Background: Developmental functions of Sphk1 and Sphk2 remain unclear in vertebrates.
Results: Maternal-zygotic sphk2 zebrafish mutant exhibited cardia bifida, whereas maternal-zygotic sphk1, maternal sphk2, and zygotic sphk2 mutants did not.
Conclusion: Maternal and zygotic Sphk2 cooperatively regulate cardiac development.
Significance: The contribution of maternally supplied lipid mediators presents as a critical requirement for maternal-zygotic Sphk2 during cardiac development.
Keywords: lipid metabolism, lipid signaling, lipid synthesis, lipid transport, sphingolipid, lipid mediator signaling, sphingosine 1-phosphate (S1P), heart development, cell migration, zebrafish, transcription activator-like effector nuclease (TALEN), lipid chromatography-tandem mass spectrometry (LC-MS/MS)
Abstract
Sphingosine 1-phosphate (S1P) is synthesized from sphingosine by sphingosine kinases (SPHK1 and SPHK2) in invertebrates and vertebrates, whereas specific receptors for S1P (S1PRs) selectively appear in vertebrates, suggesting that S1P acquires novel functions in vertebrates. Because the developmental functions of SPHK1 and SPHK2 remain obscure in vertebrates, we generated sphk1 or sphk2 gene-disrupted zebrafish by introducing premature stop codons in their coding regions using transcription activator-like effector nucleases. Both zygotic sphk1 and sphk2 zebrafish mutants exhibited no obvious developmental defects and grew to adults. The maternal-zygotic sphk2 mutant (MZsphk2), but not the maternal-zygotic sphk1 mutant and maternal sphk2 mutant, had a defect in the cardiac progenitor migration and a concomitant decrease in S1P level, leading to a two-heart phenotype (cardia bifida). Cardia bifida in MZsphk2, which was rescued by injecting sphk2 mRNA, was a phenotype identical to that of zygotic mutants of the S1P transporter spns2 and S1P receptor s1pr2, indicating that the Sphk2-Spns2-S1pr2 axis regulates the cardiac progenitor migration in zebrafish. The contribution of maternally supplied lipid mediators during vertebrate organogenesis presents as a requirement for maternal-zygotic Sphk2.
Introduction
Sphingosine 1-phosphate (S1P)3 is a bioactive lipid mediator that plays important roles in regulating crucial cellular processes, such as cell proliferation, migration, and differentiation (1, 2). S1P is intracellularly synthesized from sphingosine by sphingosine kinases (SPHK1 and SPHK2) and is released by S1P transporters, including SPNS2 (3, 4). The S1P released is recognized by G protein-coupled S1P receptors (S1PR1-S1PR5), leading to the activation of various downstream signaling pathways (5, 6). S1P also functions as an intracellular second messenger (7, 8). Conversely, various physiological roles, such as lymphocyte egress, vascular development, and bone homeostasis, are mediated by the intercellular S1P signaling pathway because these biological activities are affected in S1P receptors or transporter KO mice (9–12).
Both SPHK1 and SPHK2 contain conserved domains responsible for substrate binding and catalytic activity (13, 14). However, there are differences in cellular localization and substrate specificity (2, 6). Human SPHK1 is localized in the cytosol and translocated to the plasma membrane by various extracellular stimuli, whereas human SPHK2 localizes to the mitochondria, endoplasmic reticulum, and nucleus (5). Sphingosine and dihydro-sphingosine can be endogenous substrates of both SPHK1 and SPHK2. However, phyto-sphingosine and FTY720, which are sphingosine analogues, are preferentially catalyzed by SPHK2 (15–17); a phosphorylated form of FTY720 acts as an immunosuppressive agent through the S1PR1 receptor on mature lymphocytes (15, 16).
Sphk1 or Sphk2 KO mice have been generated, and their loss-of-function phenotypes were analyzed to reveal the physiological roles of SPHKs. Sphk1 or Sphk2 single KO mice are viable and fertile; however, Sphk1/2 double KO mice are embryonic lethal because of severe defects in the vascular and neural development (18, 19). Furthermore, Sphk1−/−Sphk2+/− female mice are infertile due to defective decidualization, whereas Sphk1 or Sphk2 single KO female mice are normally fertile (20). Taken together, SPHK1 and SPHK2 redundantly function during embryonic development.
S1P signaling is involved in cardiac and lower jaw development in zebrafish (11, 21, 22). Zygotic zebrafish mutants for s1pr2 (S1P receptor) and spns2 (S1P transporter) exhibit a defect in the cardiac progenitor migration, resulting in a two-heart phenotype known as cardia bifida (23–25). Furthermore, Gα13/RhoGEF signaling has been identified downstream of S1pr2 in the endoderm (26); however, upstream molecules in this signaling pathway and the maternal contribution of S1P signaling remain obscure. Zebrafish is an ideal model organism to investigate the contribution of maternal factors, such as mRNAs, proteins, lipids, and nutrients, because zygotic gene expression begins around the 1,000-cell stage; thus, initial embryogenesis is regulated by maternal factors stored in blastomeres and yolk (27, 28). In clear contrast, zygotic expression starts in mice from the initial stage (two-cell stage), and proteins, lipids, and nutrients are supplied from the mother through the placenta (28). Thus, it is difficult to examine the contribution of maternal factors during early mammalian embryogenesis. Several mRNAs for S1P signaling-related molecules (s1pr2, spp2, and sphk2) are maternally supplied in zebrafish (29); however, their functions as maternal factors are not completely understood.
