Abstract
Purpose
This phase III randomized trial (ClinicalTrials.gov identifier: NCT00337103) compared eribulin with capecitabine in patients with locally advanced or metastatic breast cancer (MBC).
Patients and Methods
Women with MBC who had received prior anthracycline- and taxane-based therapy were randomly assigned to receive eribulin or capecitabine as their first-, second-, or third-line chemotherapy for advanced/metastatic disease. Stratification factors were human epidermal growth factor receptor-2 (HER2) status and geographic region. Coprimary end points were overall survival (OS) and progression-free survival (PFS).
Results
Median OS times for eribulin (n = 554) and capecitabine (n = 548) were 15.9 and 14.5 months, respectively (hazard ratio [HR], 0.88; 95% CI, 0.77 to 1.00; P = .056). Median PFS times for eribulin and capecitabine were 4.1 and 4.2 months, respectively (HR, 1.08; 95% CI, 0.93 to 1.25; P = .30). Objective response rates were 11.0% for eribulin and 11.5% for capecitabine. Global health status and overall quality-of-life scores over time were similar in the treatment arms. Both treatments had manageable safety profiles consistent with their known adverse effects; most adverse events were grade 1 or 2.
Conclusion
In this phase III study, eribulin was not shown to be superior to capecitabine with regard to OS or PFS.
INTRODUCTION
Overall survival (OS) for women with metastatic breast cancer (MBC) has improved over recent decades. Long-term survival, however, remains poor,1,2 highlighting the unmet need for therapy that is effective, improves quality of life (QoL), and prolongs survival.
Anthracycline- or taxane-based regimens are commonly used in the treatment of breast cancer, often in the (neo)adjuvant and first-line metastatic settings.3 However, treatment decisions in subsequent lines are increasingly difficult.4 There is no single accepted standard of care after failure of anthracycline and taxane therapy5; capecitabine is commonly used in the first-, second-, and third-line settings for MBC. Capecitabine has also been the control arm in several phase III trials in MBC.6–9
Eribulin mesylate (International Nonproprietary Name is eribulin) is a nontaxane microtubule dynamics inhibitor belonging to the halichondrin class of antineoplastic agents.10,11 It has a mechanism of action distinct from other tubulin-targeted agents, binding predominantly to a small number of high-affinity sites on the growing plus ends of microtubules.10–14 Such highly focused end-binding may decrease the likelihood of effects from eribulin on normal physiologic microtubule functions in nonmalignant cells.15,16 In contrast to most other tubulin-targeted agents, mitotic blockade with eribulin is irreversible, and intermittent drug exposure leads to long-term loss of cell viability.17
The first phase III trial of eribulin (Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus Eribulin [EMBRACE]) compared eribulin with treatment of physician's choice (TPC) in patients with MBC who had received at least two prior chemotherapy regimens for advanced disease but no more than five cytotoxic regimens in total. In this trial, there was a significant improvement in OS for eribulin compared with TPC; this was confirmed in the updated analysis requested by European and US regulatory authorities. The median OS was 13.2 months for eribulin versus 10.5 months for TPC (hazard ratio [HR], 0.81; 95% CI, 0.67 to 0.96; nominal [analysis not prespecified] P = .01). Furthermore, eribulin had a manageable safety profile, with the most common adverse events (AEs) being asthenia or fatigue, and neutropenia.18,19
As a result, eribulin has been approved in more than 50 countries as monotherapy for patients with advanced breast cancer or MBC who have previously received at least two chemotherapeutic regimens for advanced/metastatic disease, with prior therapy having included an anthracycline and a taxane in the adjuvant or metastatic setting.20 We report results from a second phase III study comparing eribulin with capecitabine as first-, second-, or third-line therapy for advanced breast cancer or MBC. Detailed QoL and pharmacokinetic/pharmacodynamic results will be reported separately.
PATIENTS AND METHODS
Patients
Inclusion criteria included: female sex; age ≥ 18 years; histologically or cytologically confirmed breast cancer; up to three prior chemotherapy regimens and up to two prior chemotherapy regimens for advanced and/or metastatic disease; prior therapy with an anthracycline and a taxane; resolution of all chemotherapy- or radiation-related toxicities to ≤ grade 1 (except for stable sensory neuropathy ≤ grade 2 and alopecia); Eastern Cooperative Oncology Group performance status of 0 to 2; and adequate renal, bone marrow, and liver function. Measurable or nonmeasurable disease was allowed. Exclusion criteria included prior capecitabine treatment and radiation therapy encompassing more than 30% of marrow. Patients with human epidermal growth factor receptor 2 (HER2) –positive disease could have received HER2-targeted therapy before or after study treatment but not while on study treatment.
