Abstract
Prenatal exposure to alcohol can result in fetal alcohol syndrome (FAS), characterized by significant changes in the physiology, structural plasticity of hippocampal function, including long-term deficits in learning and memory. Environmental enrichment has long been known to improve motor and cognitive function levels, causes several neurochemical and morphological alterations in the brain. Therefore, the effects of environmental enrichment on the neurobehavioral and neurotrophic changes in mice exposed prenatally to alcohol were investigated in this study. The pregnant dams were given 25 % ethanol (w/v) or isocaloric sucrose by liquid diet from gestation day 7 to 20. After weaning on postnatal day 28, offspring were exposed to standard cage (CC, CFAS) or enriched living conditions (CE, EFAS) for 8 weeks. Neurobehavioral studies both on hippocampus-dependent spatial learning and place and cue learning strategy, a striatum-dependent test, were measured by the Morris water maze task. Moreover, the reverse-transcriptase polymerase chain reaction (RT-PCR) technique was also used in order to study the expression of brain-derived neurotrophic factor (BDNF) level in both the hippocampus and striatum of mice. Neurobehavioral studies show that animals exposed prenatally to alcohol were impaired as shown in both hippocampal-dependent spatial/place and striatal-dependent response/cue learning tests. Moreover, the levels of BDNF expression both in the hippocampus and striatum of mice were also decreased. Interestingly, environmental enrichment can ameliorate the effects of prenatal alcohol exposure both on the neurobehavioral and neurotrophic levels. These observations indicated that enriched environment attenuated memory impairment of prenatal alcohol exposure both in hippocampal and striatal circuitry.
Keywords: brain-derived neurotrophic factor, enriched environment, prenatal alcohol exposure, Morris water maze
Introduction
Exposure to alcohol during pregnancy can result in fetal alcohol spectrum disorders (FAS), which exhibit craniofacial dysmorphia, growth retardation, and behavioral and cognitive impairment that are thought to persist throughout life (Jones and Smith 1973[21]; Jones et al., 1973[22]). It is well established that the hippocampus is one of the brain regions that plays a crucial role in learning, memory, and cognition, and is known to be highly vulnerable to the neurotoxic effects of ethanol (for review see Berman and Hannigan, 2000[1]). Several studies have demonstrated that prenatal expose to alcohol can cause disturbance in structure and function of the hippocampus, and long-term impairment in learning and memory (Berman and Hannigan, 2000[1]; Gabriel et al., 2002[13]).
Brain-derived neurotrophic factor (BDNF) is an important molecular mediator of synaptic and morphological plasticity, and has crucial roles in neurodevelopment and survival (Gomez-Palacio-Schjetnan and Escobar, 2008[14]). Moreover, BDNF has been shown to enhance NMDA glutamate receptors (Crozier et al. 2008[9]; Ninan et al., 2010[34]; Wang et al., 2011[46]), and involved in neuroplasticity in term of long-term potentiation (LTP) and long-term depression (LTD) in the hippocampus (Bozdagi et al., 2008[2]; Chen et al. 2010[6]; Ikegaya et al. 2002[18]; Jia et al. 2010[20]). Environmental enrichment has previously been reported to increase the neurotrophin levels (Ickes et al., 2000[17]; Parks et al., 2008[38]), enhance neurogenesis (Choi et al., 2005[7]; Leggio et al., 2005[26]) and has significant consequences for both behavior and morphology of the animal (Pham et al., 1999[39]).
To determine whether environmental enrichment affects on the neurobehavioral and neurotrophic changes in mice exposed prenatally to alcohol. Neurobehavioral studies both on hippocampus-dependent spatial acquisition test, and a striatum-dependent place and cue learning strategy were measured by the Morris water maze task. Moreover, expression of BDNF mRNA levels in both the hippocampus and striatum of mice were also studied. Here we demonstrated the possibilities that environmental enrichment might attenuate some of the deficits due to prenatal alcohol exposure.
Materials and Methods
Animals
Adult C57BL/6 mice (8-10 weeks of age, 20-25 g) used in the present experiment were obtained from the National Experimental Animals Center of Mahidol University, Salaya Campus, Thailand. All animal procedures were approved by the Laboratory Animal Care and Use Committee of Mahidol University. Animals were house in the room maintains at 22 ± 2 °C, 30 % humidity, with a 12-h light, 12-h dark cycle (on at 06:00 h). Female mice were placed into male home cages for 2 h, and embryonic day 0 (E0) was identified when the vaginal plug was detected.
