Abstract
Uterine sarcomas are a rare heterogeneous group of tumors of mesenchymal origin, accounting for approximately 8% of uterine malignancies. They comprise leiomyosarcoma, endometrial stromal sarcoma, undifferentiated endometrial sarcoma, and adenosarcoma. Compared with the more common endometrial carcinomas, uterine sarcomas behave more aggressively and are associated with a poorer prognosis. Due to their distinct clinical and biological behavior, the International Federation of Gynecology and Obstetrics introduced a new staging system for uterine sarcomas in 2009, categorizing uterine carcinosarcoma as a variant of endometrial carcinoma, rather than a pure sarcoma. Magnetic resonance imaging (MRI) has a developing role in the assessment of these malignancies. Features such as tumor localization, irregular or nodular margins, necrosis, rapid growth, intense contrast enhancement, and restriction at diffusion-weighted imaging can suggest the diagnosis and help differentiate from more common leiomyomas and endometrial carcinoma. MRI is therefore extremely useful in preoperative detection and staging and, consequently, in determination of appropriate management. This pictorial review aims to discuss the clinical features of uterine sarcomas, as well as their most common appearances and distinct characteristics in MRI.
Uterine sarcomas are a rare heterogeneous group of tumors of mesenchymal origin, accounting for approximately 8% of uterine malignancies (1), although they were thought to represent only 2% to 3% of all uterine tumors in the past (2). This increased incidence may be the result of improved diagnosis, as well as a true increase in an ageing population (1).
These malignancies may originate from the smooth muscle in myometrium (leiomyosarcoma), from the endometrial stroma (endometrial stromal sarcoma [ESS] and undifferentiated endometrial sarcoma [UES]) or both (adenosarcoma) (3). According to the Gynecologic Oncology Group, uterine sarcomas can be classified into two categories: nonepithelial and mixed epithelial-nonepithelial, depending on the type of cancerous cell and its presumed tissue of origin (4).
The clinical presentation of uterine sarcomas is nonspecific and dependent of histologic subtype. Classically, they present as a rapidly growing pelvic mass, which may be accompanied by vaginal bleeding and abdominal or pelvic pain (1, 5).
Leiomyosarcoma is the most common histological variant of uterine sarcomas and is considered an aggressive tumor associated with poor prognosis, with a five-year survival rate ranging from 18.8% to 68%. ESS is relatively indolent, associated with long-term survival, but characterized by late recurrences (14%–60% of women). In contrast, UES has a very aggressive behavior and poor prognosis, with a five-year survival rate of 25%–55%. Adenosarcomas are rare mixed tumors (glandular and mesenchymal origin) with relatively low malignant potential and slow-growth pattern, with a five-year survival rate above 80% (6).
The recognition of their distinct clinical and biological behavior when compared to endometrial carcinoma, which tend to behave more aggressively and are associated with a poorer prognosis, led the International Federation of Gynecology and Obstetrics (FIGO) to develop a new staging system for uterine sarcomas in 2009 (Tables 1 and 2). One important feature of the new staging system is that carcinosarcoma (formerly referred to as “malignant mixed Müllerian tumor”) is no longer considered as part of uterine sarcomas, being classified as a dedifferentiated or metaplastic form of endometrial carcinoma (7).
Table 1.
Staging for uterine leiomyosarcoma (7)
| Stage | Definition | |
|---|---|---|
| I | Tumor limited to uterus | |
| IA | <5 cm | |
| IB | >5 cm | |
| II | Tumor extends beyond the uterus, within the pelvis | |
| IIA | Adnexal involvement | |
| IIB | Involvement of other pelvic tissues | |
| III | Tumor invades abdominal tissues (not just protruding into the abdomen) | |
| IIIA | One site | |
| IIIB | More than one site | |
| IIIC | Metastasis to pelvic and/or para-aortic lymph nodes | |
| IV | IVA | Tumor invades bladder and/or rectum |
| IVB | Distant metastasis |
Table 2.
