Abstract
Genital tuberculosis (TB) is an important cause of female infertility in the world, especially in developing countries. Majority of infertility cases are due to involvement of the fallopian tubes (92%–100%), endometrial cavity (50%), and ovaries (10%–30%); cervical and vulvovaginal TB are uncommon. Genital TB has characteristic radiological appearances based on the stage of the disease process (acute inflammatory or chronic fibrotic) and the organ of involvement. Hysterosalpingography (HSG) and ultrasonography (US) remain the main imaging modalities used in the diagnosis of genital TB. HSG is the primary modality for evaluating uterine, fallopian tube, and peritubal involvement and also helps in evaluating tubal patency. US, on the other hand, allows simultaneous evaluation of ovarian and extrapelvic involvement.
Genital tuberculosis (TB) is a cause of female infertility in up to 17% of cases, ranging from 1% in developed countries to 17% in developing countries (1, 2). The highest prevalence (75% of all cases) is seen in the reproductive age group (20–45 years), where it has the greatest impact on fertility (3). In this article, we provide an overview of pathophysiology of tuberculous involvement of various parts of the female genital tract and discuss in detail the imaging features with radiological-pathological correlation.
Fallopian tube and peritubal TB
Pathophysiology
The infection of the female genital tract is in most cases secondary to a primary infection elsewhere. The fallopian tubes are the initial sites of infection in majority of cases (1). In most cases, tuberculous involvement of the fallopian tube occurs by the hematogenous route from a TB focus elsewhere, usually pulmonary or osseous, leading to endosalpingitis (4). There can also be direct involvement of the peritoneal surface of the tubes from an adjacent intra-abdominal or peritoneal focus, leading to exosalpingitis (5). Spread by lymphatics has been reported due to a bovine TB focus in the abdominal cavity in people having a history of unpasteurized milk consumption. Tubal TB spreads to the endometrium in approximately half of the cases (5).The myometrium is usually not involved (2). Ovarian involvement can occur directly from the adjacent structures (tubes, peritoneum, lymph nodes) or by hematogenous spread. Infection of vulva, vagina, and cervix may also result from direct inoculation when the sexual partner has active genitourinary TB, with resultant ascending spread to the uterus and tubes.
Tuberculous involvement of the fallopian tube and endometrium progresses through an acute exudative stage followed by the productive-adhesive stage. Finally healing occurs with fibrosis and calcification.
Acute salpingitis
Tuberculous salpingitis initially leads to formation of granulomas in the mucosa and on the surface of the tubes. These granulomas undergo caseous ulceration leading to mucosal irregularity, which manifests as ragged contour of the lumen of the tubes and diverticular outpouchings (salpingitis isthmica nodosa [SIN] appearance) on hysterosalpingography (HSG) (Fig. 1) (5). SIN is basically characterized by nodular thickening of the isthmic and ampullary portions with accumulation of contrast in the wall of the fallopian tube giving the appearance of multiple small diverticuli. Pathologically the nodular areas in SIN are inpouchings of endosalpinx surrounded by a hypertrophied myosalpinx. SIN appearance is a descriptive term without any clear etiology; however, causative theories include postinflammatory (e.g., TB), acquired non-postinflammatory, and congenital causes.
Figure 1.
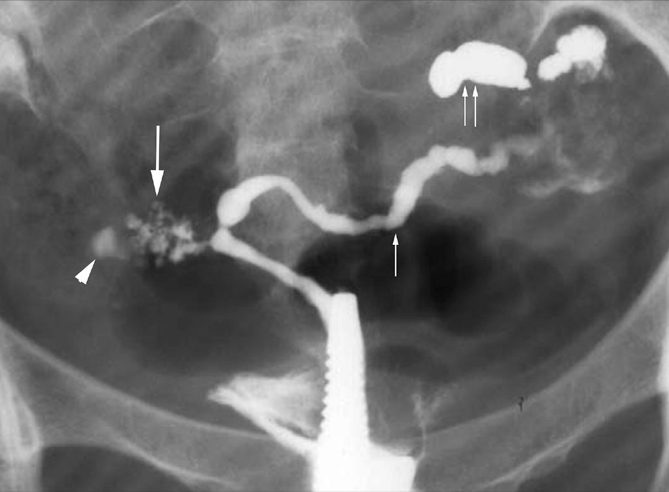
SIN-like appearance in a 23-year-old woman with infertility and genital TB. HSG oblique view shows honey-combed accumulation of contrast in the fallopian tube wall on the right side in its isthmic portion giving the appearance of multiple small diverticuli (arrow). Note that the uterus is small and scarred. There is isthmic level block on the right (arrowhead). The left tube has a rosary bead appearance with alternate dilatation and constriction (small arrow) and loculated spill of contrast (paired arrows) suggestive of peritubal adhesions.
Tuberculous salpingitis also leads to edematous thickening of the tubal walls and tubal dilatation. Tubal dilatation manifests on HSG as hydrosalpinx (Fig. 2). The tubes appear dilated and tortuous and are folded upon themselves to form a C or S shape. The hydrosalpinx may or may not be accompanied by intraperitoneal spill, depending on the presence or absence of tubal obstruction. The thickened edematous rugal folds may be seen as linear radiolucent filling defects in the contrast-filled tubes (Fig. 3) (5).
Figure 2.
Hydrosalpinx in a 28-year-old woman with infertility. HSG shows the classic tobacco pouch appearance due to disproportionate dilatation of the club-shaped ampullary ends of both fallopian tubes (arrow). The left fallopian tube is vertically oriented and fixed. There is no spill of radio-opaque contrast into the peritoneal cavity.
Figure 3.