Prenatal alcohol exposure paradigm
Pregnant female mice in the alcohol treatment group were administered 25 % (w/v) ethanol solution by ad libitum from gestation (G) 7 to G20. The pair fed group received milk with sucrose that was isocaloric and isovolumetric to the ethanol dose (1 cal/ml) (Middaugh et al., 1996[31]). One day before treatment, the alcohol-treated dams were adapted to the liquid diet by giving them the control diet (without ethanol) as their sole source of calories. All treatments were stopped on G20; the first day of birth was always defined as P0. The cages were checked regularly at 06.00, 13.00, and 22.00 h to record the time of delivery.
Environmental enrichment
The offspring were weaned at 4 weeks old then segregated and placed into four housing condition for 8 weeks after weaning: standard or enriched (1) pup from dam treated with ethanol liquid diet (ALC) in enriched environment, EFAS; (2) pup from ALC placed in standard cages, CFAS; (3) pup from dam treated with pair-fed control (PF) in enriched environment, CE (4) pup from PF in standard cages, CC. Standard housing comprised groups of four mice, in a standard mouse-housing container (7.5 cm x 11.5 cm x 5 cm; 126 cm2/mouse). Enriched conditions comprised groups of eight mice, in a standard rat-housing container (65 cm x 46 cm x 48 cm; 162 cm2/mouse). In the enriched group had a lot of toys; for example running wheel, ladder, plastic houses, tunnel and plastic boxes, which the place of food and water were changed every day. The activities of the mice were monitored to confirm similar levels of environmental interaction between groups. All mice were kept in their respective housing conditions during the entire length of the experiment until sacrificed.
Morris water maze test
Apparatus
The Morris water maze consisted of a circular pool, 150 cm in diameter and 70 cm in depth. The water in the tank was approximately 50 cm deep, temperature of 24-25 °C, and was made opaque by the addition of white nontoxic watercolor paint. The maze was divided into quadrants and four equally spaced release point designated as north (N), east (E), south (S) and west (W). A platform (10 cm in diameter) was hidden 1 cm beneath the water in the center of the northwest (NW) quadrant during training trails.
Spatial acquisition test
Mice were trained on a Morris water maze for 4 days, with four trials per day and trail interval of approximately 10 min. Mice were randomly placed at each of the four start positions (N, S, E, W) facing the pool wall, and were allowed to remain on the platform for 10 s once they found it. Animal who failed to find the platform after 60 s was placed on the platform for a period of 10 s by the experimenter. The length of swim path and the time to reach the platform (latency) were recorded by a video tracking system (Ethovision 2.2, Nodus Information Technology).
Spatial probe trial and retention test
To assess reference memory at the end of learning, at 24 h after training mice were returned to the water maze for probe trail. The hidden platform was removed and mice were allowed to swim freely for 30 s. The amount of time spent in the quadrant where the platform was previously located relative to the other three quadrants was used as an index of the mice’s memory capacity.
The remote retention test was conducted in the first and the second week after the training session. Latency to reach the platform location, the length of swim path, and time spent in each quadrant were analyzed.
Cue training
Another group of animals was trained in a cue learning test (Nicolle et al., 2003[32]). A visible platform was elevated 2 cm above the water surface and was moved to different locations in the pool between trials. One of the platform locations was centered in the middle of the tank. Animals were released from the four start points and were varied for each trial. Each mouse was given 60 s to reach the platform and was allowed to remain on it for 10 s. Animals were trained for 2 days on day 4-5 of training with 6 trials per day, 5 min of interval.
Place training
Place training was taken place on day 1-3, and day 6-9. The procedure of place training uses the same as the spatial acquisition test. The hidden platform was submerged 1 cm below the water surface and remained in the same position throughout all the training trails. There were four training trials per day for 8 days, 10 min of interval.