Recently, engineered nucleases, such as transcription activator-like effector nucleases (TALENs) and RNA-guided nucleases based on the type II bacterial clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) 9 system, have emerged and are useful in zebrafish (30, 31). These engineered nucleases induce DNA double-strand breaks in the targeted genomic locus, which could be repaired through an error-prone non-homologous end joining, leading to insertion and/or deletion mutations at the target site and to the frameshift-mediated gene disruption. To date, zebrafish is a valuable model vertebrate for maternal and/or zygotic mutant analyses, as they have both the forward and the reverse genetics.
In this study, we generated sphk1 and sphk2 mutant zebrafish using TALENs and analyzed the maternal and zygotic effects of sphk1/2 during embryonic development.
Experimental Procedures
Zebrafish Mutants
Mutant alleles of spns2ko157 and s1pr2ko322 were used (21, 23, 32). sphk1 and sphk2 mutants were generated as described below.
Construction of TALEN Plasmids
The plasmids for synthesizing TALEN mRNAs were constructed in a two-step assembly system as described previously with some modifications (32, 33). Six or fewer TAL effector repeat modules were ligated into pFUS vectors (intermediate array vectors) as the first step (34). Subsequently, the intermediate array vectors and last TAL effector repeat were ligated into the pCS2TAL3DD vector for a forward TALEN or the pCS2TAL3RR vector for a reverse TALEN as the second step (35). The amino acid sequences of the constructed TALENs are shown in Table 1.
TABLE 1.
Amino acid sequences of TALEN constructs
The repeat variable di-residue (RVD) sequences are indicated as red letters in the coding sequences of the indicated TALEN constructs.

Preparation of mRNAs and Antisense Morpholino Oligomer
The plasmids for synthesizing TALEN, zebrafish sphk2, human SPHK1, or human SPHK2 mRNA were linearized by NotI digestion, and mRNAs were transcribed using the mMESSAGE mMACHINE SP6 kit (Life Technologies) and purified using the RNeasy mini kit (Qiagen). Morpholino oligomer (MO) for sphk2 (5′-AGCTCAAGTACATTTCATACCCAGC-3′) was purchased from Gene Tools.
Microinjection
Forward and reverse TALEN mRNAs (400 pg each) were injected together into the blastomeres of one-cell stage zebrafish embryos. MO and the synthesized mRNAs were dissolved in the injection buffer (40 mm HEPES (pH 7.4), 240 mm KCl, and 0.5% phenol red). The synthesized mRNA (1 pg) or MO (5 ng) was injected into one-cell stage zebrafish embryos or the yolk syncytial layer (YSL) around high stage embryos. Injection into the YSL was confirmed by the distribution of co-injected rhodamine-dextran (Sigma).
RNA Probes and Whole-mount in Situ Hybridization
RNA probes labeled with digoxigenin for cmlc2, amhc, vmhc, and sphk2 were prepared using a RNA labeling kit (Roche Applied Science). Whole-mount in situ hybridization was performed as described previously (36).
Genotyping of Mutant Alleles
Zebrafish embryos or fin clips were incubated in 50 μl of lysis buffer (10 mm Tris-HCl (pH 8.0), 1 mm EDTA, 0.2% Triton X-100, and 200 μg/ml proteinase K) at 55 °C for 3 h. Then, the solution was incubated at 100 °C for 10 min to inactivate the proteinase K. The TALEN target loci were amplified using the supernatants as templates and the following primers: Sphk1 forward, 5′-ACCTGTGTTTGTATGCGTGTGC-3′ and Sphk1 reverse, 5′-TGTGTCTGCTCACCGTGTGTAA-3′ for the sphk1-TALEN target locus; Sphk2 forward, 5′-GCCAGATTGGGAACAAGCAATA-3′ and Sphk2 reverse, 5′-CAGCATGGTGGTTGATGGAAC-3′ for the sphk2-TALEN target locus. PCR amplicons were electrophoresed on 15% polyacrylamide gels, and individuals were genotyped by heteroduplex mobility assay as described previously (37).
Alcian Blue Staining
Embryos were fixed at 3 days post-fertilization (dpf) in 4% paraformaldehyde in PBS and washed with acid alcohol buffer (0.37% HCl and 70% ethanol). After the incubation with 0.1% Alcian blue (Sigma) in acid alcohol buffer, the embryos were washed thrice with acid alcohol buffer.
