Abstract
Objectives. This prospective longitudinal study investigated the association between baseline objectively measured sedentary time and 2-year onset of physical frailty.
Methods. We studied 1333 Osteoarthritis Initiative participants 55 to 83 years of age who were at risk for physical frailty, as assessed via low gait speed (< 0.6 m per second) or inability to perform a single chair stand. Baseline sedentary time was assessed through accelerometer monitoring. Hazard ratios (HRs) for physical frailty onset were estimated with discrete survival methods that controlled for moderate physical activity, sociodemographic characteristics, baseline gait and chair stand functioning, and health factors.
Results. The incidence of physical frailty in this high-risk group was 20.7 per 1000 person-years. Greater baseline sedentary time (adjusted HR = 1.36 per sedentary hour; 95% confidence interval [CI] = 1.02, 1.79) was significantly related to incident physical frailty after control for time spent in moderate-intensity activities and other covariates.
Conclusions. Our prospective data demonstrated a strong relationship between daily sedentary time and development of physical frailty distinct from insufficient moderate activity. Interventions that promote reductions in sedentary behaviors in addition to increases in physical activity may help decrease physical frailty onset.
New health care challenges fueled by an aging population are on the horizon. By 2030, 1 in 5 people living in the United States will be older than 65 years.1 Largely as a result of this oncoming wave of aging, a substantial increase in disability is anticipated. Physical frailty, a medical syndrome,2 is a strong risk factor for disability, dependency, and death.3–8 Physical frailty (assessed according to functional performance) reflects decreased physical reserves and vulnerability in old age.9 The demographically aging society of the United States makes prevention and delay of physical frailty a public health priority.
Regular physical activity reduces the risk of physical frailty2,8,10 and promotes health benefits, including better quality of life and reduced risk for chronic diseases.11–14 Despite the important publicized benefits of physical activity, US adults primarily engage in sedentary behaviors.15,16 In contrast to the recommendation that people engage in physical activities of moderate intensity or greater (3.0 or more metabolic equivalents), sedentary behavior involves activities at the resting level of energy expenditure (1.0–1.5 metabolic equivalents).17 Working on a computer and watching television18 are common sedentary activities. Prolonged sedentary time has been associated with an increased risk of functional decline and such conditions as obesity, metabolic syndrome, and type 2 diabetes.19–21
Physical activity interventions designed to improve health outcomes have largely focused on increasing recommended moderate-intensity activities13 and have addressed sedentary behavior only to a limited degree. Recently, however, there has been a growing interest in the role of sedentary behavior as a separate risk factor for poor health. Sedentary behavior, already associated with poor health outcomes, may have a unique relationship to the development of physical frailty or may reflect only a lack of moderate-intensity physical activity. This is an essential issue to address because if sedentary behavior is demonstrated to be an independent risk factor, reducing such behavior may increase the effectiveness of behavior interventions, particularly among people with activity-inhibiting chronic conditions such as arthritis. However, the limited available information on the relationship between sedentary behavior and physical frailty is problematic owing to a reliance on recall of sedentary behaviors or physical activity.
We tested the hypothesis that increased sedentary behavior is related to an increased risk of physical frailty independent of time spent in moderate physical activity. We focused on community-dwelling adults with or at high risk for knee osteoarthritis (OA) who participated in the multisite Osteoarthritis Initiative (OAI). Using detailed, objective physical activity accelerometer monitoring, we investigated the association between time spent in sedentary behavior and the development of physical frailty as determined via longitudinal objective function tests.
METHODS
Participants were a subcohort from the OAI (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). The OAI is an ongoing multicenter longitudinal study in the United States, in which 4796 men and women who were 45 to 79 years of age at enrollment (2004–2006) were followed over the course of 8 years.. Adults eligible for the OAI were required to have symptomatic radiographic OA in at least 1 knee (i.e., definite tibiofemoral osteophyte22 and pain, aching, or stiffness on most days for at least 1 month during the preceding 12 months) or to have at least 1 of a set of established knee OA risk factors, which included being overweight. OAI eligibility criteria have been described in detail elsewhere.23 In this study, we used data from the OAI database.24
The study sample was drawn from 2127 individuals enrolled in an OAI accelerometer monitoring substudy at the OAI 48-month visit (2008–2010),16 which was the baseline for this study (Figure A). We used baseline data and data from the subsequent 2-year follow-up visit (2010–2012). Given our focus on physical frailty onset, we restricted our analyses to 1333 participants aged 55 years or older who were free of baseline physical frailty.8,25,26 Excluded by design were participants who were younger than 55 years (n = 305), who were physically frail at baseline (n = 69), or who had less than 4 days of valid accelerometer monitoring (n = 161). For the purposes of our analysis, we also excluded 11 decedents, 59 participants who had withdrawn from the study, and 189 participants with insufficient baseline or follow-up data.