RNA isolation and RT-PCR amplification
Animals were sacrificed 1 day after the last behavioral test. The brains were immediately removed and the hippocampus and striatum were dissected and stored at -80 °C until used. Total RNA was isolated using TRIzol® Reagent. Reverse transcriptase-polymerase chain reaction (RT-PCR) was carried out in a single tube. Each 25-µl sample contained 200 µM dNTPs, 1.5 mM MgCl2, 25 pmol of each oligonucleotide primer (BDNF or β-actin), 1 unit of Taq DNA polymerase, and 10 unit of AMV reverse transcriptase. For BDNF, 2 µg total RNA was preheated with 40 pmol oligo (dT) primer at 70 °C for 10 min in the RT reaction, followed by PCR cycles consisted of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s with final extension at 72 °C for 7 min. For β-actin, AMV reverse transcriptase was activated at 42 °C for 30 min. Then denaturation was performed at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s. PCR products of BDNF and β-actin were separated by electrophoresis on a 1.5 % agarose gel and recorded by a gel documentation system (Gel Doc, Bio-Rad). The density of the BDNF band was analyzed with the Scion image software program. The amount of PCR products of hippocampus and striatum BDNF is normalized to that of PCR products of β-actin in each sample.
Data analysis
The analyses of variance (ANOVA) were performed using GraphPad Prism® software. Data are expressed as means ± standard error of the mean (SEM), P value less than 0.05 was considered significant.
Results
Morris water maze performance
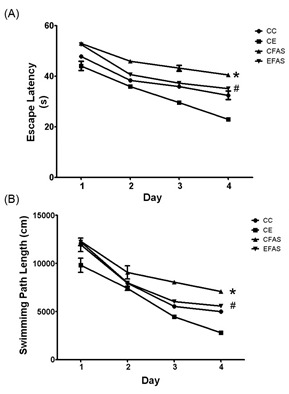
To assess the spatial acquisition test, mice were trained on a Morris water maze for 4 days, 4 trials per day and with trial interval of approximately 10 min. The retention task was tested 24 h after the last training trial. Mean latency to locate the hidden platform and mean distance traveled to reach the platform over 4-day spatial acquisition training trials were shown in Figure 1(Fig. 1). Two-way ANOVA analysis showed a significant difference between the prenatal alcohol exposure (CFAS) group and the non-treated alcohol (CC) group in the latency to reach the platform (P < 0.001) and across the 4 training days (P < 0.001). For the distance traveled to the platform, two-way ANOVA analysis showed that the offsprings from CFAS treatment group show significantly longer than in the CC group (P < 0.001) and across the 4 training days (P < 0.001). However, the environmental enrichment (EFAS) show significantly improved the spatial learning task, both in the latency and distance traveled to reach the platform, after prenatal exposure to alcohol (P < 0.001).
Figure 1. The escape latency (A) and distance traveled (B) to the hidden platform in spatial acquisition test over a 4-day trial period for the offsprings from CC, CE, CFAS, and EFAS treatment groups (n = 5-6). Data presented as mean ± SEM. * indicate P < 0.001 from CC values, # indicates P < 0.001 from CFAS group. CC, non-treated control group in standard housing; CE, non-treated control group in enriched environment; CFAS, prenatal alcohol exposure in standard housing; EFAS, prenatal alcohol exposure in enriched environmental.
To assess reference memory at the end of learning, mice were returned to the water maze for probe trail at 24 h, the first week, and the second week after the training session. The hidden platform was removed and mice were allowed to swim freely for 30 s and the time spent in each quadrant were recorded. All groups performed equally well in the probe trails and spent significantly more time in the correct quadrant (where the platform is located) than in the remaining 3 quadrants (one-way ANOVA, Turkey's multiple comparison test, P < 0.05; Figure 2(Fig. 2)).
Figure 2. Time spent in the four water maze quadrants over four probe trials occurred on day 5, the first week (R1W), and the second week (R2W) after the last training session. PF indicates the quadrant that contained the escape platform. Q1, Q2, and Q3 indicate the other quadrants of the maze.