Thickened mucosal folds in a 26-year-old woman with primary infertility. HSG exam shows that both fallopian tubes are dilated, crowded, and coiled on themselves, giving a corkscrew appearance. There are subtle radiolucent filling defects in the tubes suggestive of thickened mucosal folds (arrow). No peritoneal spill of contrast is seen.
On ultrasonography (US), the tubes are dilated and show thickened walls due to edema (Fig. 4). The tubes may be folded upon themselves and filled with clear fluid (hydrosalpinx) (Fig. 5) or show thick internal echoes with debris due to the presence of thick caseous material or pus (pyosalpinx) (Fig. 4). The thickened longitudinal mucosal folds produce the appearance of septa which do not cross the lumen completely (Fig. 5) (6). When viewed in cross section, these tubes with thickened folds resemble a cogwheel, and this is described as the “cogwheel sign.”
Figure 4.
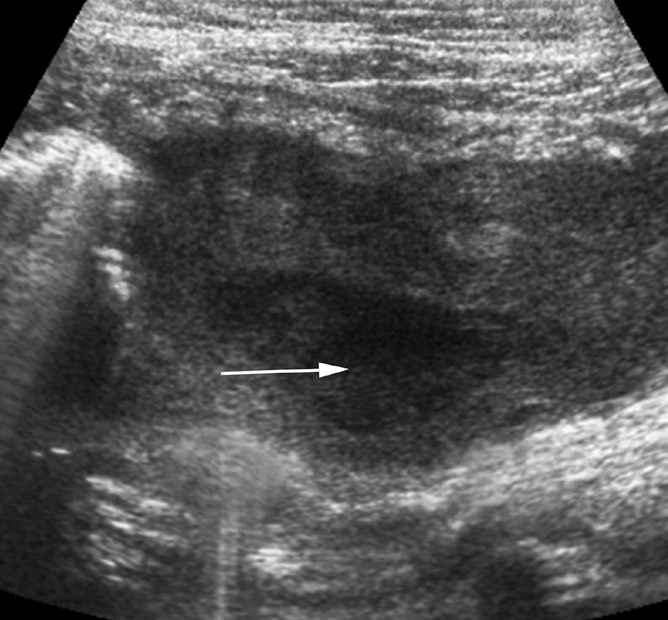
Pyosalpinx in a 23-year-old woman with chronic pelvic pain and night fevers. US image shows a dilated heterogeneously thickened fallopian tube filled with a collection having thick internal echoes (arrow). Laparoscopy-guided biopsy showed multiple caseating granulomas.
Figure 5.
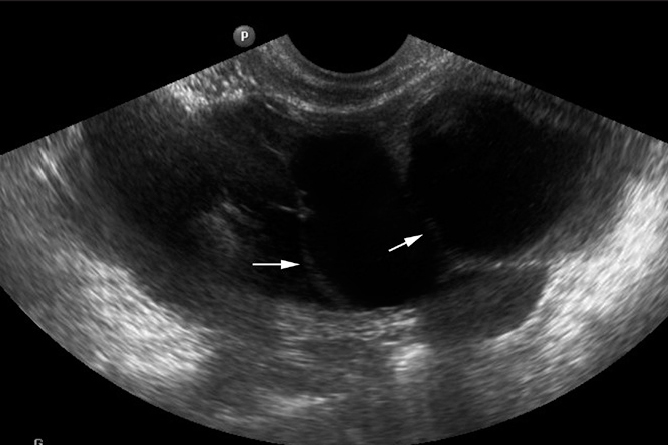
Hydrosalpinx of a 27-year-old woman with failure to conceive since six years. US pelvic image shows a thickened fallopian tube. The tube is dilated with multiple incompletely coursing septae inside, giving the cogwheel appearance (arrows). These septa are formed by the thickened mucosal folds of the tube and appear incomplete.
Chronic salpingitis
After the initial active phase of the infection, the disease process enters a chronic phase characterized by healing with fibrosis and scarring. Fibrotic scarring of the tubes leading to alternate areas of constriction along with minimal interval tubal dilatation gives rise to the rosary bead appearance of the tubes characteristic of TB (Fig. 1) (5).
Peritubal adhesions are seen in the chronic cases of tuberculous salpingitis due to peritubal scarring following repeated episodes of acute exacerbation. The HSG findings suggestive of peritubal adhesions are corkscrew configuration of the fallopian tubes, loculated spill of contrast in the peritoneal cavity, the “halo sign,” and a fixed vertically positioned tube (7). True corkscrew configuration of the tubes should be persistently visualized on both oblique views of the pelvis on HSG (Fig. 6). Loculated spillage of contrast in the peritoneal cavity is seen as oval, irregular, or bizarre shaped collections of contrast near the fimbrial end of the tube. A radiolucent halo may be seen separating the loculated peritubal collection from the dilated tube, known as the “halo sign” (Fig. 7). This radiolucent halo represents the thickened wall of the tube. The adhesions also cause fixity of the tubes (Figs. 8, 9). They often appear vertical in orientation, being directed upward or downward (Fig. 10). The vertically oriented tubes with ampullary dilatation give them a “sperm head” appearance (Fig. 10), where the dilated ampulla represents the head of a sperm and the rest of the tube represents the body and tail (8).