Staging for uterine endometrial stromal sarcoma and adenosarcoma (7)
| Stage | Definition | |
|---|---|---|
| I | Tumor limited to uterus | |
| IA | Tumor limited to endometrium/endocervix with no myometrial invasion | |
| IB | Less than half or half myometrial invasion | |
| IC | More than half myometrial invasion | |
| II | Tumor extends beyond the uterus, within the pelvis | |
| IIA | Adnexal involvement | |
| IIB | Involvement of other pelvic tissues | |
| III | Tumor invades abdominal tissues (not just protruding into the abdomen) | |
| IIIA | One site | |
| IIIB | More than one site | |
| IIIC | Metastasis to pelvic and/or para-aortic lymph nodes | |
| IV | IVA | Tumor invades bladder and/or rectum |
| IVB | Distant metastasis |
The distinction among different subtypes of uterine sarcomas and other uterine tumors (especially leiomyoma and endometrial carcinoma) cannot be made on clinical grounds. Therefore, imaging, particularly MRI, has a developing role in the assessment of these malignancies, being useful in the evaluation of pelvic masses at presentation, adequate staging (assessment of invasion depth, spread to adjacent organs and lymph nodes), and consequently, determination of appropriate management.
Leiomyosarcoma
Clinical features
Leiomyosarcoma is the most common histological subtype of uterine sarcomas. The great majority arise de novo, but rarely (in 0.2% of cases) it may result from a sarcomatous transformation in a benign leiomyoma (2). It is characterized by an aggressive behavior (even when confined to the uterus), with a five-year survival rate ranging from 18.8% to 68%, which varies widely according to different stages. Low-grade and serosal involvement seem to be significant prognostic factors. The reported risk of recurrence varies from 45% to 73% (1, 6, 8).
Most leiomyosarcomas occur in women over 40 years of age, with a median age of 60 years. There is a two-fold incidence of leiomyosarcoma in African-American women; long-term tamoxifen use and prior pelvic radiation seem to be associated with a small increase in risk (4). Signs and symptoms are similar to those occurring with leiomyomas, and include abnormal vaginal bleeding (56%), palpable pelvic mass (54%) and pelvic pain (22%). Less frequently, they can present as hemoperitoneum (due to tumor rupture), or symptoms resulting from extra-uterine extension or metastases (8). Besides that, although “rapid growth” of a presumed leiomyoma is considered a suspicious finding, the definition of the latter remains controversial. Therefore, preoperative distinction between benign leiomyomas and malignant leiomyosarcomas is very difficult (if not impossible) based solely on clinical features, and remains a challenge for clinicians (1, 8).
MRI features
Leiomyosarcomas are often difficult to differentiate from leiomyomas (the most common myometrial tumor), based on clinical features and even endometrial biopsy or dilatation and fractional curettage. Furthermore, recent advances in leiomyoma management (i.e., development of uterus-preserving treatments, such as gonadotropin-releasing hormone analogues, uterine arterial embolization, and focused ultrasound surgery) have significantly raised the importance of pretreatment imaging diagnosis of uterine sarcomas (3, 9, 10).
On MRI, leiomyosarcomas commonly manifest as large infiltrating myometrial mass of heterogeneous hypointensity on T1-weighted images, with irregular and ill-defined margins. On T2-weighted images, they usually show intermediate-to-high signal intensity, with central hyperintensity indicative of extensive necrosis (present in >50% of cases) (Fig. 1a, 1b). Hemorrhage is common, and foci of calcifications may be present. After contrast administration, they present early heterogeneous enhancement, due to the aforementioned areas of necrosis and hemorrhage (Fig. 1c) (1–3, 10, 11).
Figure 1.
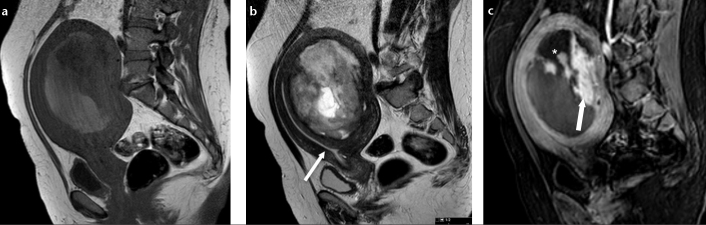
a–c. Leiomyosarcoma in a 52-year-old woman. Sagittal T1-weighted image (a), T2-weighted image (b), and gadolinium-enhanced T1-weighted image with fat suppression (c) show marked uterine enlargement due to a heterogeneous myometrial tumor. The lesion demonstrates central hyperintensity on T1-weighted image (a) attributable to extensive hemorrhage, a central area of high signal on T2-weighted image (b) representing cystic necrosis, and early intense enhancement in solid areas of the tumor (c, arrow), as compared with normal myometrium. Irregular central zones of low signal intensity (asterisk) suggest extensive tumor necrosis. Endometrial cavity is pushed anteriorly by the tumor (b, arrow).