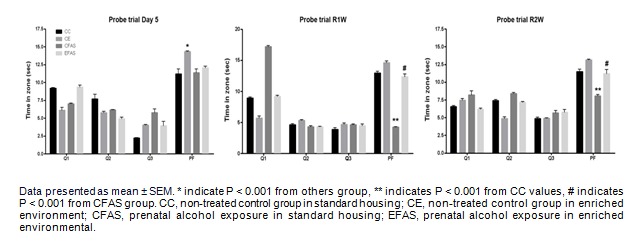
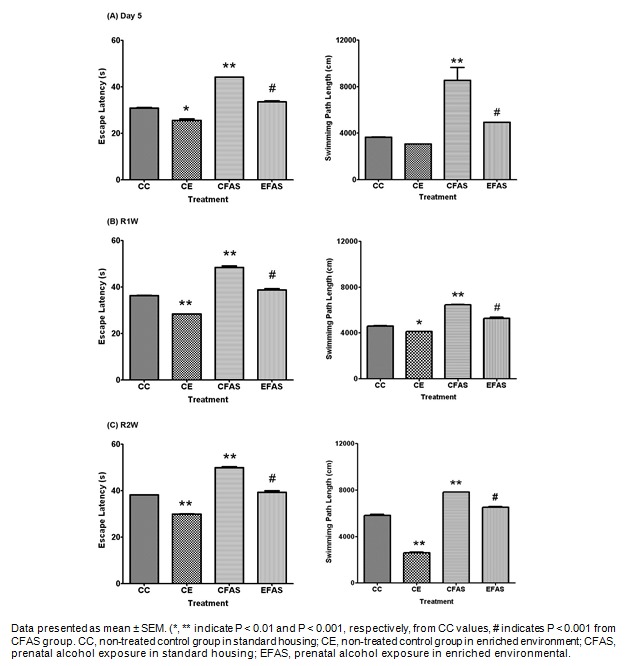
In addition, the latency and distance traveled to reach the platform were analyzed at 24 h (Figure 3A(Fig. 3)), the first week (Figure 3B(Fig. 3)), and the second week (Figure 3C(Fig. 3)) after the training session. One-way ANOVA followed by Turkey's multiple comparison test showed a significant difference between the prenatal alcohol exposure (CFAS) group and the non-treated alcohol (CC) group in the latency to reach the platform (P < 0.001) over 3 testing sessions. As well as the distance traveled to the platform, statistical analysis showed that the CFAS treatment group show significantly longer than in the CC group (P < 0.001) and across the 3 testing session (P < 0.001). However, the environmental enrichment (EFAS) show significantly improved the retention task in the latency and distance traveled to reach the platform, and time spent in each quadrant after prenatal exposure to alcohol (P < 0.001; Figure 2(Fig. 2) and Figure 3(Fig. 3)).
Figure 3. The escape latency (left) and distance traveled (right) to the hidden platform in retention test. Mice from CC, CE, CFAS, and EFAS treatment groups (n=5-6) were trained in spatial acquisition test for 4 consecutive days (four trails per day) and tested the retention task on Day 5, the first week, and the second week, after the last training session.
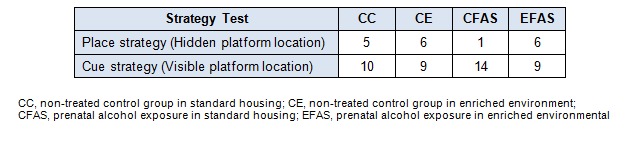
Another group of animals was trained in place and cue strategy learning (Figure 4(Fig. 4) and Figure 5(Fig. 5), respectively). Place training was take place on day 1-3, and day 6-9. The hidden platform was submerged 1 cm below the water surface and remained in the same position throughout all the training trails. There were four training trials per day for 8 days, 10 min of interval. For cue strategy test, a visible platform was elevated 2 cm above the water surface and was moved to different locations in the pool between trials. Each mouse was given 60 s to reach the platform and was allowed to remain on it for 10 s. Animals were trained for 2 days on day 4-5 of training with 6 trials per day, 5 min of interval. All groups improved during the course of training on both visible and hidden trials. Statistical analysis showed that both the latency and distance traveled to reach the hidden platform in place strategy test were significant difference between CFAS treatment group and the CC group (P < 0.001; Figure 4A(Fig. 4), 4B(Fig. 4)). For the cue strategy test, the latency to reach the visible platform showed a significantly increased in CFAS treatment group compared to CC group (P < 0.05; Figure 5(Fig. 5)). However, the environmental enrichment (EFAS) show significantly improved both the place (P < 0.001; Figure 4(Fig. 4)) and cue (P < 0.05; Figure 5(Fig. 5)) strategy learning, after prenatal exposure to alcohol. Interestingly, CFAS mice were impaired both on place learning task and cue learning task, and were used a cue strategy on the strategy probe test. In contrast, CC, CE and EFAS mice were more evenly divided between the use of place and cue strategies (Table 1(Tab. 1)).