Physical Frailty
Consistent with the literature, we used low gait speed (< 0.6 m per second) or inability to rise from a chair without using one’s arms to assess physical frailty at baseline and the 2-year follow-up.8,25,26 Reduced functional performance capabilities may reflect decreased physical reserves in old age.9 Our definition captured people with deterioration in physical performance essential to their daily life functioning.
Participants’ gait speed was measured in meters per second on the basis of average speed over a pair of 20-meter walking tests. A single chair stand test was used to assess participants’ ability to stand up without using their arms.
Physical Activity Measures
The ActiGraph GT1M uniaxial accelerometer was used to measure physical activity.27 Participants were instructed to wear the unit on a belt at their natural waistline (on their right hip in line with the right axilla) upon awakening in the morning until they went to bed at night for 7 consecutive days, except during water activities.
Accelerometer data were analytically filtered via validated methods.28,29 Nonwear periods were defined as intervals of at least 90 minutes with zero activity counts (allowing for 2 consecutive interrupted minutes with counts < 100). We defined participants with 4 to 7 valid monitoring days (i.e., ≥ 10 hours of daily wear or monitoring time) as having reliable physical activity estimates.30 We applied intensity thresholds used by the National Cancer Institute on a minute-by-minute basis to identify sedentary (< 100 counts per minute) and moderate-intensity (≥ 2020 counts per minute) activity.30 Data on daily sedentary time and time spent in moderate-intensity activities were accumulated over the monitoring period and averaged across valid monitored days. Sedentary behaviors were measured according to average daily sedentary time in hours and average daily percentage of monitoring time registering as sedentary. For simplicity, we use the terms waking time and monitoring time interchangeably.
Covariates
Covariates measured at baseline included functioning, sociodemographic characteristics, and health factors, which have been recognized in the literature as potential confounders.3,8,31–33 Gait speed and chair stand rate were used to assess functioning. The time required for 5 repetitions of rising from a chair and sitting down was converted to a chair stand rate (number of stands per minute). Sociodemographic characteristics included age, gender, self-reported race/ethnicity (African American, White, or other), marital status, education, and income.
Finally, health factors included body mass index (BMI, defined as weight in kilograms divided by the square of height in meters), comorbidity, depressive symptoms, radiographic knee OA, hip OA, knee symptoms, hip symptoms, general pain, smoking status, and alcohol consumption. BMI was calculated as normal (18.5–24.9), overweight (25.0–29.9), or obese (≥ 30). Comorbidity was assessed with the Charlson comorbidity index.34 A high depressive symptom level was defined as a Center for Epidemiological Studies Depression Scale score of 16 or greater.35
Participants with a Kellgren and Lawrence grade of 2 or greater36 in at least 1 knee were classified as having radiographic knee OA, and participants who reported a diagnosis of hip osteoarthritis or degenerative arthritis were classified as having hip OA. Chronic knee or hip symptoms were established via participants’ self-reports of “pain, aching, or stiffness on most days of a month during the past year” in either knee or on either side of the hip. General pain was measured (on a 5-point Likert scale) with an SF-12 Health Survey question on the extent to which pain interfered with participants’ housework and work outside the home. In the 1.8% of cases in which data on a baseline health factor were missing, information from the most recent annual assessment was used as a proxy.
Statistical Analysis
We used kernel density plots to graphically illustrate the distributions of baseline daily sedentary time and percentage of sedentary time by presence and absence of physical frailty onset over the 2 years of follow-up. To investigate the relationship between baseline sedentary behavior and physical frailty over 2 years, we used discrete-time survival regression by applying a generalized linear model with a complementary log-log link.37 We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) for 1-hour increases in sedentary time and 10% increases in sedentary behaviors. Also, we conducted a survival analysis that hierarchically adjusted for average daily time spent in moderate-intensity activity, baseline functioning, sociodemographic characteristics, and health factors. Because sedentary time was related to monitoring time, all analyses involving sedentary hours as a predictor also adjusted for average daily monitoring time.