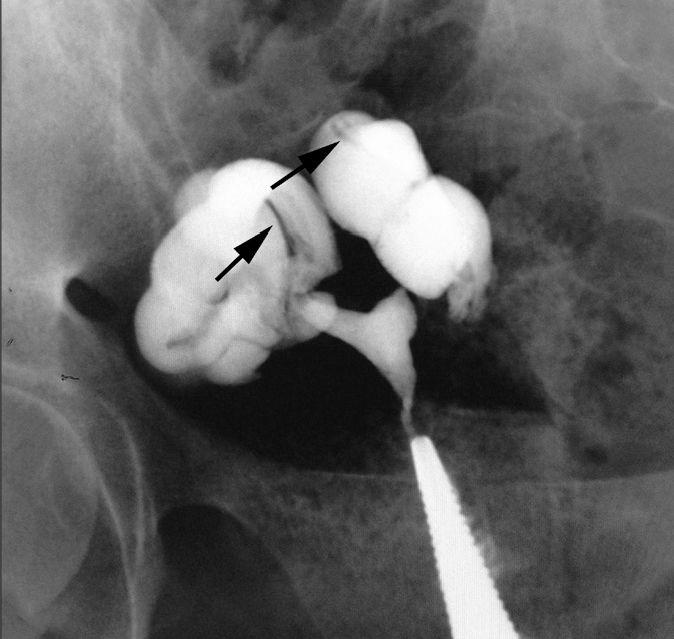
Figure 6.
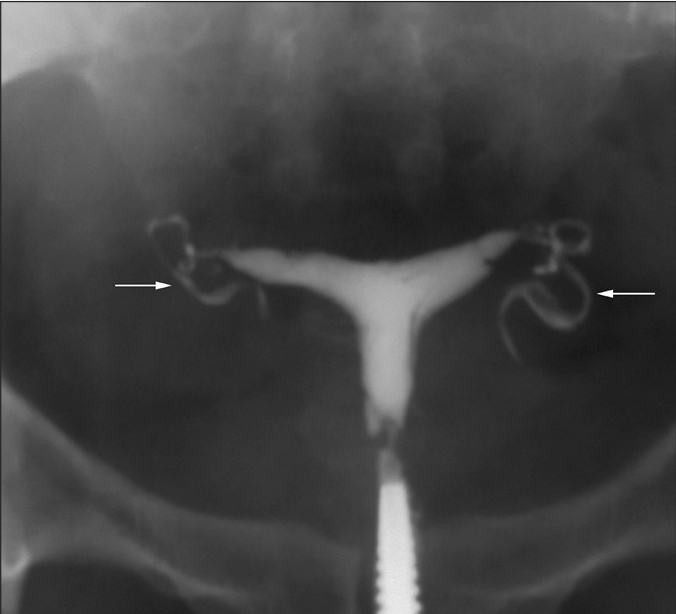
HSG of a 25-year-old woman with primary infertility shows classic cork screw fallopian tubes on both sides (arrows).
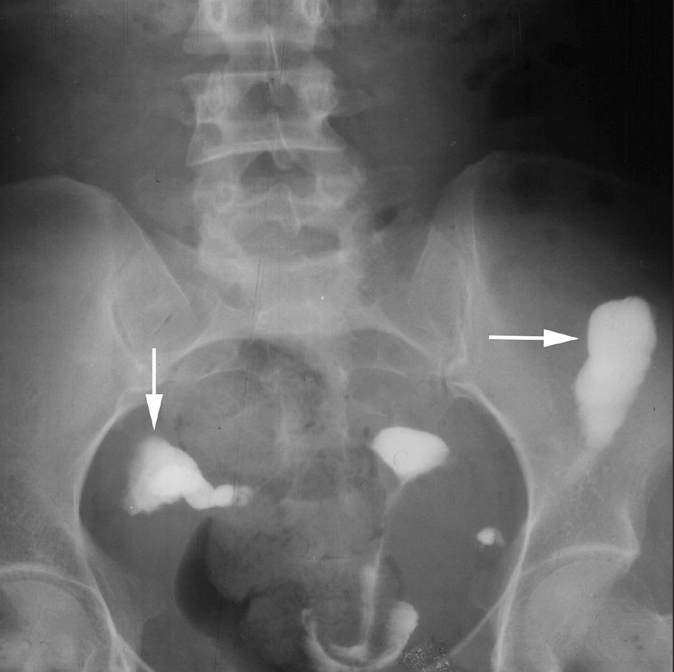
Figure 7.
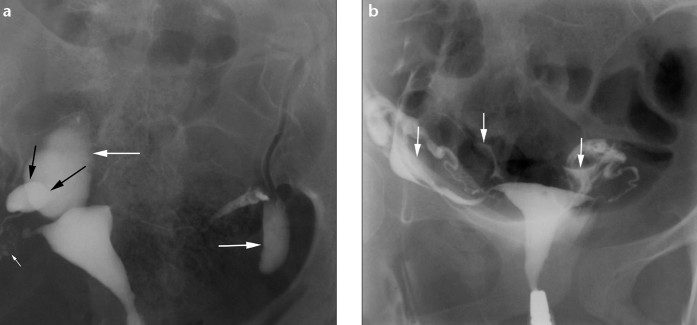
a, b. Loculated spill in a 26-year-old woman with primary infertility. HSG frontal view (a) shows loculated spill of contrast (large white arrows) on both sides. The right fallopian tube is convoluted on itself. A clear halo sign (black arrows) is seen between the loculated spill and the dilated ampullary end of the right fallopian tube. Also note SIN on the right side (small arrow). Compare with HSG frontal view (b) showing free intraperitoneal spillage of contrast lining the bowel loops (arrows).
Figure 8.
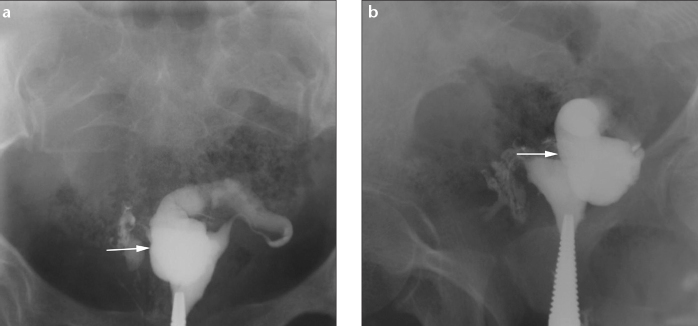
a, b. Peritubal adhesions and hydrosalpinx in a 26-year-old woman with chronic pelvic pain and infertility. HSG frontal (a) and oblique (b) views show that the left tube (arrow) courses abnormally, is folded upon itself, and is related to the anterior uterine surface. The right tube is also convoluted with loculated spillage of contrast. Laparoscopy showed extensive peritubal adhesions with left-sided hydrosalpinx and the left tube adherent to the broad ligament and uterus.