Figure 4. The escape latency (A) and swimming path length (B) to reach the hidden platform from day 1-3 and day 4-9 in the place strategy test.
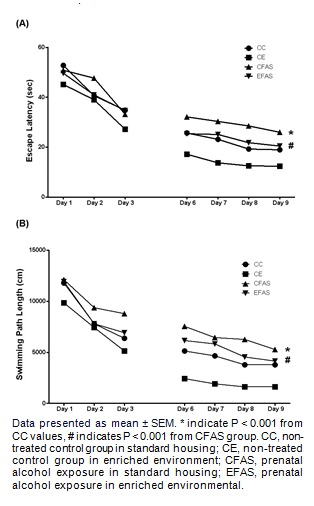
Figure 5. The escape latency to reach the visible platform in cue strategy test. Animals were trained for 2 days on day 4 of training and on day 5, with 6 trials per day.
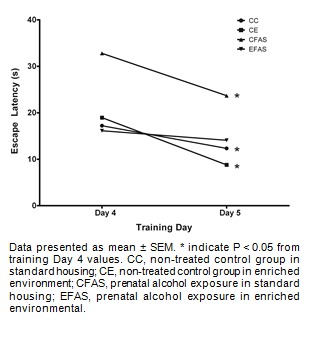
Table 1. Number of mice using place or cue strategies on the first strategy probe trial test.
Brain-derived neurotrophic factor mRNA level
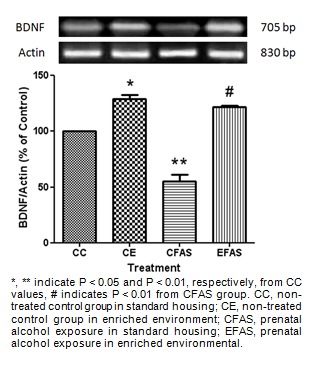
To investigate the effect of enriched environment on BDNF mRNA expression after prenatal exposure to alcohol, the hippocampus and striatum were collected from the CC, CE, CFAS, and EFAS treatment animals 1 day after the last probe trial test. Semi-quantitative analysis showed that prenatal exposure to alcohol (CFAS) caused a significant decrease in BDNF mRNA expression both in the hippocampus (P < 0.05; Figure 6(Fig. 6)) and the striatum (P < 0.01; Figure 7(Fig. 7)) of offsprings compared to the non-treated alcohol (CC) group. Animals housed in the enriched environment (CE) showed a significant increase in BDNF mRNA level in both areas. Moreover, the environmental enrichment significantly (P < 0.01) attenuated the decrease of BDNF mRNA level caused by prenatal exposure to alcohol both in the hippocampus (Figure 6(Fig. 6)) and the striatum (Figure 7(Fig. 7)).
Figure 6. The mRNA expression of BDNF in the hippocampus. Total mRNAs were extracted from CC, CE, CFAS and EFAS treatment groups. The BDNF RT-PCR products were normalized to the level of actin expression within the same sample and represented as a percentage of the values of CC group, indicated by Mean ± SEM (n = 5-6).
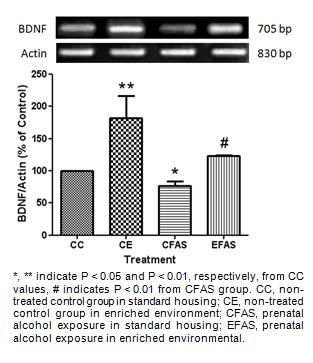
Figure 7. The mRNA expression of BDNF in the striatum. Total mRNAs were extracted from CC, CE, CFAS and EFAS treatment groups. The BDNF RT-PCR products were normalized to the level of actin expression within the same sample and represented as a percentage of the values of CC group, indicated by Mean ± SEM (n = 5-6).