We used SAS version 9.3 (SAS Institute Inc, Cary, NC) in conducting our analyses. Kernel density plots were generated with the sm package in Rstudio.38 Statistical testing was conducted at a nominal 5% significance level.
RESULTS
At baseline all 1333 participants were, by design, free of physical frailty (they could complete at least 1 chair stand without using their arms and had a gait speed ≥ 0.6 m per second). The participants were primarily female (55%), White (84%), married (69%), and college educated (87%); the mean age of the sample was 66.7 years (Table 1). More than 70% of the participants were either overweight (39%) or obese (35%), and most had radiographic knee OA (61%). On average, the cohort spent 9.9 hours each day (66% of waking time) in sedentary behavior and less than 20 minutes in moderate-intensity activities. The average daily monitoring time was 14.9 hours.
TABLE 1—
Baseline Characteristics of Participants: Osteoarthritis Initiative, United States, 2008–2010
| Characteristic | Overall (n = 1333), % or Mean (SD) | Women (n = 730), % or Mean (SD) | Men (n = 603), % or Mean (SD) |
| Sociodemographic characteristics | |||
| Age, y | 66.7 (7.7) | 67.2 (7.5) | 66.1 (7.9) |
| Female | 54.8 | 100.0 | 0.0 |
| White | 84.3 | 79.7 | 89.7 |
| Married | 69.2 | 59.0 | 81.4 |
| > high school education | 87.4 | 84.0 | 91.5 |
| Yearly income < $50 000a | 36.4 | 45.6 | 25.2 |
| Functioning | |||
| Gait speed, m/sec | 1.3 (0.2) | 1.3 (0.2) | 1.4 (0.2) |
| Chair stand rate, repetitions/min | 30.9 (9.3) | 30.2 (9.2) | 31.9 (9.4) |
| Health factors | |||
| Body mass index | |||
| Normal (18.5–24.9 kg/m2) | 26.2 | 31.2 | 20.2 |
| Overweight (25.0–29.9 kg/m2) | 38.9 | 34.5 | 44.1 |
| Obese (≥ 30 kg/m2) | 34.9 | 34.3 | 35.7 |
| Comorbiditiesb | 29.5 | 28.1 | 31.2 |
| High depressive symptom levelc | 11.0 | 12.3 | 9.3 |
| Chronic knee symptomsd | 38.3 | 37.1 | 39.6 |
| Radiographic knee OAe | 61.4 | 60.8 | 62.2 |
| Chronic hip symptomsf | 23.3 | 26.0 | 19.9 |
| Hip OA | 13.7 | 18.0 | 8.5 |
| Moderate or worse general pain | 18.9 | 20.8 | 16.6 |
| Current smoker | 6.0 | 2.9 | 9.8 |
| Any alcohol consumption | 81.6 | 77.1 | 86.9 |
| Physical activity measures | |||
| Sedentary h/d | 9.9 (1.5) | 9.7 (1.4) | 10.1 (1.4) |
| Daily percentage of sedentary time | 66.4 (8.6) | 65.4 (8.6) | 67.6 (8.5) |
| Moderate-intensity activity, min/d | 17.3 (18.4) | 13.7 (15.4) | 21.7 (20.7) |
Note. OA = osteoarthritis.
Or not reported.
Charlson comorbidity score > 0.
Center for Epidemiological Studies Depression Scale score > 16.
Affirmed pain, aching, or stiffness most days of the month over preceding 12 months in either or both knees.
Kellgren–Lawrence grade of ≥ 2 in one or both knees.
Affirmed pain, aching, or stiffness most days of the month over preceding 12 months on either or both sides of the hip.
The physical frailty rate was 20.7 per 1000 person-years at the 2-year follow-up. The rate among women was more than twice that among men (28.1 vs 11.2 per 1000 person-years). Men tended to spend more time in both sedentary behaviors (10.1 vs 9.7 hours per day) and moderate-intensity activities (21.7 vs 13.7 minutes per day) than women. There were no significant interactions between sedentary behavior and gender.