Figure 9.
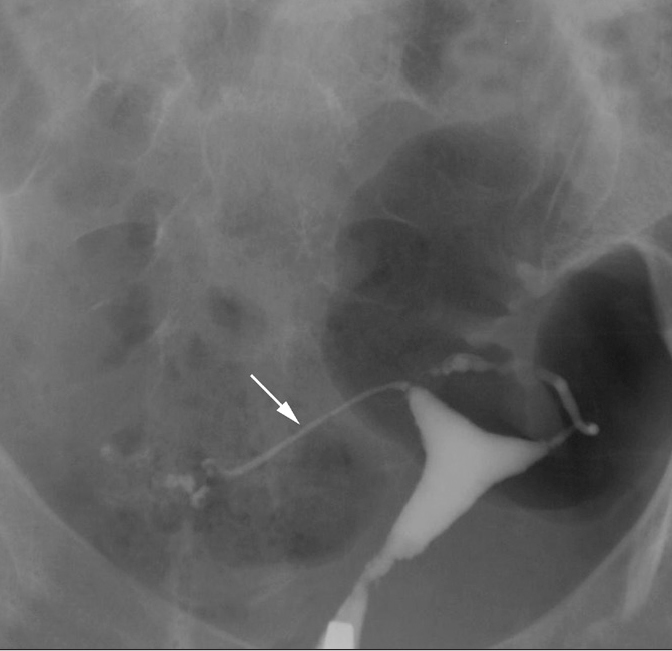
Rigid pipe stem fallopian tubes in a 31-year-old woman with primary infertility. HSG shows narrowed rigid pipe stem-like right fallopian tube (arrow) which is displaced downward and fixed deep within the pelvis. The left tube is also irregularly narrowed.
Figure 10.
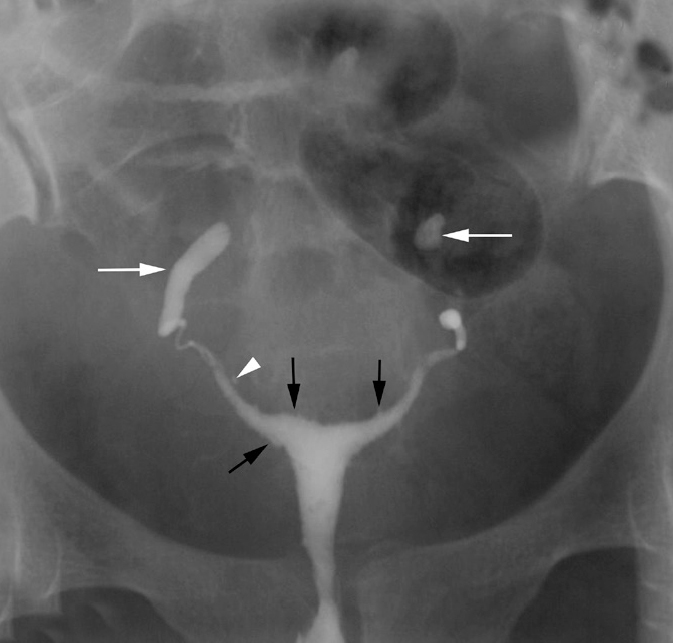
Vertically fixed tubes secondary to TB in a 29-year-old woman with infertility. HSG shows that both tubes are vertically oriented (white arrows) and fixed. This abnormal orientation is due to peritubal adhesions. Few small outpouchings filled with contrast are present on the right side consistent with SIN (arrowhead). There is bilateral ampullary level block with a sperm head appearance of the tubes. Also note irregularity of the endometrial cavity (black arrows) secondary to associated endometritis.
Intraluminal scarring due to adhesions can give rise to a cobblestone pattern in hydrosalpinges. This finding is more likely to be associated with infertility (5). Inflammatory fibrosis can eventually lead to complete obstruction of the fallopian tubes, which can be seen with or without tubal dilatation. Tubal obstruction is the most common HSG finding encountered in TB (9). It can occur at multiple sites; however, TB characteristically causes obstruction of the isthmico-ampullary segment of the tubes (10). If the site of obstruction is at the distal ampulla, it leads to dilatation of the fallopian tube with a club-like appearance to the ampulla giving rise to the characteristic “tobacco pouch” appearance on HSG (Fig. 2).
Other causes of tubal obstruction and focal contrast spillage include pelvic inflammatory disease due to gonococci or chlamydia, previous surgery, endometriosis, inflammatory bowel disease, and actinomycosis. Endometriosis, inflammatory bowel disease, and previous surgery cause tubal obstruction due to pelvic adhesions. Fimbriae are the usual site of obstruction in cases of tubal obstruction due to chlamydia or actinomycosis (7). Intrauterine contraceptive device use is associated with actinomycotic infection of the female genital tract, which can lead to the formation of tubo-ovarian mass and cause tubal obstruction.
The tubal and peritubal fibrotic scarring finally leads to the conversion of the fallopian tubes from compliant, mobile conduits to rigid, noncompliant, and fixed structures encased in a fibrous connective tissue scar. These tubes show a narrowed, rigid pipe stem appearance on HSG (Fig. 9) (5).