Discussion
Prenatal alcohol exposure through maternal alcohol administration can cause the behavioral impairment and neurochemical alteration in postnatal offspring that persist into adulthood. The present findings demonstrate that the post-weaning environmental enrichment can improve both the behavioral performance and neurochemical disturbance of animals exposed prenatally to alcohol. In the spatial acquisition of the Morris water maze test, all groups of animal demonstrate learning by showing a decrease in latency and distance traveled to the hidden platform over the 4 days of training. This learning ability was supported by their spending most time in the target quadrant during the probe trial occurred at 24 h, the first week, and the second week after the last training session. However, the prenatal alcohol-exposed mice show the memory impairment demonstrated by markedly increase in the latency and the distance to reach the hidden platform in the test on day 5 compared to the non-alcohol treated mice. Moreover, this group demonstrated the long-term memory deficit by showing a less time spend in the target quadrant in the probe trial occurred at the first week, and the second week after the last training session, compared to the non-alcohol treated group. These impairments in acquisition in the water maze task after chronic prenatal alcohol exposure thereby suggesting hippocampal dysfunction (Berman and Hannigan, 2000[1]; Byrnes et al., 2004[3]; Gabriel et al., 2002[13]; Iqbal et al., 2004[19]; Kim et al., 1997[24]; Matthews and Simson, 1998[28]; McAdam et al., 2008[29]; Richardson et al. 2002[40]; Shea et al., 2012[42]).
However, the present study demonstrates that these neurobehavioral impairments caused by prenatal alcohol exposure can be reversed by enriched environmental housing in post-weaning period. Environmental enrichment has been reported to enhance behavioral performance, stimulate CNS development, and facilitate recovery of CNS function (for review see Hannigan et al., 2007[16]; Kelly et al., 2009[23]). Improvements in spatial memory following environmental enrichment have been previously reported (Diniz et al., 2010[11]; Kovesdi et al., 2011[25]; Leggio et al., 2005[26]; Lui et al., 2011[27]; Nilsson et al. 1999[33]; Simao et al., 2012[43]; Speisman et al., 2013[44]). Our findings show significant different in spatial memory acquisition patterns between mice exposed prenatally to alcohol housed in standard cages and housed in enriched environments after weaning. These results are similar to the earlier studies that post-weaning environmental enrichment improved spatial learning deficits due to prenatal alcohol exposure (Hannigan et al., 1993[15]; Wainwright et al., 1993[45]).
In addition to spatial acquisition task, strategy preference was also assessed in this study by evaluating cue and place acquisition task. Evidence suggests that different navigational tasks are likely to be mediated by different neural system depending upon the type of learning involved (Colombo et al., 2003[8]; Devan and White, 1999[10]; McDonald and White, 1994[30]; Oliveira et al., 1997[35]; Packard 1999[36]; Packard and McGaugh, 1996[37]). A spatial/place strategy is dependent upon intact hippocampal circuitry, whereas a response/cue strategy is dependent upon intact striatal circuitry (McDonald and White, 1994[30]; Nicolle et al., 2003[32]). Our study demonstrates that cue and spatial learning abilities were maintained in all groups; however, only the prenatal alcohol-exposed mice exhibited a significant bias toward the cue strategy test. With parallel brain systems underlying place and cue strategies, it is possible that the cue bias in the prenatal alcohol-exposed mice reflects a shift of information processing away from the hippocampal formation toward the dorsal striatal system. These data are supported by several previous works demonstrated that the prenatal ethanol exposure led to the persistent abnormal synaptic plasticity in the striatal dopaminergic system (Carneiro et al., 2005[5]; Schneider et al., 2005[41]; Zhou et al., 2012[47]). However, the novel finding of our experiments is that the post weaning environmental enrichment can ameliorate the impairment of both cue and place strategy tests in animals exposed prenatally to alcohol.