All patients provided written informed consent. Approval was obtained from independent ethics committees and regulatory authorities in participating countries. The study was conducted in accordance with the World Medical Association Declaration of Helsinki, guidelines of the International Conference for Harmonisation/Good Clinical Practice, and local ethical and legal requirements.
Study Design
This phase III, open-label, parallel, two-arm, multicenter trial (study No. E7389-G000-301; ClinicalTrials.gov identifier: NCT00337103) stratified patients by geographic region (Latin America, Western Europe/Australia, Eastern Europe, North America, Asia, or South Africa) and the HER2 status of their cancer (positive, negative, or unknown). Patients were randomly assigned (1:1) using a central interactive voice-response system to receive eribulin mesylate 1.4 mg/m2 (equivalent to eribulin 1.23 mg/m2 [expressed as free base]) intravenously over 2 to 5 minutes on days 1 and 8, or capecitabine 1.25 g/m2 orally twice per day on days 1 to 14, both in 21-day cycles. Patients received study treatment until disease progression, unacceptable toxicity, or patient/investigator request to discontinue. Grade 3 and 4 toxicities and certain grade 2 toxicities for capecitabine were managed by treatment interruption and/or dose reduction and symptomatic treatment. Use of colony-stimulating factors and erythropoietin was allowed according to American Society of Clinical Oncology guidelines or local practice.
Study Objectives
Coprimary end points, as used in other clinical trials,21 were OS and progression-free survival (PFS). Secondary end points included objective response rate (ORR); duration of response; 1-, 2-, and 3-year survival; safety; QoL; and population pharmacokinetic/pharmacodynamic relationships.
Study Assessments
OS was measured from date of random assignment until date of death from any cause or last date known alive/data cutoff (censored). PFS was measured from date of random assignment to date of recorded disease progression or death from any cause.
Tumor response was determined according to RECIST (version 1.0), censored at last tumor assessment before subsequent anticancer therapy or before two or more missed scheduled tumor assessments,22 and confirmed by a second assessment at least 4 weeks after first observation of response. An independent radiology review was performed; in a protocol amendment requested by the US Food and Drug Administration, a bone scan was required to confirm tumor response. Duration of response was defined as the time from first documented complete or partial response until disease progression, death from any cause, or censoring at date of last tumor assessment. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3).
QoL Analyses
QoL was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (version 3.0) and breast module Quality of Life Questionnaire BR23 (version 1.0) at baseline, at 6 weeks, and at 3, 6, 12, 18, and 24 months or until disease progression or initiation of other antitumor treatment. The principal prespecified outcome was overall QoL, expressed as change from baseline in Global Health Status (GHS)/QoL measured on a 0 (worst) to 100 (best) scale.
Statistical Analyses
Because there were coprimary end points, the total type I error was split, 0.04 for OS and 0.01 for PFS. Sample size was based on a superiority test of OS; when 905 events (deaths) were observed, the two-sided log-rank test had 90% power to detect a 3-month increase in median survival over a 12-month median survival for capecitabine (HR, 0.80). Planned enrollment was 1,100 patients with a maximum of 55 patients per study site.
Primary efficacy analysis used the intent-to-treat population comprising all randomly assigned patients. The safety population included all patients who received at least one dose of treatment. Tumor assessments were obtained from an independent radiology review (primary analysis) and an investigator radiology review (secondary analysis).
The coprimary end points, OS and PFS, were compared between treatment groups using two-sided, stratified (geographic region and HER2 status) log-rank tests. Interim planned OS analyses were performed after 453 and 603 deaths. To maintain an overall level of 0.04, α spending for sequential analyses of OS was based on Lan-DeMets implementation of the O'Brien-Fleming spending function23; the nominal significance levels of the first and second interim analyses and final analysis were P = .002, P = .0081, and P = .0372, respectively. The study would be defined as positive if, at final analysis, either OS with eribulin was statistically significantly better (P ≤ .0372) versus capecitabine or PFS with eribulin was statistically significantly better (P ≤ .01) versus capecitabine, and the HR for OS (eribulin/capecitabine) was less than 1. ORRs were compared between treatment groups using Fisher's exact test. As prespecified in the statistical analyses plan, exploratory analyses of OS and PFS by the stratification factors of HER2 status and geographic region were also performed.
For the principal QoL outcome, longitudinal analyses were carried out using linear mixed model and pattern-mixture model techniques. An independent data monitoring committee reviewed safety and efficacy data from interim analyses. The sponsor (Eisai, Woodcliff Lake, NJ) collected and analyzed all data with the exception of the QoL analyses, which were conducted by Clinical Outcomes Solutions (Evergreen, CO).