Dystrophic calcification in healed tuberculous granulomas on the tubes or ovaries can be seen as an ancillary finding. Tubal calcification can be in the form of linear streaks or tiny nodules along the course of fallopian tubes, while calcification in pelvic lymph nodes may be round or irregular (11). The differential diagnosis of pelvic calcifications would include a calcified leiomyoma or a calcified dermoid.
Endometrial TB
Acute endometritis
Acute endometritis can be identified on HSG as irregularity of the contour of the endometrial cavity (Figs. 10, 11). An indirect sign of endometritis is intravasation of contrast into the vascular and lymphatic system (Fig. 12). Acute endometritis is seen as thickened hypoechoic endometrium on US (Fig. 13).
Figure 11.
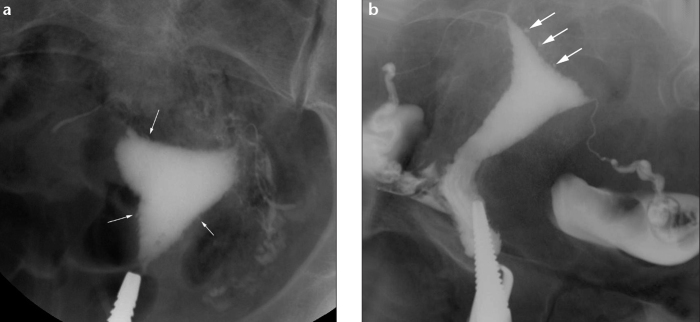
a, b. Tuberculous endometritis in a 27-year-old woman with oligomenorrhea, chronic pelvic pain, and primary infertility. HSG oblique view (a) shows irregular endometrial cavity (arrows). Fallopian tubes have an irregular contour with an ampullary level obstruction on the right side. Compare with HSG oblique view (b) which shows adenomyosis as multiple contrast-filled outpouchings of the endometrium into the myometrium (arrows), rather than mucosal irregularity. In contrast to TB, both tubes in (b) are of normal morphology with free intraperitoneal spill.
Figure 12.
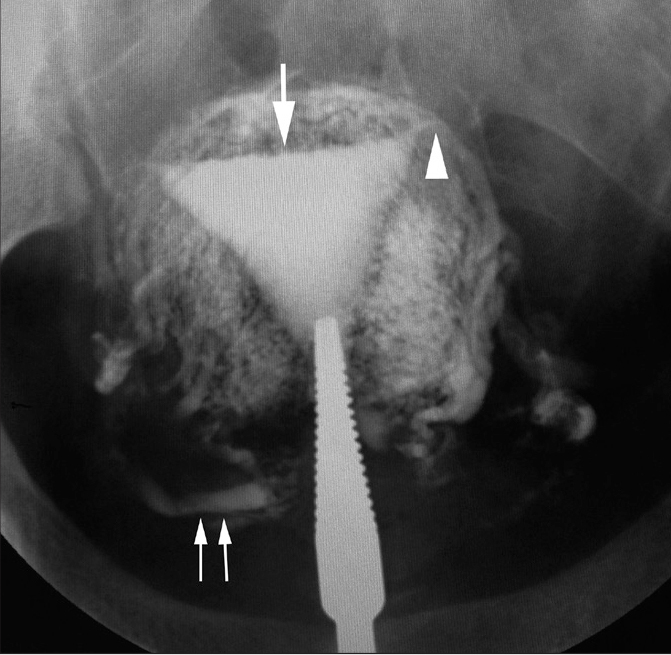
Tuberculous endometritis with intravasation in a 29-year-old woman with primary infertility. HSG shows left cornual level obstruction (arrowhead). The endometrial cavity is irregular in outline (large arrow). There is extensive intravasation of contrast into the myometrial vascular and lymphatic channels. The ovarian veins are well opacified with radio opaque contrast on both sides (paired arrows).
Figure 13.
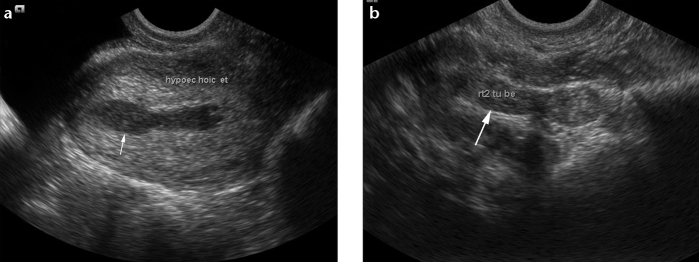
a, b. Tuberculous endometritis with salpingitis in a 26-year-old woman with primary infertility and oligomenorrhea. Transvaginal pelvis US shows a thickened hypoechoic endometrium (a, arrow) and a thickened undilated right fallopian tube (b, arrow). Endometrial curettage performed later, yielded positive tuberculous culture.
Chronic endometritis
Chronic endometritis is characterized by fibrosis, scarring, and calcification. This can be detected on HSG as calcification of the endometrium on plain film and irregularity of the endometrial cavity on contrast film. US shows a heterogeneous appearance of the endometrium with the presence of hyperechoic areas that represent foci of calcification or fibrosis (Fig. 14). The irreversible sequelae of fibrosis and scarring include intrauterine adhesions (synechiae) and a distorted uterine cavity.
Figure 14.
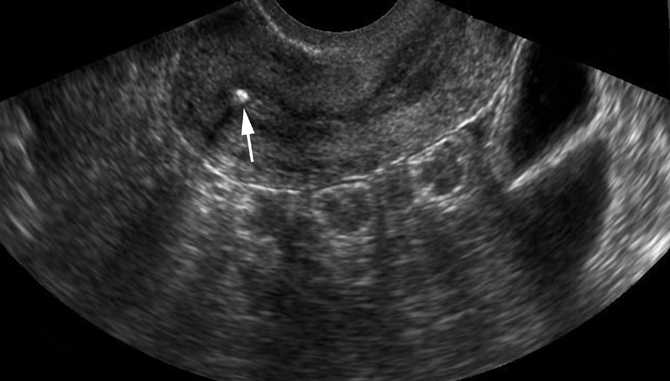
Chronic tuberculous endometritis in a 28-year-old woman with primary infertility. Transvaginal US image of the uterus shows a heterogeneous endometrium with a tiny focus of calcification (arrow).