As expected based on cognitive study in Morris water maze task, the present findings demonstrate that the prenatal alcohol exposure caused a significant decrease of BDNF mRNA expression both in the hippocampal formation and the striatum. In this study, the hippocampus and the striatum were chosen because BDNF was highly expressed in these brain regions and because these brain structures were particularly vulnerable to prenatal alcohol treatments (Caldwell et al., 2008[4]; Feng et al., 2005[12]; Parks et al., 2008[38]). BDNF is an important molecular mediator of synaptic and morphological plasticity, and has crucial roles in neurodevelopment and survival (Gomez-Palacio-Schjetnan and Escobar, 2008[14]). Prenatal ethanol exposure associated with reduced BDNF mRNA level in hippocampal formation has previously been reported (Caldwell et al., 2008[4]). However, the reduction of BDNF protein level in the hippocampus appeared to be limited to male rat (Parks et al., 2008[38]). In the striatum, previous study showed that prenatal alcohol exposure at the dose of 1 g/kg/day did not significantly affect BDNF protein levels but at the dose of 3 g/kg/day markedly reduced levels of BDNF protein and mRNA in the hippocampus of offspring (Feng et al., 2005[12]). Since BDNF has been shown to enhance NMDA glutamate receptors (Crozier et al., 2008[9]; Ninan et al., 2010[34]; Wang et al., 2011[46]), and involved in neuroplasticity in term of LTP and LTD in the hippocampus (Bozdagi et al., 2008[2]; Chen et al., 2010[6]; Ikegaya et al., 2002[18]; Jia et al., 2010[20]). Therefore, decrease of BDNF transcription might lead to impairment in a memory test, as shown in the acquisition Morris water maze task in this study. Environmental enrichment has previously been reported to increase the neurotrophin levels in rat brain (Ickes et al., 2000[17]) and have significant consequences for both behavior and morphology of the animal (Pham et al., 1999[39]). Our findings is the first time demonstrated the effect of postweaning environmental enrichment can attenuate the decreased of BDNF mRNA level both in the hippocampal formation and the striatum.
In conclusion, these results suggest that postnatal environment can ameliorate the effects of alcohol-related memory impairment in mice, as well as the expression of BDNF mRNA in the hippocampus and the striatum. This environmental enrichment might be considered as the postnatal factor to attenuate some of the deficits due to prenatal alcohol exposure.
Notes
Corresponding authors: Rungpiyada Tipyasang and Naiphinich Kotchabhakdi
Acknowledgements
This work was supported by a Mahidol University Research Grant to NK.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28:9857–9869. doi: 10.1523/JNEUROSCI.3145-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt-prenatal ethanol exposure. Neurotoxicol Teratol. 2004;26:543–551. doi: 10.1016/j.ntt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, et al. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol Biochem Behav. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, et al. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen LY, Rex CS, Pham DT, Lynch G, Gall CM. BDNF signaling during learning is regionally differentiated within hippocampus. J Neurosci. 2010;30:15097–15101. doi: 10.1523/JNEUROSCI.3549-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- 8.Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crozier RA, Bi C, Han YR, Plummer MR. BDNF modulation of NMDA receptors is activity dependent. J Neurophysiol. 2008;100:3264–3274. doi: 10.1152/jn.90418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diniz DG, Foro CA, Rego CM, Gloria DA, de Oliveira FR, Paes JM, et al. Environmental impoverishment and aging alter object recognition, spatial learning, and dentate gyrus astrocytes. Eur J Neurosci. 2010;32:509–519. doi: 10.1111/j.1460-9568.2010.07296.x. [DOI] [PubMed] [Google Scholar]
- 12.Feng MJ, Yan SE, Yan QS. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring. Brain Res. 2005;1042:125–132. doi: 10.1016/j.brainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel KI, Johnston S, Weinberg J. Prenatal ethanol exposure and spatial navigation: effects of postnatal handling and aging. Develop Psychobiol. 2002;40:345–357. doi: 10.1002/dev.10023. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Palacio-Schjetnan A, Escobar ML. In vivo BDNF modulation of adult functional and morphological synaptic plasticity at hippocampal mossy fibers. Neurosci Lett. 2008;445:62–67. doi: 10.1016/j.neulet.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 15.Hannigan JH, Berman RF, Zajac CS. Environmental enrichment and the behavioral effects of prenatal exposure to alcohol in rats. Neurotoxicol Teratol. 1993;15:261–266. doi: 10.1016/0892-0362(93)90007-b. [DOI] [PubMed] [Google Scholar]