RESULTS
Patients
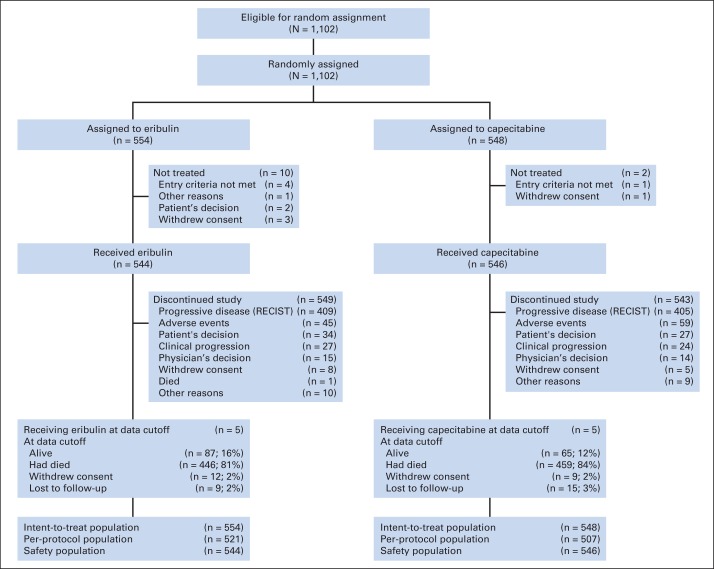
From September 2006 to September 2009, 1,102 patients were randomly assigned, 554 to eribulin and 548 to capecitabine (Fig 1). Baseline patient demographics and disease characteristics were generally well balanced (Table 1); there were small differences in the percentages of patients who had estrogen receptor–positive and triple-negative disease (46.8% v 50.7%, and 27.1% v 24.5% for eribulin and capecitabine, respectively). Overall, 68.5% of patients had HER2-negative disease. Twenty percent, 52.0%, and 27.2% of patients received study therapy as first-line, second-line, and third-line treatment, respectively, for advanced disease.
Fig 1.
CONSORT diagram.
Table 1.
Patient Demographic and Baseline Clinical Characteristics (intent-to-treat population)
| Characteristic | Eribulin (n = 554) |
Capecitabine (n = 548) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 54.0 | 53.0 | ||
| Range | 24-80 | 26-80 | ||
| Race | ||||
| White | 496 | 89.5 | 495 | 90.3 |
| Asian/Pacific Islander | 18 | 3.2 | 18 | 3.3 |
| Black or African American | 15 | 2.7 | 16 | 2.9 |
| Other | 25 | 4.5 | 19 | 3.5 |
| Geographic region | ||||
| Eastern Europe | 307 | 55.4 | 305 | 55.7 |
| Latin America | 105 | 19.0 | 104 | 19.0 |
| Western Europe | 80 | 14.4 | 77 | 14.1 |
| North America | 44 | 7.9 | 43 | 7.8 |
| Asia | 13 | 2.3 | 12 | 2.2 |
| South Africa | 5 | 0.9 | 7 | 1.3 |
| ECOG performance status | ||||
| 0 | 250 | 45.1 | 230 | 42.0 |
| 1 | 293 | 52.9 | 301 | 54.9 |
| 2 | 11 | 2.0 | 16 | 2.9 |
| 3 | 0 | 0 | 1 | 0.2 |
| No. of prior chemotherapy regimens | ||||
| 0 | 1 | 0.2 | 0 | 0 |
| 1 | 147 | 26.5 | 153 | 27.9 |
| 2 | 319 | 57.6 | 314 | 57.3 |
| 3 | 84 | 15.2 | 78 | 14.2 |
| 4 | 3 | 0.5 | 2 | 0.4 |
| 5 | 0 | 0 | 1 | 0.2 |
| No. of prior chemotherapy regimens for advanced disease | ||||
| 0 | 116 | 20.9 | 104 | 19.0 |
| 1 | 280 | 50.5 | 293 | 53.5 |
| 2 | 154 | 27.8 | 146 | 26.6 |
| > 2 | 4 | 0.7 | 5 | 0.9 |
| Refractory to treatment with:* | ||||
| Taxane | 250 | 45.1 | 260 | 47.4 |
| Anthracycline | 134 | 24.2 | 139 | 25.4 |
| Taxane and anthracycline | 91 | 16.4 | 103 | 18.8 |
| HER2 status | ||||
| Positive | 86 | 15.5 | 83 | 15.1 |
| Negative | 375 | 67.7 | 380 | 69.3 |
| Not done | 93 | 16.8 | 85 | 15.5 |
| ER status | ||||
| Positive | 259 | 46.8 | 278 | 50.7 |
| Negative | 233 | 42.1 | 216 | 39.4 |
| Not done | 62 | 11.2 | 54 | 9.9 |
| PgR status | ||||
| Positive | 227 | 41.0 | 234 | 42.7 |
| Negative | 262 | 47.3 | 248 | 45.3 |
| Not done | 65 | 11.7 | 66 | 12.0 |
| Triple (HER2/ER/PgR) negative | 150 | 27.1 | 134 | 24.5 |
| Most common metastatic sites† | ||||
| Bone | 299 | 54.0 | 308 | 56.2 |
| Lung | 279 | 50.4 | 280 | 51.1 |
| Lymph nodes | 268 | 48.4 | 274 | 50.0 |
| Liver | 247 | 44.6 | 271 | 49.5 |
| No. of organs involved | ||||
| 1 | 113 | 20.4 | 92 | 16.8 |
| 2 | 174 | 31.4 | 177 | 32.3 |
| 3 | 153 | 27.6 | 149 | 27.2 |
| ≥ 4 | 114 | 20.6 | 129 | 23.5 |
| Missing | 0 | 0 | 1 | 0.2 |
| Site of disease‡ | ||||
| Visceral | 467 | 84.3 | 483 | 88.1 |
| Nonvisceral only | 81 | 14.6 | 61 | 11.1 |
| Missing | 6 | 1.1 | 4 | 0.7 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
Refractory was defined as progression within 60 days after taking the last dose.