Leiomyosarcomas are generally larger and show more rapid growth than leiomyomas (3). On the other hand, common benign leiomyomas can show areas of increased signal intensity on T2-weighted images as well, due to various types of degeneration or cellular histologic subtypes (9, 10). Some authors have suggested the presence of irregular margins, necrosis, and rapid growth as the most suggestive features of malignancy (2).
Diffusion-weighted imaging (DWI) has the potential to delineate malignant lesions as hyperintense areas with excellent tissue contrast, providing quantitative measurements of apparent diffusion coefficient (ADC) values (Fig. 2). Tamai et al. (10) reported significant differences in mean ADC values of leiomyosarcomas, compared with normal myometrium and degenerated leiomyomas, without any overlap. Namimoto et al. (9) showed that overlap in ADC values between leiomyosarcomas and ordinary leiomyomas (attributed to the “T2 blackout effect,” i.e., hypointensity on DWI caused by hypointensity on T2-weighted images) could be resolved with the evaluation of tumor-myometrium contrast ratio on T2-weighted images. Thomassin-Naggara et al. (12) reported that by combining the analysis of T2 signal intensity, b1000 images and ADC map, MRI achieved 92.4% accuracy in distinguishing benign and uncertain or malignant myometrial tumors. Therefore, they concluded that DWI may limit misdiagnosis of uterine sarcomas as benign leiomyomas, and should be the first criterion to help radiologists characterize a unique uterine tumor. Based on these results, the use of DWI must be recommended in the setting of myometrial lesions, especially when high signal intensity is seen on T2-weighted images.
Figure 2.
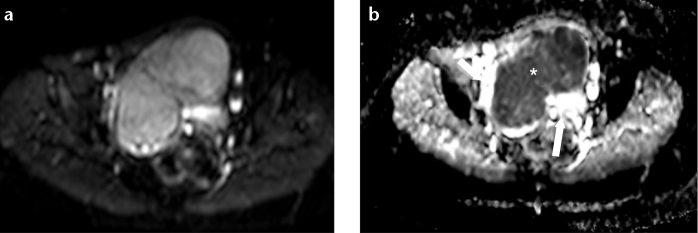
a, b. Leiomyosarcoma in a 54-year-old woman. Axial DWI on b1000 (a) demonstrates a hyperintense mass. The mass appears hypointense on ADC map (b, asterisk), with the normal myometrium seen as an area of hyperintensity (arrows).
Endometrial stromal sarcoma
Clinical features
According to the World Health Organization classification, an endometrial stromal tumor is composed of cells that resemble endometrial stromal cells of the proliferative endometrium (13). ESS corresponds to the former “low-grade ESS”, and UES replaces the term “high-grade ESS”. This new classification is a result of the recognition of the very distinct biological behaviors and clinical outcomes of these tumors (3, 4, 6, 8).
ESS is a rare tumor, accounting for 0.2% of all malignant uterine tumors and 10%–15% of uterine malignancies with a mesenchymal component (3, 6, 8). It is considered to be a low-grade, well-differentiated tumor without significant cellular atypia (4). ESS is therefore a relatively indolent lesion, generally with a favorable prognosis, with five- and 10-year survival rates of 98% and 89% for stage I disease, which corresponds to the majority of patients at presentation (3, 8). However, the outcome is largely dependent on the extent of the tumor at presentation, and stage seems to be the most significant indicator for survival (3, 6, 8). For stages II and III, five-year survival significantly drops to 50% and 65%, respectively (3). ESS is also characterized by late recurrences (14%–60% of patients), even in patients with stage I disease (6, 8). Therefore, long-term follow-up is mandatory.
ESS occurs more commonly in women between 40 and 55 years of age (8). There has been a reported association with tamoxifen and estrogen use, although data are limited (3, 14). They usually present with abnormal vaginal bleeding, pelvic pain, and dismenorrhea; however, as many as 25% of patients are asymptomatic (1, 3, 8).
According to the new FIGO staging for uterine sarcomas, ESS is staged in the same manner as adenosarcoma (Table 2).
