in six (R1) and two cases (R2), respectively. There was one unconfident concordance for R1.
The vertebral bodies from Th11 to L1 and the intervertebral discs lying in between were measured in a subcohort of 12 patients (six males and six females; mean age, 49.8 years; sagittal CT multiplanar reconstruction, 6±1 mm).
In terms of anatomical variants, a common drainage of the RAV and an accessory hepatic vein was observed in 7% of patients (5/69) in angiography (Fig. 5). In MDCT, this condition was detected by both readers in three cases: two variants were identified concordantly, and additionally each reader found one variant, which was not detected by the other. Among six patients with unsuccessful AVS, the above mentioned variant was found in one patient in concordance and in another patient by R1, however, these findings were not proven by the gold standard.
Figure 5.
a–d. Contrast-enhanced coronal CT image (a) depicts the drainage of the right adrenal vein (RAV, open arrow) into an accessory hepatic vein as a normal variant. Axial CT image (b) shows the accessory hepatic vein (open arrow). Selective angiogram of the accessory hepatic vein (c) shows a small notch representing the entry of the RAV. Superselective angiogram of the RAV (d).
Discussion
Bilateral AVS is the method of choice for distinguishing between uni- and bilateral excessive aldosterone secretion in patients with PA. However, this intervention is strikingly often not successful. It is well known to interventional radiologists that particularly the cannulation of the RAV is technically more demanding than the catheterization of the left: the RAV is shorter, usually drains directly into the IVC and has a diameter of not more than 2–3 mm (17). Moreover, the location of the RAV’s orifice is variable and must be anticipated in a range between the 11th thoracic and the 2nd lumbar vertebra (16), quite a distance, considering the very small size of the RAV. Not surprisingly, the unsatisfying success rates of bilateral AVS even in specialized centers (70%–90%) are mainly attributed to missing blood samples from the RAV (8–10, 13). Therefore, catheterization of the RAV is crucial to the success of AVS.
All patients should undergo CT or MRI imaging prior to AVS in the diagnostic workup of PA (2), MDCT mapping (i.e., the search for the adrenal veins prior to AVS) is implicated within our routine procedure and it is even compulsory in our opinion. However, because the RAV is often a very tiny structure, we were motivated to investigate the quality of its visualization and the concordance between MDCT mapping and the angiographic findings of the RAV. Only two previous publications considered the possible benefit of reading MDCT images for the planning of AVS, but without comparing it to an angiographic standard (12). Consequently it was not clear so far, if a structure identified in MDCT matches the RAV and whether MDCT is useful in AVS or may, on the contrary, even be misleading. The purpose of this study was to systematically evaluate MDCT images in PA patients to determine the reliability and feasibility of indirect MDCT mapping for facilitating AVS. The gold standard regarding the position of the RAV consisted of selective angiographic finding of the RAV and was additionally corroborated by laboratory testing of selectively withdrawn blood samples. As a reader might get biased when performing both the sampling and the interpretation of the MDCT mapping directly one after another, we decided to provide a time interval of at least 12 months between AVS and the retrospective MDCT mapping.
We found that the orifices of the RAVs were mostly (in about 70% of cases) located in the center between the level of the intervertebral disc Th11/12 and the 12th thoracic vertebra. In about 30% of cases, the orifice was located further cranially or caudally to the typical position. The correct position of the RAV’s confluence into the IVC could be identified in the majority of patients, both by the junior reader (70%) and the experienced reader (88%) with good and excellent agreement with the gold standard. In particular, the verification of the RAV’s position in the center may facilitate AVS and thereby reduce the time required for fluoroscopy. Moreover, the “off-center” confluences could be visualized in a substantial proportion of cases (65%–90%), allowing for improved planning of the interventional procedure. This is most remarkable since these locations are more difficult to visualize due to their variant position. On the other hand, a considerable number of RAV orifices was located unconfidently (14% R1, 4% R2) or falsely (16% R1, 7% R2). This implies that there is also potential for “MDCT misguidance” in AVS, especially for readers with limited experience.
The most frequently described variant consists of a common trunk that drains the blood of the adrenal vein and an accessory hepatic vein into the IVC. In an autopsy study, its incidence was reported as approximately 10% (18). In angiographic studies this condition was described sporadically (19) and in frequencies of up to 12% (17). In our series, five cases (7%) had this variant, three of which were detected in MDCT. This variant is of particular importance because superselective cannulation of the RAV is necessary to obtain appropriate blood samples without major dilution by hepatic blood (17). Although the numbers are low and further confirmation is required, the detection of anatomical variants prior to AVS might be an additional benefit of MDCT mapping.