Reported by at least 20% of the total population.
Visceral/nonvisceral was determined by independent assessment.
Efficacy
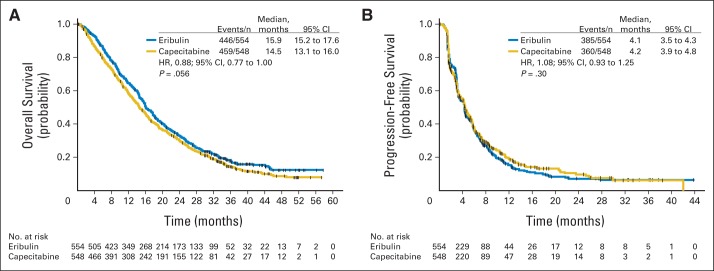
Median OS was 15.9 months (95% CI, 15.2 to 17.6 months) for eribulin compared with 14.5 months (95% CI, 13.1 to 16.0 months) for capecitabine (Fig 2A), resulting in an HR of 0.88 (95% CI, 0.77 to 1.00; P = .056). Median PFS was 4.1 months (95% CI, 3.5 to 4.3 months) for eribulin and 4.2 months (95% CI, 3.9 to 4.8 months) for capecitabine (HR, 1.08; 95% CI, 0.93 to 1.25; P = .30; Fig 2B). By investigator review, median PFS times were 4.2 months (95% CI, 3.9 to 4.3 months) and 4.1 months (95% CI, 3.7 to 4.5 months) for eribulin and capecitabine, respectively (HR, 0.98; 95% CI, 0.86 to 1.11; P = .74).
Fig 2.
Kaplan-Meier curve for (A) overall survival and (B) progression-free survival (independent review; intent-to-treat population). HR, hazard ratio. One-, 2-, and 3-year survival rates were 64.4% and 58.0% (P = .04), 32.8% and 29.8% (P = .32), and 17.8% and 14.5% (P = .18) for eribulin and capecitabine, respectively.
ORRs by independent review were 11.0% (95% CI, 8.5% to 13.9%) and 11.5% (95% CI, 8.9% to 14.5%; P = .85) for eribulin and capecitabine, respectively (Table 2). ORRs by investigator review were 16.1% (95% CI, 13.1% to 19.4%) and 19.9% (95% CI, 16.6% to 23.5%; P = .10) for eribulin and capecitabine, respectively.
Table 2.