MRI features
Imaging (and particularly MRI) may be a powerful tool in the management of these tumors, allowing characterization of the initial stage and also providing differentiation from the more common endometrial carcinoma (3).
ESS more frequently appears as polypoid endometrial mass, with low signal on T1-weighted images and heterogeneously increased high T2 signal (3, 11, 15). It typically shows myometrial involvement, either sharply demarcated or in a more diffuse and destructive manner (the latter is far more common with UES) (Fig. 3a). These tumors have a tendency for lymphatic and vascular invasion, showing worm-like extension bands of low signal intensity within areas of myometrial involvement on T2-weighted images (“bag of worms”), corresponding to preserved bundles of myometrium (Fig. 4) (2, 3, 11, 15–17). After contrast administration, enhancement is moderate and commonly heterogeneous (Fig. 3b) (3, 15).
Figure 3.
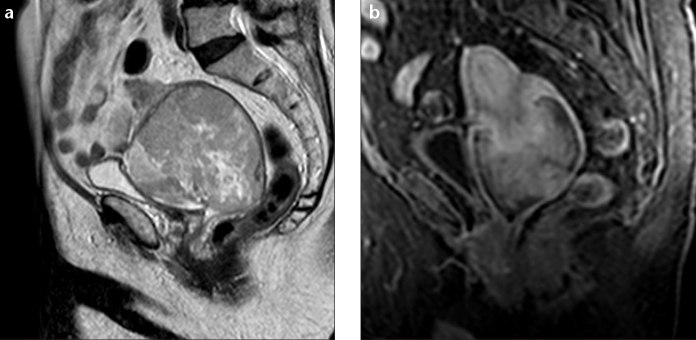
a, b. Endometrial stromal sarcoma in an 82-year-old woman. Sagittal T2-weighted image (a) and sagital T1-weighted image with fat suppression, after contrast administration (b) show a very large lesion centered at cervix region, infiltrating uterine body superiorly and superior half of the vagina inferiorly. The tumor shows multiple foci of hyperintense signal on T2-weighted image due to extensive necrosis, as well as moderate and mildly heterogeneous contrast enhancement.
Figure 4.
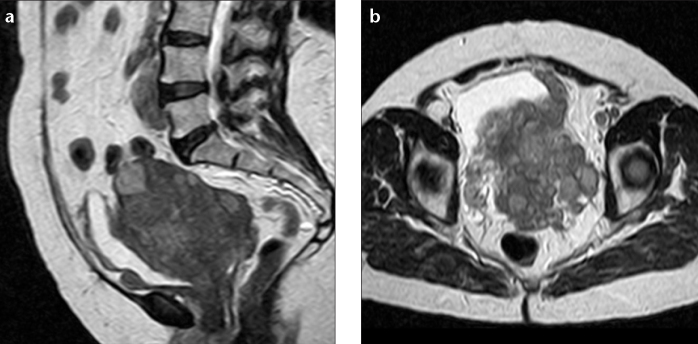
a, b. Sagittal (a) and axial (b) T2-weighted images show an endometrial stromal sarcoma in a 64-year-old woman. The lesion shows heterogeneous signal with extensive nodular invasion into the myometrium and marked marginal irregularity and nodularity (attributable to tumor extension along vessels and lymphatics).
Compared to endometrial carcinoma, ESS usually shows larger size, more contrast enhancement, irregular margin, nodular extension into the myometrium, and marginal nodularity due to tumor extension along vessels and lymphatics (2, 3, 15, 16).
Rarely, ESS can appear as a myometrial mass mimicking intramural leiomyoma with cystic degeneration. In these cases, intramyometrial ESS can be differentiated based on their rapid and invasive growth, lower degree of enhancement, lymphatic and vascular invasion, higher incidence of necrosis, peripheral hypointense rim on T2-weighted images, and enhanced marginal irregularity (3, 11, 15, 18).
Lymph node metastases are seen in 10% of patients, being more frequent locally within the pelvis or vagina, followed by the lung parenchyma (3).