Our study had some limitations. First, the success of AV, and therefore, the validation of the gold standard depend on the definition of the selectivity index (20). The selectivity indices in our study was ≥ 2. In other studies, selectivity index of 1.1–5.0 were used (7). The higher sensitivity indices most commonly were applied with concomitant infusion of adrenocorticotropic hormone (21). Second, RAV sampling was not successful in six patients. Consequently, position of the RAV could not be verified by angiography and results of MDCT mapping could not be correlated. We therefore only evaluated 69 patients who had bilaterally successful AVS, which might bias the analysis. However, this potential bias is small as the results of 92% of patients could be analyzed as intended. The success rate was at the upper end of the reported results (13). The true role of preprocedural assessment of the RAV from cross-sectional images, has to be investigated in a second step. Such studies should compare the success rates and the dose exposure of AVS with and without the use of MDCT mapping. Third, the venograms of the RAV were acquired during normal breathing whereas CT examinations were performed during expiration. According to our experience, the movement of the adrenals during normal breathing is in the range of 1–2 cm, while the maximal vertical motion can reach up to 4 cm (22). To take this fact into account, correct detection of the confluence was assumed if the location detected in MDCT did not differ more than one level compared to the angiographic gold standard. Although the extent of movement is not exactly known, this approach led to a good concordance with respect to the correct positioning of the RAV orifice in the majority of cases. Fourth, about a quarter of RAVs were not identified by CT mapping. However, a CT scan is recommended in the Endocrine Society Clinical Practice Guideline (2), and there is no additional radiation exposure to the patients. The impact of MDCT mapping with regards to radiation exposure during AVS, time for fluoroscopy and amount of applied contrast agent may be subject to prospective randomized trials. Lastly, as the evaluation included subjective ratings, the significance of results with respect to visibility of the RAV may be limited. However, there was good interobserver agreement between the readers (kappa value 0.75).
In conclusion, MDCT mapping identified the RAV orifices, as well as potential off-center locations and anatomic variants in good concordance with angiography, in the majority of cases. Although this study does not provide evidence for improvement of AVS success rates by MDCT mapping, the initial results are promising to facilitate precise catheterization during AVS and may justify prospective, controlled trials.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 3.Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–1926. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura K, Fujii K, Oniki H, Oka M, Iida M. Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens. 2006;19:13–18. doi: 10.1016/j.amjhyper.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Rossi GP, Bernini G, Desideri G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 6.Mulatero P, Bertello C, Verhovez A, et al. Differential diagnosis of primary aldosteronism subtypes. Curr Hypertens Rep. 2009;11:217–223. doi: 10.1007/s11906-009-0038-1. [DOI] [PubMed] [Google Scholar]
- 7.Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005;25(Suppl 1):S143–158. doi: 10.1148/rg.25si055514. [DOI] [PubMed] [Google Scholar]
- 9.Plank C, Wolf F, Langenberger H, Loewe C, Schoder M, Lammer J. Adrenal venous sampling using Dyna-CT-A practical guide. Eur J Radiol. 2012;81:2304–2307. doi: 10.1016/j.ejrad.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf) 2009;70:14–17. doi: 10.1111/j.1365-2265.2008.03450.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham UM, Mullan KR, Hunter SJ, Atkinson AB. Comment on Stewart PM, Allolio B. Adrenal vein sampling for primary aldosteronism: time for a reality check. Clin Endocrinol (Oxf) 2010;73:551–552. doi: 10.1111/j.1365-2265.2010.03825.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura T, Takase K, Ota H, et al. Radiologic anatomy of the right adrenal vein: preliminary experience with MDCT. AJR Am J Roentgenol. 2008;191:402–408. doi: 10.2214/AJR.07.3338. [DOI] [PubMed] [Google Scholar]
- 13.Schirpenbach C, Segmiller F, Diederich S, et al. The diagnosis and treatment of primary hyperaldosteronism in Germany: results on 555 patients from the German Conn Registry. Dtsch Arztebl Int. 2009;106:305–311. doi: 10.3238/arztebl.2009.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur A, Kemp CD, Dutta U, et al. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg. 2010;211:384–390. doi: 10.1016/j.jamcollsurg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulatero P, Bertello C, Rossato D, et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93:1366–1371. doi: 10.1210/jc.2007-2055. [DOI] [PubMed] [Google Scholar]
- 16.Monkhouse WS, Khalique A. The adrenal and renal veins of man and their connections with azygos and lumbar veins. J Anat. 1986;146:105–115. [PMC free article] [PubMed] [Google Scholar]
- 17.Miotto D, De Toni R, Pitter G, et al. Impact of accessory hepatic veins on adrenal vein sampling for identification of surgically curable primary aldosteronism. Hypertension. 2009;54:885–889. doi: 10.1161/HYPERTENSIONAHA.109.134759. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 1981;152:43–50. [PubMed] [Google Scholar]
- 19.Sebe P, Peyromaure M, Raynaud A, Delmas V. Anatomical variations in the drainage of the principal adrenal veins: the results of 88 venograms. Surg Radiol Anat. 2002;24:222–225. doi: 10.1007/s00276-002-0021-x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008;26:989–997. doi: 10.1097/HJH.0b013e3282f9e66a. [DOI] [PubMed] [Google Scholar]
- 21.Betz MJ, Degenhart C, Fischer E, et al. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur J Endocrinol. 2011;165:301–306. doi: 10.1530/EJE-11-0287. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LH, Richaud J, Buffat L, Touboul E, Schlienger M. Kidney mobility during respiration. Radiother Oncol. 1994;32:84–86. doi: 10.1016/0167-8140(94)90452-9. [DOI] [PubMed] [Google Scholar]