Best Overall Tumor Response As Assessed by Independent and Investigator Review (intent-to-treat population)
| Response | Independent Review |
Investigator Review |
||
|---|---|---|---|---|
| Eribulin (n = 554) | Capecitabine (n = 548) | Eribulin (n = 554) | Capecitabine (n = 548) | |
| Tumor response | ||||
| CR | ||||
| No. of patients | 1 | 0 | 4 | 10 |
| % | 0.2 | 0 | 0.7 | 1.8 |
| PR | ||||
| No. of patients | 60 | 63 | 85 | 99 |
| % | 10.8 | 11.5 | 15.3 | 18.1 |
| Stable disease | ||||
| No. of patients | 313 | 303 | 332 | 278 |
| % | 56.5 | 55.3 | 59.9 | 50.7 |
| Progressive disease | ||||
| No. of patients | 125 | 133 | 99 | 126 |
| % | 22.6 | 24.3 | 17.9 | 23.0 |
| Not evaluable | ||||
| No. of patients | 11 | 6 | 34 | 35 |
| % | 2.0 | 1.1 | 6.1 | 6.4 |
| Unknown | ||||
| No. of patients | 44 | 43 | 0 | 0 |
| % | 7.9 | 7.8 | 0 | 0 |
| Unconfirmed CR/PR* | ||||
| No. of patients | — | — | 21 | 16 |
| % | 3.8 | 2.9 | ||
| Objective response rate† | ||||
| No. of patients | 61 | 63 | 89 | 109 |
| % | 11.0 | 11.5 | 16.1 | 19.9 |
| 95% CI | 8.5 to 13.9 | 8.9 to 14.5 | 13.1 to 19.4 | 16.6 to 23.5 |
| P‡ | .85 | .10 | ||
| Clinical benefit rate§ | ||||
| No. of patients | 145 | 147 | 182 | 188 |
| % | 26.2 | 26.8 | 32.9 | 34.3 |
| 95% CI | 22.6 to 30.0 | 23.2 to 30.7 | 29.0 to 36.9 | 30.3 to 38.4 |
| P‡ | .84 | .61 | ||
| Duration of response, months | ||||
| Median | 6.5 | 10.8 | 6.5 | 6.7 |
| 95% CI | 4.9 to 9.0 | 6.8 to 17.8 | 4.9 to 7.6 | 5.8 to 7.9 |
| P‖ | .01 | .45 | ||
Abbreviations: CR, complete response; PR, partial response.
PR/CR was confirmed as per RECIST in no less than 4 weeks, but bone scan was missing at confirmation visit required by a protocol amendment.
Objective response rate included CR and PR.
Fisher's exact test.
Clinical benefit rate was an exploratory end point and included CR, PR, or stable disease of at least 6 months in duration.
Unstratified log-rank test.
Analyses by stratification factors.
Prespecified exploratory analyses were conducted to assess an effect of eribulin according to HER2 status. Although a possible benefit according to HER2 status was suggested for OS, an interaction test showed no benefit for eribulin when comparing patients with HER2-negative disease and all other patients (HER2-positive and unknown HER2 status).
Safety
For eribulin, the median number of treatment cycles was six (range, one to 65 cycles), and the median duration of treatment was 4.1 months (range, 0.7 to 45.1 months). For capecitabine, the median number of treatment cycles was five (range, one to 61 cycles), and the median duration of treatment was 3.9 months (range, 0.7 to 47.4 months). Relative dose-intensity was 87% for eribulin and 86% for capecitabine.
AEs were reported in 94.1% and 90.5% of patients treated with eribulin and capecitabine, respectively. Serious AEs were reported in 17.5% of those receiving eribulin and 21.1% of those receiving capecitabine; these were life-threatening AEs in 2.2% and 3.5% of patients, respectively, and required or prolonged hospitalization in 13.4% and 17.0% of patients, respectively. AEs leading to discontinuation, reduction, or delay in treatment occurred in 7.9%, 32.0%, and 31.8% of patients receiving eribulin and in 10.4%, 31.9%, and 35.7% of those receiving capecitabine, respectively. Fatal AEs (within 30 days of last dose) occurred in 4.8% of patients receiving eribulin and 6.6% of patients receiving capecitabine. These were reported as treatment-related AEs for five patients treated with eribulin (sepsis, pericardial effusion, sudden death, toxic hepatitis, and renal failure) and four patients treated with capecitabine (sepsis, pneumonia, cardiogenic shock, and pancytopenia).
The most common AEs with eribulin were neutropenia, alopecia, leukopenia, global peripheral neuropathy, and nausea. The most common AEs with capecitabine were hand-foot syndrome, diarrhea, and nausea (Table 3). Febrile neutropenia occurred at low incidence with both eribulin (2.0%) and capecitabine (0.9%). Most AEs were grade 1 or 2. The most common grade 3 or 4 AEs were neutropenia, leukopenia, asthenia, and global peripheral neuropathy for eribulin, and hand-foot syndrome, diarrhea, neutropenia, dyspnea, and asthenia for capecitabine. Grade 3 or 4 global peripheral neuropathy occurred in 7.0% of patients receiving eribulin and 0.9% of patients receiving capecitabine (Table 3). In the eribulin group, the incidences of grade 3 or 4 peripheral motor neuropathy, peripheral sensorimotor neuropathy, and polyneuropathy were 0.7% (all grade 3), 0.6% (all grade 3), and 0.6% (0.4% grade 3, 0.2% grade 4), respectively; these AEs did not occur at grade 3 or 4 in the capecitabine group. The most common AEs leading to discontinuation (occurring in > 1% of patients) were neutropenia (1.7%) with eribulin and hand-foot syndrome (2.2%) and dyspnea (1.1%) with capecitabine. Colony-stimulating factors were received by 14.6% and 3.6% of patients in the eribulin and capecitabine arms, respectively.