Undifferentiated endometrial sarcoma
Clinical features
UES, previously referred to as “high-grade ESS”, is an aggressive tumor lacking specific differentiation, and showing no endometrial stromal features. It is characterized by myometrial invasion, severe nuclear polymorphism, high mitotic activity, and/or tumor cell necrosis (4, 8). UES shows an aggressive behavior, with five-year survival rates of 25%–55% (6). The most significant prognostic factor seems to be the presence of vascular invasion, decreasing five-year survival to as low as 17% (19). Local recurrences and distant metastases (due to hematogenous spread) are also associated with high mortality and poor outcome (8, 14).
UES tends to occur in an older age group, when compared to ESS, with a mean age of 61 years at the time of diagnosis (2, 11). Signs and symptoms resemble those of leiomyosarcoma and include vaginal bleeding, palpable pelvic mass, and pelvic pain (16).
MRI features
UES typically appears as a large polypoid mass in an expanded endometrial cavity, showing heterogeneous signal intensity on both T1- and T2-weighted images (Fig. 5a). The latter is attributable to the high frequency of hemorrhage and necrosis within the tumor (11, 14, 17). UES tends to infiltrate the myometrium in a more destructive and extensive manner than ESS, due to marked vascular and lymphatic invasion (3, 11). Contrast enhancement is generally heterogeneous, and iso- or hyperintense when compared with normal myometrium, allowing differentiation from endometrial carcinoma (Fig. 5b). Hyperenhancement, the presence of irregular margins, multiple marginal tumor nodules, intramyometrial worm-like extension, and multiple nodular mass formation, are more frequently seen in UES than ESS (2, 3, 15).
Figure 5.
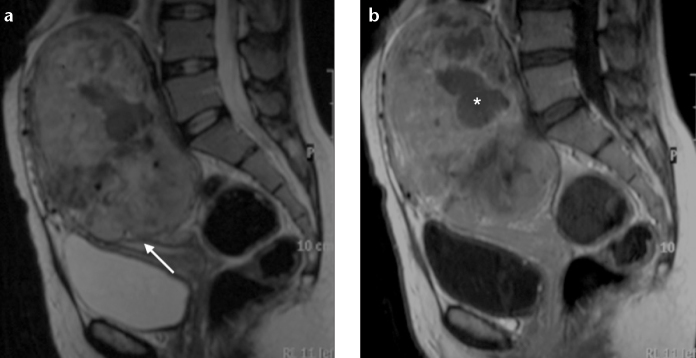
a, b. Undifferentiated endometrial sarcoma in a 36-year-old woman. Sagittal T2-weighted image (a) and T1-weighted image after gadolinium administration (b) show marked uterine enlargement due to a large polypoid heterogeneous tumor, with some nodular marginality (arrow). The lesion shows intense and heterogeneous contrast uptake (uncommon for endometrial carcinoma), with a hypointense area (asterisk) suggestive of necrosis.
Adenosarcoma
Clinical features
Adenosarcoma is a rare uterine malignancy, accounting for 5.5%–9% of all uterine sarcomas (6). It is a mixed tumor, composed of a benign (but occasionally atypical) epithelial component, and a malignant stromal (sarcomatous, usually low-grade) element (4, 6, 16). Adenosarcoma is considered a mixed Müllerian tumor, intermediate between adenofibroma and carcinosarcoma; it was even suggested that some adenofibromas are in fact well-differentiated adenosarcomas (3, 6, 8). It is a slow-growing tumor, with low malignant potential and good prognosis. Majority of women are diagnosed at stage I (>60%), with an overall five-year survival over 80% (3, 4, 6). More than 70% of adenosarcomas occur in the endometrium, but they can also be found in the myometrium (probably from adenomyosis), cervix, and extra-uterine tissues such as the ovaries. Extra-uterine location is more common in adolescents and young women (6, 8).
Adenosarcoma with sarcomatous overgrowth is defined as an adenosarcoma with the sarcomatous component constituting more than 25% of the tumor; it occurs in 8%–54% of uterine adenosarcomas, and 30% of ovarian adenosarcomas. It carries a worse prognosis, with mortality reaching 50% at five years. Other proposed factors associated with a poorer outcome and higher risk of recurrence are advanced age, myometrial invasion (found in 15% of cases), and lymphovascular extension (3, 4, 6, 8, 16). Late recurrence can be seen in one-third of the women at five years, therefore, long-term imaging follow-up is mandatory (3).
Adenosarcoma most commonly presents with abnormal vaginal bleeding; some women also complain of pelvic pain, palpable pelvic mass, or vaginal discharge (8).