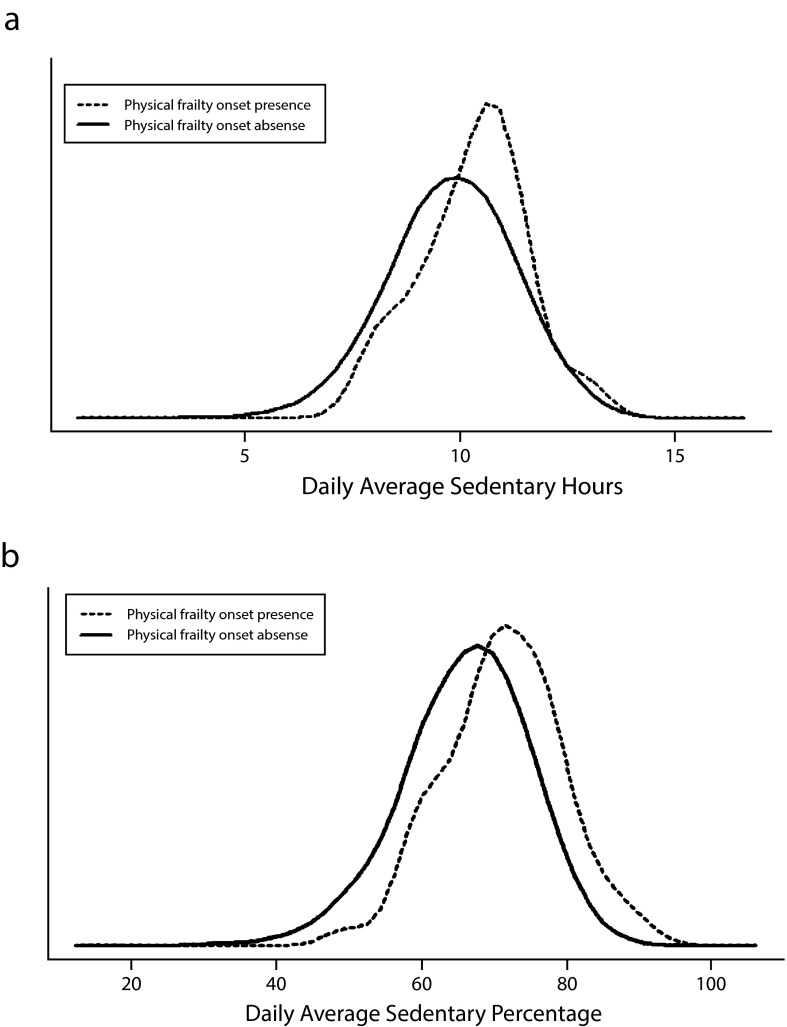
Figure 1 shows the distributions of baseline sedentary time and percentage of daily waking time spent in sedentary behavior according to the presence or absence of physical frailty 2 years later. These graphs indicate that participants who developed physical frailty tended to spend more time in sedentary behavior at baseline than those who remained free of physical frailty.
FIGURE 1—
Distributions, by physical frailty onset, of (a) daily average sedentary hours and (b) daily average sedentary percentage: Osteoarthritis Initiative, United States, 2008–2012.
Table 2 shows that higher average number of daily sedentary hours at baseline was significantly related to higher risk of physical frailty over 2 years (unadjusted HR = 1.27 per sedentary hour; 95% CI = 1.05, 1.53). This association remained statistically significant after control for moderate-intensity activity, baseline functioning, monitoring time, sociodemographic characteristics, and health factors (adjusted HR = 1.36; 95% CI = 1.02, 1.79). In addition to sedentary time, baseline chair stand rate, comorbidities, and hip OA were significant predictors of development of physical frailty. The effect of daily moderate physical activity on physical frailty onset was strong but statistically insignificant (adjusted HR = 0.18 per hour; 95% CI = 0.02, 1.73) after sedentary time and the covariates had been taken into account, possibly owing to the low levels of moderate physical activity achieved in this cohort.
TABLE 2—
Relationship of Baseline Average Daily Sedentary Hours to Physical Frailty Onset Over 2 Years of Follow-Up: Osteoarthritis Initiative, United States, 2008–2012
| Model Type | HRa (95% CI) | P |
| Unadjusted | 1.27 (1.05, 1.53) | .014 |
| Adjusted for moderate activity, baseline functioning, and monitoring time | 1.35 (1.05, 1.73) | .021 |
| Adjusted for moderate activity, baseline functioning, monitoring time, sociodemographic characteristics, and health factors | 1.36 (1.02, 1.79) | .033 |
Note. CI = confidence interval; HR = hazard ratio. Sedentary time analyses controlled for monitoring time as a design variable.