Table 3.
Most Common Adverse Events (incidence of > 10% for all grades or > 2% for ≥ grade 3 in either arm; safety population)
| Adverse Event | Eribulin (n = 544) |
Capecitabine (n = 546) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Hematologic | ||||||||||||
| Neutropenia | 295 | 54.2 | 134 | 24.6 | 115 | 21.1 | 87 | 15.9 | 23 | 4.2 | 4 | 0.7 |
| Leukopenia | 171 | 31.4 | 73 | 13.4 | 9 | 1.7 | 57 | 10.4 | 10 | 1.8 | 1 | 0.2 |
| Anemia | 104 | 19.1 | 11 | 2.0 | 0 | 0 | 96 | 17.6 | 5 | 0.9 | 1 | 0.2 |
| Febrile neutropenia | 11 | 2.0 | 8 | 1.5 | 3 | 0.6 | 5 | 0.9 | 2 | 0.4 | 3 | 0.5 |
| Nonhematologic | ||||||||||||
| Alopecia | 188 | 34.6 | 22 | 4.0 | ||||||||
| Global peripheral neuropathy* | 149 | 27.4 | 35 | 6.4 | 3 | 0.6 | 75 | 13.7 | 5 | 0.9 | 0 | 0 |
| Nausea | 121 | 22.2 | 1 | 0.2 | 0 | 0 | 133 | 24.4 | 9 | 1.6 | 0 | 0 |
| Fatigue | 91 | 16.7 | 11 | 2.0 | 0 | 0 | 84 | 15.4 | 12 | 2.2 | 1 | 0.2 |
| Asthenia | 83 | 15.3 | 22 | 4.0 | 1 | 0.2 | 79 | 14.5 | 20 | 3.7 | 0 | 0 |
| Diarrhea | 78 | 14.3 | 6 | 1.1 | 0 | 0 | 157 | 28.8 | 28 | 5.1 | 1 | 0.2 |
| Pyrexia | 70 | 12.9 | 2 | 0.4 | 0 | 0 | 31 | 5.7 | 3 | 0.5 | 0 | 0 |
| Headache | 69 | 12.7 | 4 | 0.7 | 0 | 0 | 57 | 10.4 | 2 | 0.4 | 1 | 0.2 |
| Decreased appetite | 68 | 12.5 | 3 | 0.6 | 0 | 0 | 81 | 14.8 | 9 | 1.6 | 0 | 0 |
| Vomiting | 65 | 11.9 | 1 | 0.2 | 1 | 0.2 | 92 | 16.8 | 12 | 2.2 | 0 | 0 |
| Dyspnea | 56 | 10.3 | 10 | 1.8 | 2 | 0.4† | 59 | 10.8 | 16 | 2.9 | 5 | 0.9‡ |
| Back pain | 56 | 10.3 | 8 | 1.5 | 0 | 0 | 43 | 7.9 | 3 | 0.5 | 0 | 0 |
| Bone pain | 50 | 9.2 | 10 | 1.8 | 1 | 0.2 | 43 | 7.9 | 4 | 0.7 | 1 | 0.2 |
| ALT increased | 46 | 8.5 | 18 | 3.3 | 0 | 0 | 23 | 4.2 | 3 | 0.5 | 0 | 0 |
| Hypokalemia | 19 | 3.5 | 5 | 0.9 | 0 | 0 | 25 | 4.6 | 9 | 1.6 | 2 | 0.4 |
| Hand-foot syndrome | 1 | 0.2 | 0 | 0 | 0 | 0 | 246 | 45.1 | 79 | 14.5 | 0 | 0 |
NOTE. If a patient had ≥ two adverse events in the same system organ class or with the same preferred term with different Common Terminology Criteria for Adverse Events grades, the event with the highest grade was used for that patient.
Defined as Standardized Medical Dictionary for Regulatory Activities Queries narrow and broad terms.
Grade 5 events also occurred in four patients (0.7%).
Grade 5 events also occurred in three patients (0.5%).
QoL Analyses
Almost all (> 95%) QoL data were available at baseline for both arms; completion rates over time decreased similarly in both arms (Data Supplement). GHS/QoL scores were low at baseline in both the eribulin and capecitabine arms (mean ± standard deviation, 56.3 ± 22.2 and 54.7 ± 21.7, respectively). Over time, average GHS/QoL scores improved in both arms, but the linear mixed model and pattern-mixture model showed no significant difference between the groups (linear mixed model: estimated treatment effect, −0.068; P = .958; pattern-mixture model: estimated treatment effect, 0.082; P = .949).