According to the new FIGO staging for uterine sarcomas, adenosarcoma is staged in the same manner as ESS (Table 2).
MRI features
Adenosarcoma is typically seen as a large well-demarcated polypoid mass arising within the endometrial cavity and protruding through the cervical os, causing marked enlargement of the uterus with a thin myometrium (3, 20, 21). This polypoid mass usually shows a multiseptated cystic appearance, with multiple heterogeneous solid components that fill the endometrial cavity, and may mimic the appearance of gestational trophoblastic disease (Fig. 6a) (3, 20). On T2-weighted images, small hyperintense foci may be seen scattered within the mass, representing glandular epithelial components or necrosis (8, 22). After administration of gadolinium, there is heterogeneous enhancement, with solid components of the mass showing enhancement similar to that of the myometrium (Fig. 6b) (3, 20, 21).
Figure 6.
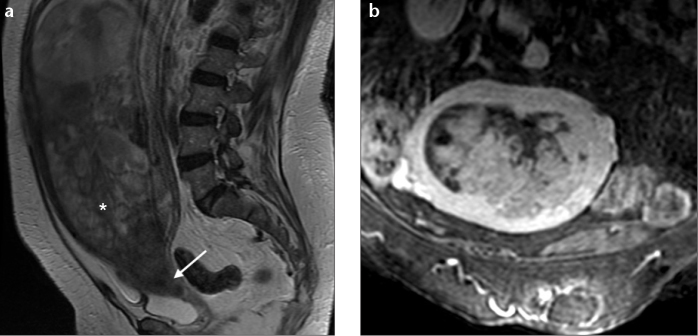
a, b. Adenosarcoma in a 76-year-old woman. Sagittal T2-weighted image (a) and oblique coronal T1-weighted image with fat suppression, after contrast administration (b) show a very large polypoid mass with heterogeneous high signal intensity arising within the endometrial cavity and protruding into the cervical os (arrow), causing marked enlargement of the uterus. The tumor demonstrates a multicystic appearance (asterisk), with solid areas demonstrating enhancement similar to myometrium.
Adenosarcoma with sarcomatous overgrowth may present with myometrial invasion and areas of hemorrhage and necrosis. However, on DWI, they usually show relatively low signal intensity on high b-value, reflecting its low grade nature (21, 22).
Conclusion
Uterine sarcomas are a rare and aggressive heterogeneous group of tumors of mesenchymal origin, with nonspecific clinical features. MRI is useful in lesion detection and characterization, as well as assessment of disease staging. Although the radiologic findings of these lesions can overlap, characteristic features (summarized in Table 3) can help narrow the differential diagnosis and guide adequate treatment selection and follow-up. In addition to morphological features, DWI seems to be a potentially useful tool in characterization of large uterine lesions.
Table 3.
MRI features of uterine sarcomas, leiomyoma, and endometrial carcinoma
| LMS | ESS | UES | AS | Leiomyoma | Endometrial carcinoma | |
|---|---|---|---|---|---|---|
| Localization | Myometrium | Generally endometrium; can be located in myometrium | Generally endometrium; can be located in myometrium | Endometrium | Myometrium | Endometrium |
| Margins | Irregular and ill-defined | Irregular and nodular | Markedly irregular and nodular | Regular and well demarcated | Regular | Regular or irregular |
| T1 signal | Hypointense and heterogeneous (hemorrhage, calcifications) | Hypointense | Heterogeneous | Predominantly hypointense, heterogeneous | Low-to-intermediate signal; high signal foci – hemorrhagic degeneration | Hypo-to-isointense signal to normal endometrium |
| T2 signal | Intermediate-to-high signal | Hyperintense and heterogenous; bands of low signal corresponding to preserved myometrium | Heterogeneous (extensive hemorrhage and necrosis) | Multiseptated cystic appearance; can show multiple small hyperintense foci | Low signal (non-degenerated); high signal – cystic, myxoid degeneration | Hyperintense and heterogeneous relative to normal endometrium |
| Contrast enhancement | Early and heterogenous | Moderate (more intense than endometrial carcinoma) and heterogeneous | Marked (generally more intense than normal myometrium) and heterogeneous | Marked (generally isointense compared to normal myometrium) and heterogeneous | Variable | Hypointense compared to normal myometrium |
| DWI | Generally more restriction (lower ADC values) than leiomyomas | High signal and low ADC | High signal and low ADC | Low signal (low grade nature) | Variable; generally higher ADC values than LMS | High signal and low ADC |