For physical frailty onset (per 1-hour increase in sedentary time).
The results were similar when we assessed sedentary behavior as average daily percentage of waking time spent in sedentary activity. Table 3 summarizes hazard ratios associated with each 10% increase in sedentary behavior, which approximated 1.5 sedentary hours during a day of 15 waking hours. A higher percentage of sedentary behavior was strongly associated with a higher risk of physical frailty (adjusted HR = 1.55 per 10% increase; 95% CI = 1.04, 2.32) independent of moderate-intensity activity and after other risk factors had been controlled. Other significant predictors were baseline chair stand rate, comorbidities, and hip OA. Greater daily moderate physical activity was associated with a decreased risk of physical frailty onset (adjusted HR = 0.21 per hour; 95% CI = 0.02, 1.91), but this relationship did not reach statistical significance.
TABLE 3—
Relationship of Baseline Daily Percentage of Sedentary Behavior to Physical Frailty Onset Over 2 Years of Follow-Up: Osteoarthritis Initiative, United States, 2008–2012
| Model Type | HRa (95% CI) | P |
| Unadjusted | 2.17 (1.53, 3.08) | < .001 |
| Adjusted for moderate activity and baseline functioning | 1.55 (1.08, 2.23) | .018 |
| Adjusted for moderate activity, baseline functioning, sociodemographic characteristics, and health factors | 1.55 (1.04, 2.32) | .033 |
Note. CI = confidence interval; HR = hazard ratio.
For physical frailty onset (per 10% increase in time spent in sedentary behavior during waking time).
We conducted sensitivity analyses assessing the contribution of physical frailty onset to death and study withdrawal at 2 years. We also conducted additional analyses that were restricted to participants aged 65 years or older. The findings of our sensitivity analyses were similar to the results shown in Tables 2 and 3.
DISCUSSION
The primary finding from this longitudinal study focusing on community-dwelling adults with or at high risk for knee OA was a significant relationship between sedentary behavior and an increased risk of incident physical frailty. The risk of physical frailty increased 36% (adjusted HR = 1.36) for each additional hour spent in sedentary behavior during daily waking time.
Furthermore, we found that the association between sedentary time and physical frailty was independent of time spent in moderate-intensity activity. This finding supports sedentary time as an independent risk factor for physical frailty, in contrast to merely reflecting insufficient moderate activity. It also lends weight to the importance of reducing sedentary time in addition to increasing moderate-intensity activities, the current focus of many behavior interventions. As expected, poorer baseline functioning and the presence of comorbidities increased participants’ risk of physical frailty onset. However, sedentary behavior was a significant predictor of the development of physical frailty after control for these known risk factors.
Studies have demonstrated a relationship between verifiable accelerometer sedentary time and poor health outcomes such as obesity, insulin resistance, and metabolic syndrome.19,20,39,40 However, these cross-sectional investigations may reflect the impact of poor health outcomes on sedentary behavior, and vice versa. Two longitudinal studies focusing on verifiable sedentary time produced mixed evidence related to cardiometabolic biomarkers such as insulin resistance.41,42 There is limited evidence based on self-reported activity measures supporting a longitudinal relationship between a sedentary lifestyle and physical frailty.8 Self-reported assessments are known to overestimate physical activity and potentially underestimate sedentary time.43 To our knowledge, this study is the first in which verifiable data on sedentary activity have been used to demonstrate that daily sedentary time predicts the future development of physical frailty.
The development of physical frailty is particularly concerning as it often leads to adverse health outcomes, including death. The Health, Aging, and Body Composition Study examined physical frailty onset rates per 1000 person-years among adults aged 70 to 79 years, and the results showed rates of 8.3 among men and 16.6 among women after 3 years of follow-up.8 Using the same physical frailty definition as that study, we examined adults aged 55 years or older with chronic conditions. Although our participants were younger, they had even higher physical frailty onset rates: 11.2 and 28.1 per 1000 person-years among men and women, respectively, after 2 years of follow-up. Our cohort largely comprised overweight or obese adults, many of whom had knee OA, typical of a large portion of community-dwelling older patients seen in clinical practice. Individuals with these conditions may need to be specifically targeted for interventions to prevent or delay development of physical frailty.