- 16.Hannigan JH, O'Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci Biobehav Rev. 2007;31:202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- 18.Ikegaya Y, Ishizaka Y, Matsuki N. BDNF attenuates hippocampal LTD via activation of phospholipase C: implications for a vertical shift in the frequency-response curve of synaptic plasticity. Eur J Neurosci. 2002;16:145–148. doi: 10.1046/j.1460-9568.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal U, Dringenberg HC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters hippocampal GABA(A) receptors and impairs spatial learning in the guinea pig. Behav Brain Res. 2004;150:117–125. doi: 10.1016/S0166-4328(03)00246-8. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 22.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- 23.Kelly SJ, Goodlett CR, Hannigan JH. Animal models of fetal alcohol spectrum disorders: impact of the social environment. Dev Disabil Res Rev. 2009;15:200–208. doi: 10.1002/ddrr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, et al. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behav Neurosci. 1997;111:985–995. doi: 10.1037//0735-7044.111.5.985. [DOI] [PubMed] [Google Scholar]
- 25.Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, et al. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Lui CC, Wang JY, Tain YL, Chen YC, Chang KA, Lai MC, et al. Prenatal stress in rat causes long-term spatial memory deficit and hippocampus MRI abnormality: differential effects of postweaning enriched environment. Neurochem Int. 2011;58:434–441. doi: 10.1016/j.neuint.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DB, Simson PE. Prenatal exposure to ethanol disrupts spatial memory: effect of the training-testing delay period. Physiol Behav. 1998;64:63–67. doi: 10.1016/s0031-9384(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 29.McAdam TD, Brien JF, Reynolds JN, Dringenberg HC. Altered water-maze search behavior in adult guinea pigs following chronic prenatal ethanol exposure: lack of mitigation by postnatal fluoxetine treatment. Behav Brain Res. 2008;191:202–209. doi: 10.1016/j.bbr.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 30.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 31.Middaugh LD, Boggan WO, Bingel SA, Patrick KS, Xu W. A murine model of prenatal cocaine exposure: effects on the mother and the fetus. Pharmacol Biochem Behav. 1996;55:565–574. doi: 10.1016/s0091-3057(96)00250-x. [DOI] [PubMed] [Google Scholar]
- 32.Nicolle MM, Prescott S, Bizon JL. Emergence of a cue strategy preference on the water maze task in aged C57B6 x SJL F1 hybrid mice. Learn Mem. 2003;10:520–524. doi: 10.1101/lm.64803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira MG, Bueno OF, Pomarico AC, Gugliano EB. Strategies used by hippocampal- and caudate-putamen-lesioned rats in a learning task. Neurobiol Learn Mem. 1997;68:32–41. doi: 10.1006/nlme.1996.3761. [DOI] [PubMed] [Google Scholar]
- 36.Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 38.Parks EA, McMechan AP, Hannigan JH, Berman RF. Environmental enrichment alters neurotrophin levels after fetal alcohol exposure in rats. Alcohol Clin Exp Res. 2008;32:1741–1751. doi: 10.1111/j.1530-0277.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- 40.Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- 41.Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, et al. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res. 2005;29:1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- 42.Shea KM, Hewitt AJ, Olmstead MC, Brien JF, Reynolds JN. Maternal ethanol consumption by pregnant guinea pigs causes neurobehavioral deficits and increases ethanol preference in offspring. Behav Pharmacol. 2012;23:105–112. doi: 10.1097/FBP.0b013e32834ed866. [DOI] [PubMed] [Google Scholar]
- 43.Simao F, Porto JA, Nunes ML. Effects of enriched environment in spatial learning and memory of immature rats submitted to early undernourish and seizures. Int J Dev Neurosci. 2012;30:363–367. doi: 10.1016/j.ijdevneu.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Speisman RB, Kumar A, Rani A, Pastoriza JM, Severance JE, Foster TC, et al. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol Aging. 2013;34:263–274. doi: 10.1016/j.neurobiolaging.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wainwright PE, Levesque S, Krempulec L, Bulman-Fleming B, McCutcheon D. Effects of environmental enrichment on cortical depth and Morris-maze performance in B6D2F2 mice exposed prenatally to ethanol. Neurotoxicol Teratol. 1993;15:11–20. doi: 10.1016/0892-0362(93)90040-u. [DOI] [PubMed] [Google Scholar]
- 46.Wang FJ, Li CM, Hou XH, Wang XR, Zhang LM. Selective upregulation of brain-derived neurotrophic factor (BDNF) transcripts and BDNF direct induction of activity independent N-methyl-D-aspartate currents in temporal lobe epilepsy patients with hippocampal sclerosis. J Int Med Res. 2011;39:1358–1368. doi: 10.1177/147323001103900422. [DOI] [PubMed] [Google Scholar]
- 47.Zhou R, Wang S, Zhu X. Prenatal ethanol exposure alters synaptic plasticity in the dorsolateral striatum of rat offspring via changing the reactivity of dopamine receptor. PloS One. 2012;7:42443. doi: 10.1371/journal.pone.0042443. [DOI] [PMC free article] [PubMed] [Google Scholar]