DISCUSSION
Although eribulin is an active single agent in patients with MBC, it was not superior to capecitabine with regard to either OS or PFS. Our results contrast with those of EMBRACE, in which a statistically significant improvement in OS was seen with eribulin compared with TPC.18 The reasons for this apparent difference are unclear. It is possible that treatment earlier in the course of MBC is less likely to impact OS, as a consequence of such patients typically receiving further lines of cytotoxic or other therapy. Even if therapeutically more active, a first- or second-line regimen may not impact on OS when multiple subsequent lines of effective treatment are administered.
The influence of postprogression therapies on OS is often discussed in studies of MBC, particularly when cross over is imbalanced, and usually in the context of differences in PFS being more apparent than those in OS (which did not occur in our study). In this trial, more patients went on to receive further anticancer treatment after study treatment in the eribulin arm (70.4%) than in the capecitabine arm (62.0%). Specifically, patients in the eribulin arm could cross over and receive capecitabine (49.6%), whereas cross over from capecitabine to eribulin (0.4%) was limited by eribulin only being approved toward the end of the study. Nevertheless, no differences in OS were seen in this study.
The OS data in patients with HER2-negative disease were similar to those reported in EMBRACE,18 and there was no significant difference in PFS between treatment groups in the HER2 subgroups.
Although PFS and OS are similar to other studies in this setting,7,8 ORRs in this study are low. This may be explained, at least in part, by only 88% of patients having disease evaluable for response; the remainder had no baseline scan per independent review (1%), a baseline scan of any type only (7%), or a RECIST response but no confirmatory bone scan (3%).
Eribulin had a manageable tolerability profile, consistent with previous studies; neutropenia, alopecia, leukopenia, and peripheral neuropathy were the most common AEs.18,24–27 For patients receiving eribulin, the incidences of hematologic and grade 3 or 4 AEs were similar to those in EMBRACE, except for febrile neutropenia. The total incidence of febrile neutropenia with eribulin was lower in this trial (2% with eribulin v 0.9% with capecitabine) than in EMBRACE (5%), in which patients had received more prior lines of chemotherapy.18 Neutropenia was managed with dose delays, reductions, and growth factors according to local practice. The use of colony-stimulating factors was higher in the eribulin group than in the capecitabine group (14.6% v 3.6%, respectively), consistent with the greater incidence of neutropenia. There were, however, no deaths as a result of neutropenia in either treatment group. AEs experienced with capecitabine, particularly hand-foot syndrome and diarrhea, were also consistent with known AEs.10,8,28 Even though this study used the approved dose of capecitabine (1.25 g/m2 twice per day), these AEs were generally within the range observed for capecitabine administered at 1.0 g/m2 twice per day,29–35 a dose commonly used in clinical practice.36 Furthermore, dose-intensity was high for both eribulin and capecitabine in this study. Although incidences of alopecia and peripheral neuropathy were higher for eribulin compared with capecitabine, incidences of diarrhea and vomiting were lower. In summary, the AE profiles of both treatments in this phase III trial were predictable, manageable, and, overall, clinically acceptable. From the patients' perspective, average GHS/QoL scores generally improved in both treatment arms with no evidence of a difference between treatments.
In conclusion, this trial did not demonstrate superiority of eribulin versus capecitabine for either OS or PFS. The effects on QoL in this population of patients with MBC and the AE profiles of eribulin and capecitabine were consistent with their known AEs.
Supplementary Material
Acknowledgment
We thank all of the patients and investigators who participated in this study. We also thank Jantien Wanders for contributions to the development of the article and input into the study. In addition, we thank Stacie Hudgens from Clinical Outcomes Solutions for conducting the quality-of-life analyses. Editorial support was provided by Annette Smith, PhD, of Complete Medical Communications. Additional editorial support during article revisions was provided by Oxford PharmaGenesis, United Kingdom. Funding for all editorial support was provided by Eisai.
Glossary Terms
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- overall survival:
the duration between random assignment and death.
- progression-free survival:
time from random assignment until death or first documented relapse, categorized as either locoregional (primary site or regional nodes) failure or distant metastasis or death.
Footnotes
Supported by Eisai (Woodcliff Lake, NJ).
Presented in part at the 2012 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2012; the British Breast Group Meeting, Glasgow, United Kingdom, February 1, 2013; and the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00337103.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Martin S. Olivo, Eisai (C); Corina E. Dutcus, Eisai (C) Consultant or Advisory Role: Peter A. Kaufman, Eisai (C); Ahmad Awada, Eisai (C); Chris Twelves, Eisai (C); Galina Velikova, Eisai (U); Javier Cortes, Roche, Novartis, Celgene (C) Stock Ownership: None Honoraria: Peter A. Kaufman, Eisai; Chris Twelves, Eisai; Galina Velikova, Eisai; Javier Cortes, Roche, Novartis, Celgene, Eisai Research Funding: Peter A. Kaufman, Eisai; Louise Yelle, Eisai Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Galina Velikova, Eisai
AUTHOR CONTRIBUTIONS
Conception and design: Peter A. Kaufman, Chris Twelves, Corina E. Dutcus, Javier Cortes
Provision of study materials or patients: Javier Cortes
Collection and assembly of data: Peter A. Kaufman, Ahmad Awada, Chris Twelves, Louise Yelle, Edith A. Perez, Corina E. Dutcus, Javier Cortes
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Cancer facts and figures 2011. http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011.