Targeting people at risk for physical frailty for interventions designed to reduce sedentary behaviors and promote physical activity may provide a means of improving health outcomes. Exercise and increasing activity have been shown to delay the development of physical frailty.8 Furthermore, higher leisure time physical activity levels during midlife have been associated with a lower prevalence of physical frailty 20 years later.10 A large body of literature has investigated exercise interventions designed to restore functioning or prevent further functional decline among people with physical frailty. Accumulating evidence supports the use of exercise interventions among older adults with physical frailty to reduce functional decline and improve muscle strength, fat-free mass, functional ability (gait speed), balance, and performance of activities of daily living.25,44,45 Even though physical activity has proven benefits, older adults with mobility-limiting conditions, such as knee or hip OA, may be reluctant to engage in such activity and may adopt a sedentary lifestyle.
It is important to convey the potential benefits of reducing sedentary time to older adults who are at an elevated risk of physical frailty owing to chronic conditions and whose physical ability to exercise may be limited. Reducing sedentary behavior includes increasing light levels of activity (i.e., energy expenditures in the range of 1.5 to 2.9 metabolic equivalents), which encompass many activities common to such older adults. Convincing adults with arthritis to decrease the amount of time they spend in sedentary activities may increase the effectiveness of behavior interventions promoting weight control and physical activity. Our results indicate that replacing as little as 1 hour of sedentary time with nonsedentary physical activity such as a casual walk or easy gardening could substantially reduce the risk of physical frailty onset.
Strengths and Limitations
The strengths of this study include the use of prospective data and objective accelerometer physical activity monitoring as well as the sample’s large size and age and gender diversity. Also, we used verifiable assessments of both sedentary behavior and physical frailty.
However, some limitations should be noted. The physical frailty definition we used is one among many definitions in the literature. Although this definition captures vulnerability in strength, consistent with other research,8,9,25 our findings may not be generalizable to other definitions. In addition, our observational results may have been confounded by factors associated with unmeasured physical frailty at baseline, influencing both low levels of physical activity and increased physical frailty risk. To mitigate this concern, we restricted our analyses to participants who were not physically frail at baseline and controlled for their baseline gait and chair stand functioning, which may reflect early unmeasured physical frailty.
Our results may also have been confounded by factors associated with being sedentary and developing physical frailty. However, our analyses controlled for major confounders including sociodemographic characteristics, BMI, depressive symptoms, pain, functioning, and comorbidities to minimize this concern. We recognize that the accelerometer used for monitoring had limitations. For example, we were not able to distinguish sitting from lying down for sleeping or leisure purposes.
Conclusions
We found that, among adults with or at high risk for knee OA, greater time spent in sedentary behavior was related to an increased risk of physical frailty. This relationship was observed independent of time spent in moderate-intensity activities. Our findings support sedentary behavior as a risk factor for physical frailty distinct from insufficient moderate activity. Future public health initiatives may be more effective if, in addition to promoting physical activity, they encourage adults with or at high risk of knee OA to reduce sedentary behaviors.
Acknowledgments
This study was funded in part by the National Institute for Arthritis and Musculoskeletal Diseases (grants R01-AR054155, R21-AR059412, and P60-AR064464) and by the Falk Medical Trust. The publicly released Osteoarthritis Initiative data were funded through a public–private partnership consisting of 5 contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) awarded by the National Institutes of Health. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer Inc. Private-sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the private funding partners, or the Osteoarthritis Initiative.
Human Participant Protection
Institutional review board approval was obtained at the participating sites and from Northwestern University. Participants provided written informed consent.
References
- 1.He W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. Available at: http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed February 10, 2015.
- 2.Morley JE, Vellas B, van Kan GA et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Guralnik JM, Studenski S et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottenbacher KJ, Graham JE, Al Snih S et al. Mexican Americans and frailty: findings from the Hispanic Established Populations Epidemiologic Studies of the Elderly. Am J Public Health. 2009;99(4):673–679. doi: 10.2105/AJPH.2008.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson MJ, Giuliani C, Morey MC et al. Physical activity as a preventative factor for frailty: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64(1):61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallucci M, Ongaro F, Amici GP, Regini C. Frailty, disability and survival in the elderly over the age of seventy: evidence from “The Treviso Longeva (TRELONG) Study.”. Arch Gerontol Geriatr. 2009;48(3):281–283. doi: 10.1016/j.archger.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Savela SL, Koistinen P, Stenholm S et al. Leisure-time physical activity in midlife is related to old age frailty. J Gerontol A Biol Sci Med Sci. 2013;68(11):1433–1438. doi: 10.1093/gerona/glt029. [DOI] [PubMed] [Google Scholar]
- 11.Hughes SL, Seymour RB, Campbell RT, Whitelaw N, Bazzarre T. Best-practice physical activity programs for older adults: findings from the National Impact Study. Am J Public Health. 2009;99(2):362–368. doi: 10.2105/AJPH.2007.131466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. Available at: http://www.health.gov/paguidelines/pdf/paguide.pdf. Accessed February 10, 2015.