HSG is a useful modality to detect synechiae, which are seen as irregular filling defects with well-defined borders (Fig. 15a). The filling defects may be linear, angulated, or stellate shaped and allow contrast to flow around them in one dimension. This is in contrast to polyps, myomas, and other masses that have round, smooth borders and allow contrast to flow around them in two dimensions (Fig. 15c) (12). With extensive synechiae, the uterine cavity fails to distend in the area of adhesion and presents with severe reduction in its volume and capacity (Fig. 16).
Figure 15.
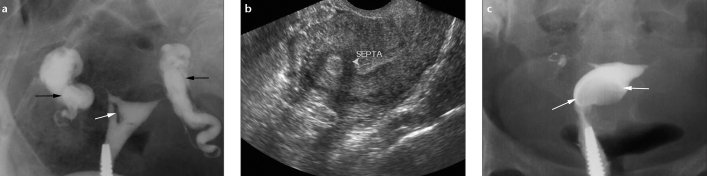
a–c. Uterine synechiae in a 27-year-old woman. HSG (a) shows linear, irregular, well-defined filling defect in the right half of uterine cavity (white arrow). Transvaginal US in grey scale (b) depicts the synechiae (arrowhead) quite well. Also note in (a), tubes are dilated on both sides, the right tube is convoluted on itself, and the left tube is oriented vertically. There is loculated contrast spill on both sides. A clear halo can be seen between the tubes and loculated spill (black arrows). Compare with HSG frontal view (c) showing a submucosal fibroid (arrows) in the left lateral wall which is seen as a round, smooth-bordered filling defect.
Figure 16.
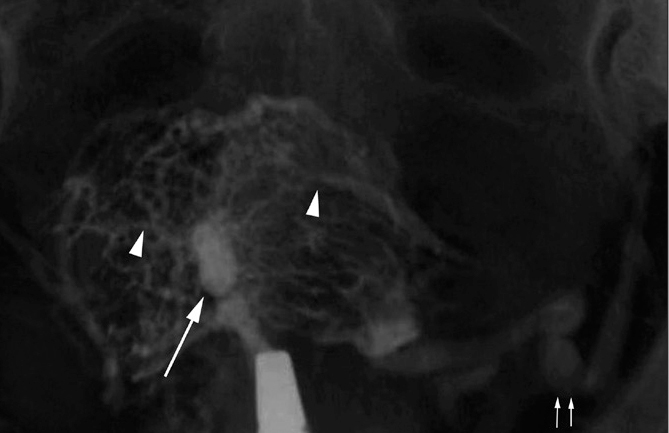
Asherman’s syndrome due to TB in a 31-year-old woman with primary infertility and hypomenorrhea. HSG shows a small scarred uterine cavity with multiple filling defects (arrow) due to extensive intrauterine synechiae leading to Asherman’s syndrome. Also note the extensive intravasation of contrast (arrowheads). The left uterine vein is also well opacified (paired arrows).
On US, synechiae appear as defects disrupting the continuity of the endometrial lining or as irregularities in the endometrium surrounded by cystic spaces (Fig. 15b, 15c). The association of extensive endometrial synechiae with infertility is known as Ashermann’s syndrome (Fig. 17) (13). Sonohysterography (saline infusion sonography) can also be used for better evaluation of endometrial lining. It is an excellent technique for diagnosis of uterine synechiae, which appear as linear echogenic bridges in the fluid filled endometrial cavity. Other conditions that can be better evaluated with sonohysterography include polyps, fibroid, and malignant lesions.
Figure 17.
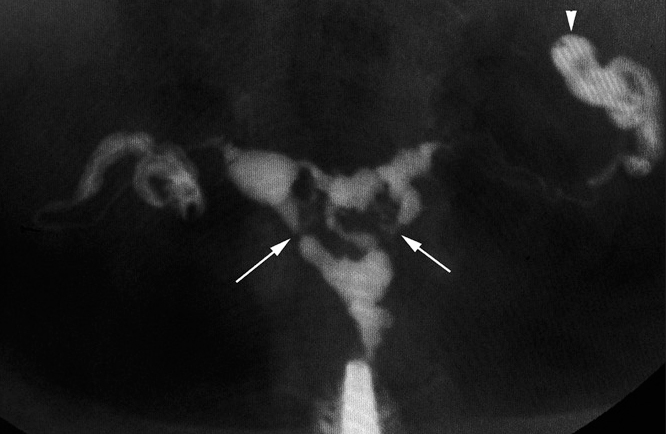
Extensive intrauterine synechiae in a 28-year-old woman with primary infertility. HSG shows multiple irregular filling defects in the uterus due to extensive intrauterine synechiae (arrows). There is loculated spill of contrast on the left side due to peritubal adhesions (arrowhead).
Deformed uterine cavity can give rise to various abnormal shapes visualized on HSG. Scarring involving one half of the endometrium may lead to unilateral obliteration of the endometrial cavity. The HSG appearance may mimic a unicornuate uterus and is called a “pseudounicornuate” uterus (Fig. 18). Scarring along both long and short axes of the uterus leads to transformation of the triangular-shaped uterine cavity into a “T-shaped uterus” (Fig. 19).
Figure 18.