- 2.National Cancer Institute. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, based on November 2012 SEER data submission, posted to the SEER Web site. http://seer.cancer.gov/csr/1975_2010.
- 3.Andreopoulou E, Sparano JA. Chemotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer: An overview. Curr Breast Cancer Rep. 2013;5:42–50. doi: 10.1007/s12609-012-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Aspitia A, Perez EA. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin Proc. 2009;84:533–545. doi: 10.4065/84.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Breast Cancer Version 3. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 6.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Gomez HL, Li RK, et al. Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes. Breast Cancer Res Treat. 2010;122:409–418. doi: 10.1007/s10549-010-0901-4. [DOI] [PubMed] [Google Scholar]
- 8.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 9.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 10.Kuznetsov G, Towle MJ, Cheng H, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64:5760–5766. doi: 10.1158/0008-5472.CAN-04-1169. [DOI] [PubMed] [Google Scholar]
- 11.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 12.Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 13.Okouneva T, Azarenko O, Wilson L, et al. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7:2003–2011. doi: 10.1158/1535-7163.MCT-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JA, Wilson L, Azarenko O, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozniak KM, Nomoto K, Lapidus RG, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res. 2011;71:3952–3962. doi: 10.1158/0008-5472.CAN-10-4184. [DOI] [PubMed] [Google Scholar]
- 16.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: Boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–4615. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: Implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res. 2011;71:496–505. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 18.Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 19.Twelves C, Cortes J, Vahdat LT, et al. Phase III trials of eribulin mesylate (E7389) in extensively pretreated patients with locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2010;10:160–163. doi: 10.3816/CBC.2010.n.023. [DOI] [PubMed] [Google Scholar]
- 20.Eisai. Halaven prescribing information. http://www.halaven.com.
- 21.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Lan K, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 24.Goel S, Mita AC, Mita M, et al. A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res. 2009;15:4207–4712. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 25.Synold TW, Morgan RJ, Newman EM, et al. A phase I pharmacokinetic and target validation study of the novel anti-tubulin agent E7389: A California Cancer consortium trial. J Clin Oncol. 2005;23(suppl 16s):200s. abstr 3036. [Google Scholar]
- 26.Tan AR, Rubin EH, Walton DC, et al. Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4213–4429. doi: 10.1158/1078-0432.CCR-09-0360. [DOI] [PubMed] [Google Scholar]
- 27.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 28.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajetta E, Procopio G, Celio L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol. 2005;23:2155–2161. doi: 10.1200/JCO.2005.02.167. [DOI] [PubMed] [Google Scholar]
- 30.El-Helw L, Coleman RE. Reduced dose capecitabine is an effective and well-tolerated treatment in patients with metastatic breast cancer. Breast. 2005;14:368–374. doi: 10.1016/j.breast.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Hennessy BT, Gauthier AM, Michaud LB, et al. Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: Retrospective analysis of patients treated at M. D. Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol. 2005;16:1289–1296. doi: 10.1093/annonc/mdi253. [DOI] [PubMed] [Google Scholar]
- 32.Rossi D, Alessandroni P, Catalano V, et al. Safety profile and activity of lower capecitabine dose in patients with metastatic breast cancer. Clin Breast Cancer. 2007;7:857–860. doi: 10.3816/CBC.2007.n.050. [DOI] [PubMed] [Google Scholar]
- 33.Sezgin C, Kurt E, Evrensel T, et al. Efficacy of lower dose capecitabine in patients with metastatic breast cancer and factors influencing therapeutic response and outcome. South Med J. 2007;100:27–32. doi: 10.1097/01.smj.0000252968.87824.19. [DOI] [PubMed] [Google Scholar]
- 34.Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498–4504. doi: 10.1200/JCO.2010.33.9101. [DOI] [PubMed] [Google Scholar]
- 35.Yap YS, Kendall A, Walsh G, et al. Clinical efficacy of capecitabine as first-line chemotherapy in metastatic breast cancer: How low can you go? Breast. 2007;16:420–424. doi: 10.1016/j.breast.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: Confused, clarified or confirmed? Ann Oncol. 2010;21:2145–2152. doi: 10.1093/annonc/mdq069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.