- 14.Dunlop DD, Song J, Semanik PA, Sharma L, Chang RW. Physical activity levels and functional performance in the Osteoarthritis Initiative: a graded relationship. Arthritis Rheum. 2011;63(1):127–136. doi: 10.1002/art.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews CE, Chen KY, Freedson PS et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlop DD, Song J, Semanik PA et al. Objective physical activity measurement in the Osteoarthritis Initiative: are guidelines being met? Arthritis Rheum. 2011;63(11):3372–3382. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Herrmann SD et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 18.Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary.”. Exerc Sport Sci Rev. 2008;36(4):173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- 19.Bankoski A, Harris TB, McClain JJ et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy GN, Wijndaele K, Dunstan DW et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 22.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(suppl A):3–70. [PubMed] [Google Scholar]
- 23.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging—the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8(10):622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osteoarthritis Initiative. Complete clinical data. Available at: http://oai.epi-ucsf.org/datarelease/DataClinical.asp. Accessed February 10, 2015.
- 25.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995;50(5):M235–M241. doi: 10.1093/gerona/50a.5.m235. [DOI] [PubMed] [Google Scholar]
- 27.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Semanik P, Song J, Chang RW, Manheim L, Ainsworth B, Dunlop D. Assessing physical activity in persons with rheumatoid arthritis using accelerometry. Med Sci Sports Exerc. 2010;42(8):1493–1501. doi: 10.1249/MSS.0b013e3181cfc9da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Semanik P, Sharma L et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62(12):1724–1732. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Chang RW, Ehrlich-Jones L, Kwoh CK, Nevitt M, Semanik PA et al. Sedentary behavior and physical function: objective evidence from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2014 doi: 10.1002/acr.22432. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra D, Felson DT, Silliman RA et al. Knee osteoarthritis and frailty: findings from the Multicenter Osteoarthritis Study and Osteoarthritis Initiative. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu102. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miguel RC, Dias RC, Dias JM, da Silva SL, Menicucci Filho PR, Ribeiro TM. Frailty syndrome in the community-dwelling elderly with osteoarthritis. Rev Bras Reumatol. 2012;52(3):331–347. [PubMed] [Google Scholar]
- 34.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Pandya R, Metz L, Patten SB. Predictive value of the CES-D in detecting depression among candidates for disease-modifying multiple sclerosis treatment. Psychosomatics. 2005;46(2):131–134. doi: 10.1176/appi.psy.46.2.131. [DOI] [PubMed] [Google Scholar]
- 36.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prentice R, Gloeckler L. Regression analysis of group survival data with applications to breast cancer. Biometrics. 1978;34(1):57–67. [PubMed] [Google Scholar]
- 38.R Foundation for Statistical Computing. R: a language and environment for statistical computing. Available at: http://www.R-project.org. Accessed February 10, 2015.
- 39.Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med. 2011;41(2):216–227. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Healy GN, Dunstan DW, Salmon J et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 41.Ekelund U, Brage S, Griffin SJ, Wareham NJ. Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care. 2009;32(6):1081–1086. doi: 10.2337/dc08-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahjibi E, Heude B, Dekker JM et al. Impact of objectively measured sedentary behaviour on changes in insulin resistance and secretion over 3 years in the RISC study: interaction with weight gain. Diabetes Metab. 2013;39(3):217–225. doi: 10.1016/j.diabet.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Irwin ML, Ainsworth BE, Conway JM. Estimation of energy expenditure from physical activity measures: determinants of accuracy. Obes Res. 2001;9(9):517–525. doi: 10.1038/oby.2001.68. [DOI] [PubMed] [Google Scholar]
- 44.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93(2):237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Binder EF, Yarasheski KE, Steger-May K et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60(11):1425–1431. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]