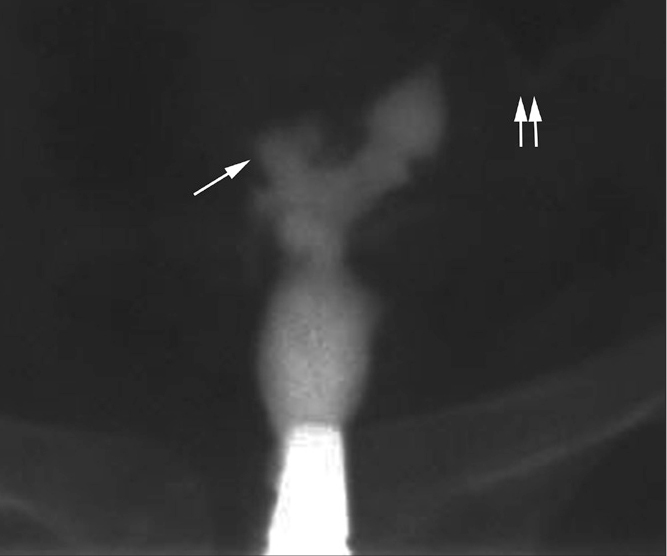
Pseudounicornuate uterus in a 29-year-old woman with previously treated abdominal TB and failure to conceive since six years. HSG shows opacification of the left fallopian tube (paired arrows) with blunting of the right cornu (arrow). Non-visualization of the right tube gives rise to a pseudounicornuate appearance.
Figure 19.
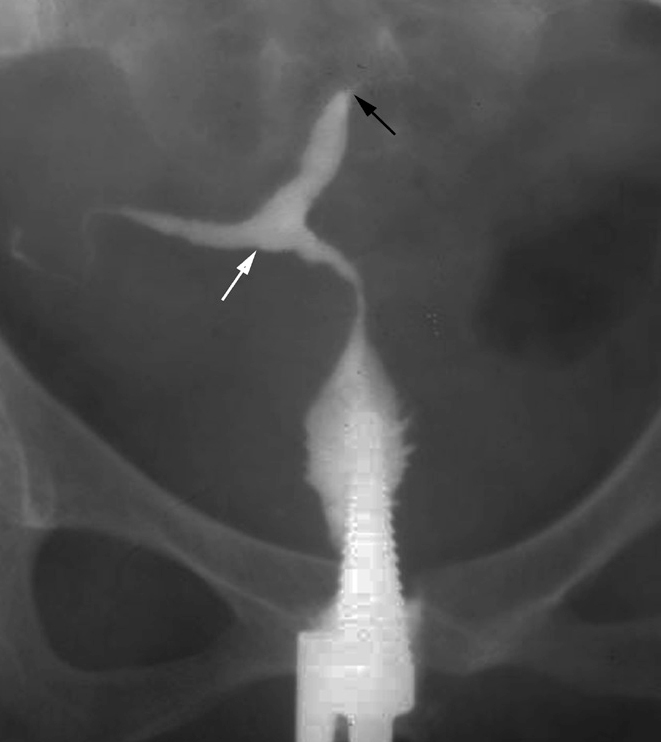
T-shaped uterus in a 29-year-old woman with infertility and previously documented genital TB. HSG shows a deformed T-shaped uterus (white arrow) due to endometrial scarring. There is obstruction at isthmic level on the left side (black arrow).
Based on the above HSG findings, female genital TB can be diagnosed with great probability using a set of criteria established by Klein et al. (14):
Calcified lymph nodes or smaller irregular linear or nodular calcifications in the adnexal area
Obstruction of the fallopian tubes in the zone of transition between the isthmus and ampulla
Multiple constrictions along the course of the fallopian tubes
Endometrial adhesions and/or deformity or obliteration of the endometrial cavity in the absence of history of curettage or surgical termination of pregnancy.
Ovarian TB and tubo-ovarian mass or abscess formation
Ovarian involvement in TB may occur in two forms, namely perioophoritis and oophoritis. Perioophoritis is the more common form and results from the spread of infection to the ovaries directly from the tubes. Oophoritis is relatively rare and results from hematogenous spread to the ovaries. US may reveal echogenic areas of calcification involving the ovaries and tubes, which represent healed calcified granulomas (Fig. 20).
Figure 20.
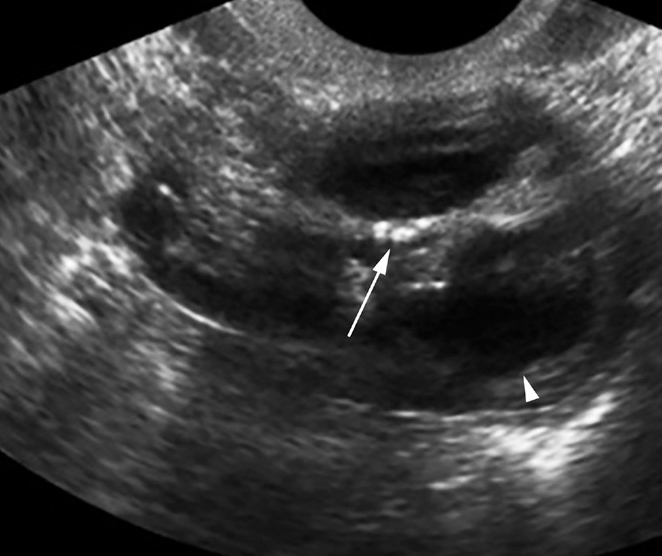
Chronic ovarian TB in a 28-year-old woman with primary infertility. Transvaginal US image of the uterus shows tiny foci of calcification in the ovary (arrow). Ovarian follicle is pointed (arrowhead).