LMS, leiomyosarcoma; ESS, endometrial stromal sarcoma; UES, undifferentiated endometrial sarcoma; AS, adenosarcoma; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Wu TI, Yen TC, Lai CH. Clinical presentation and diagnosis of uterine sarcoma, including imaging. Best Pract Res Clin Obstet Gynaecol. 2011;25:681–689. doi: 10.1016/j.bpobgyn.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–740. doi: 10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 3.Shaan SH, Jagannathan JP, Krajewski K, O’Regen KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012;199:213–223. doi: 10.2214/AJR.11.7287. [DOI] [PubMed] [Google Scholar]
- 4.Memarzadeh S, Mundt AJ, Berek JS. Uterine sarcoma: Classification, clinical manifestations, and diagnosis. In: Goff B, Falk SJ, editors. UpToDate. 2012. [Google Scholar]
- 5.Seddon BM, Davda R. Uterine sarcomas – recent progress and future challenges. Eur J Radiol. 2011;78:30–40. doi: 10.1016/j.ejrad.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 6.Tse KY, Crawford R, Ngan HYS. Staging of uterine sarcomas. Best Pract Res Clin Obstet Gynaecol. 2011;25:733–749. doi: 10.1016/j.bpobgyn.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 7.FIGO Committee on Gynecologic Oncology. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;106:277. [Google Scholar]
- 8.D’Angelo A, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Namimoto T, Yamashita Y, Awai K, et al. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2009;19:2756–2764. doi: 10.1007/s00330-009-1471-x. [DOI] [PubMed] [Google Scholar]
- 10.Tamai K, Koyama T, Saga T, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723–730. doi: 10.1007/s00330-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 11.Rha SE, Byun JY, Jung SE, et al. CT and MRI of Uterine Sarcomas and their mimickers. AJR Am J Roentgenol. 2003;181:1369–1374. doi: 10.2214/ajr.181.5.1811369. [DOI] [PubMed] [Google Scholar]
- 12.Thomassin-Naggara I, Dechoux S, Bonneau C, et al. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol. 2013;23:2306–2314. doi: 10.1007/s00330-013-2819-9. [DOI] [PubMed] [Google Scholar]
- 13.Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. World Health Organization classification of tumours. [Google Scholar]
- 14.Lenhard SM, Untch M, Himsl I, et al. The high-grade endometrial sarcoma: a rare entity. Arch Gynecol Obstet. 2006;274:56–59. doi: 10.1007/s00404-005-0100-4. [DOI] [PubMed] [Google Scholar]
- 15.Ueda M, Otsuka M, Hatakenaka M, et al. MR imaging findings of uterine endometrial stromal sarcoma: differentiation from endometrial carcinoma. Eur Radiol. 2001;11:28–33. doi: 10.1007/s003300000541. [DOI] [PubMed] [Google Scholar]
- 16.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 17.Ueda M, Otsuka M, Hatakenaka M, Torii Y. Uterine endometrial stromal sarcoma located in uterine myometrium: MRI appearance. Eur Radiol. 2000;10:780–782. doi: 10.1007/s003300051004. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa R, Akahane M, Yamada H, et al. Endometrial stromal sarcoma located in the myometrium with a low-intensity rim on T2-weighted images: report of three cases and literature review. J Magn Reson Imaging. 2010;31:975–979. doi: 10.1002/jmri.22126. [DOI] [PubMed] [Google Scholar]
- 19.Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 20.Szklaruk J, Tamm EP, Choi H, Varavithya V. MR imaging of common and uncommon large pelvic masses. Radiographics. 2003;23:403–424. doi: 10.1148/rg.232025089. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizako T, Wada A, Kitagaki H, Ishikawa N, Miyasaki K. MR imaging of uterine adenosarcoma: case report and literature review. Magn Reson Med Sci. 2011;10:251–254. doi: 10.2463/mrms.10.251. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi M, Matsuzaki K, Yoshida S, et al. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009;33:244–247. doi: 10.1016/j.clinimag.2008.11.003. [DOI] [PubMed] [Google Scholar]