Tubo-ovarian mass or abscess is a sequela to tuberculous salpingitis with secondary perioophoritis (15). The ovary is distinguishable in a tubo-ovarian mass, while it is not seen separately in an abscess. US exam reveals a heterogeneous complex mass in the adnexa. The fallopian tube appears distended with thickened walls (>5 mm) and is adherent to or engulfs the ovary (Fig. 21). The ovary appears enlarged and edematous. A tubo-ovarian abscess is seen as a heterogeneous hypoechoic collection with moving internal echoes and echogenic debris in the adnexa, with the ovary not being recognized separately.
Figure 21.
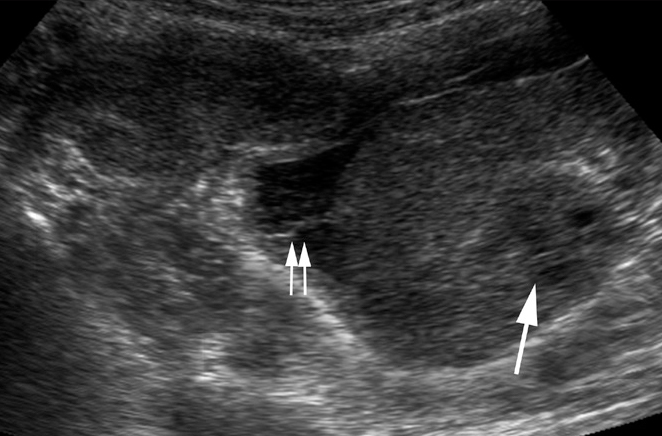
Tubo-ovarian mass in a 30-year-old woman with infertility. Pelvic US shows a complex tubo-ovarian mass encasing the ovary (arrow). Minimal fluid with septations, is seen adjacent to the mass (paired arrows).
Cervical and vulvovaginal TB
Cervical TB is rare, seen in 5%–24% of cases, of which approximately half suffer from infertility (16). Chronic infection may result in cervical canal narrowing and stenosis, which is defined as an internal os diameter of less than 1 mm on HSG.
Vulvovaginal TB is the rarest form of genital TB. It is usually not associated with infertility (16).
Conclusion
Female genital TB is an important cause of female infertility especially in the developing countries. Recognition and understanding the spectrum of the characteristic HSG and US features enables timely diagnosis and management. Although imaging findings may be highly suggestive of tuberculosis, mycobacterial cultures or histopathological analyses are still required to make a definitive diagnosis in most cases.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Schaefer G. Female genital TB. Clin Obstet Gynecol. 1976;19:223–239. doi: 10.1097/00003081-197603000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Thankam R, Varma MD. Tuberculosis of the female genital tract. Global Library of Women’s Medicine; Available at http://www.glowm.com. Accessed March, 2014. [Google Scholar]
- 3.Qureshi RN, Sammad S, Hamd R, Lakha SF. Female genital tuberculosis revisited. J Pak Med Assoc. 2001;51:16–18. [PubMed] [Google Scholar]
- 4.Simpson WL, Jr, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006;26:419–431. doi: 10.1148/rg.262055109. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadi F, Zafarani F, Shahrzad G. Hysterosalpingographic appearances of female genital tract tuberculosis: part I. Fallopian tube. Int J Fertil Steril. 2014;7:245–252. [PMC free article] [PubMed] [Google Scholar]
- 6.Benjaminov O, Atri M. Sonography of the abnormal fallopian tube. AJR Am J Roentgenol. 2004;183:737–742. doi: 10.2214/ajr.183.3.1830737. [DOI] [PubMed] [Google Scholar]
- 7.Winfield AC, Wentz AC. Hysterosalpingography of the fallopian tube. In: Grayson TH, editor. Diagnostic imaging in infertility. 2nd ed. Baltimore: Williams and Wilkins; 1992. pp. 184–186. [Google Scholar]
- 8.Bhojani P. Obstetrics and gynecology. 2nd ed. Gurgaon: Elsevier; 2012. p. 313. [Google Scholar]
- 9.Chavhan GB, Hira P, Rathod K, et al. Female genital tuberculosis: hysterosalpingographic appearances. Br J Radiol. 2004;77:164–169. doi: 10.1259/bjr/27379200. [DOI] [PubMed] [Google Scholar]
- 10.Winfield AC, Wentz AC. Hysterosalpingography of the fallopian tube. In: Grayson TH, editor. Diagnostic imaging in infertility. 2nd ed. Baltimore: Williams and Wilkins; 1992. p. 172. [Google Scholar]
- 11.Merchant SA. Genital tract tuberculosis. In: Subbarao K, Banerjee S, editors. Diagnostic radiology and imaging. 1st ed. New Delhi: Jaypee Brothers; 1997. pp. 637–646. [Google Scholar]
- 12.Dixit R. Imaging in female infertility. In: Khandelwal N, Chowdhury V, Gupta AK, editors. Diagnostic radiology-genitourinary imaging. 3rd ed. New Delhi: Jaypee Brothers; 2009. pp. 470–483. [Google Scholar]
- 13.Ahmadi F, Siahbazi S, Akhbari F, Eslami B, Voshough A. Hysterosalpingography finding in intra uterine adhesion (Asherman’s Syndrome): a pictorial essay. Int J Fertil Steril. 2013;7:155–160. [PMC free article] [PubMed] [Google Scholar]
- 14.Klein TA, Ruchmond JA, Mishell DR. Pelvic tuberculosis. Obstet Gynecol. 1976;48:99–104. [PubMed] [Google Scholar]
- 15.Kim SH, Kim SH, Yang DM, Kim KA. Unusual causes of tubo-ovarian abscess: CT and MR imaging findings. Radiographics. 2004;24:1575–1589. doi: 10.1148/rg.246045016. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh K, Ghosh K, Chowdhury JR. Tuberculosis and female reproductive health. J Postgrad Med. 2011;57:307–313. doi: 10.4103/0022-3859.90082. [DOI] [PubMed] [Google Scholar]