Lysosphingolipid Analysis by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
Ten and five embryos were collected in a 1.5-ml siliconized tube at the two-cell stage and 5 dpf stage, respectively, and homogenized with a ultrasonic homogenizer (Smurt NR-50M, Microtech) in 200 μl of methanol in the presence of 1 μm dihydro-S1P (C17) as an internal standard. After further bath sonication for 10 min, the samples were centrifuged at 15,000 rpm for 10 min at 4 °C. The resulting supernatants were passed through a filter (0.22-μm pore size), and 10 μl of the filtrate was subjected to the LC-MS/MS analysis as described previously with some modifications (38, 39). LC separation was performed using a CAPCELLPAK C18 ACR 1.5 × 100-mm column (Shiseido) with a gradient elution of solvent A (5 mm ammonium formate in water, pH 4.0) and solvent B (5 mm ammonium formate in 95% (v/v) acetonitrile, pH 4.0) at 250 μl/min. The initial conditions were set to 50% solvent B. The following solvent gradient was applied: maintain 50% solvent B for 0.2 min, followed by a linear gradient to 95% solvent B from 0.2 to 2.8 min, and hold at 95% solvent B for 2.2 min. Subsequently, the mobile phase was immediately returned to the initial conditions and maintained for 1.4 min. The ratio of an analyte peak area to the internal standard peak area was used for quantification. Lipid content was expressed as pmol per embryo.
Results
Generation of sphk1 and sphk2 Mutant Zebrafish
S1P is synthesized from sphingosine by SPHK1 and SPHK2. Because the developmental functions of SPHK1 and SPHK2 during vertebrate embryogenesis are not completely understood, we generated sphk1 and sphk2 gene-disrupted zebrafish using TALENs. The sphk1 and sphk2 TALENs were designed to target the third and fourth exons, respectively (Fig. 1A). Their in vivo activities of inducing insertion and/or deletion mutations at the targeted genomic loci were assessed by heteroduplex mobility assay (Fig. 1D). Individual TALEN-injected F0 founders were mated with wild-type zebrafish to establish the individual mutant lines. We identified the TALEN-mediated frameshift mutations in the sphk1ko811 and sphk2ko822 mutants: the sphk1ko811 allele, with 13 deleted bases, composed of 74 correct amino acids from the start codon and 39 incorrect amino acids (Figs. 1B and 2), and the sphk2ko822 allele, with two deleted bases and three inserted bases, composed of 257 correct amino acids from the start codon and 16 incorrect amino acids (Figs. 1C and 2). Characteristic domains, such as ATP-binding domain and sphingosine-binding domain, are present in mammalian SPHK1 and SPHK2, and both domains are conserved in zebrafish (13, 14). The 13-base deletion of the sphk1ko811 allele occurred inside the ATP-binding domain. Both the sphk1ko811 and the sphk2ko822 alleles lacked the sphingosine-binding domain (Fig. 2). Therefore, Sphk1ko811 and Sphk2ko822 were expected to lose their inherent S1P producing activities.
FIGURE 1.
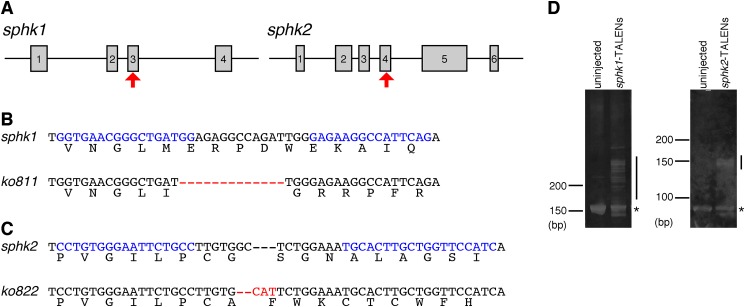
Generation of sphk1 and sphk2 mutant zebrafish. A, schematic representation of the sphk1 and sphk2 genes. Exons are shown as gray boxes with numbers. The TALEN-targeted genomic sites are indicated by red arrows. B and C, sequence alignment of the TALEN-generated alleles for sphk1 (B) and sphk2 (C). The 13 residues in sphk1ko811 indicated as red bars were deleted. Two residues in sphk2ko822 were deleted (red bars), and three residues were inserted (red letters). TALEN-recognizing sequences are indicated by blue letters. D, heteroduplex mobility assay to detect sphk1 and sphk2 TALEN-induced insertion and/or deletion mutations. Individual TALENs were injected into zebrafish embryos, and individual TALEN target regions were amplified from the genomic DNA of uninjected and TALEN-injected embryos. Individual PCR products were separated by 15% polyacrylamide gel electrophoresis. The expected homoduplex and multiple heteroduplex bands are indicated by asterisks and lines, respectively.
FIGURE 2.

Amino acid sequence alignments of mammalian and zebrafish sphingosine kinases. The ATP-binding domain and sphingosine (Sph)-binding domain are highlighted in yellow and blue, respectively. The frameshift-derived incorrectly translated amino acids are shown in red. hSPHK1, human SPHK1; hSPHK2, human SPHK2; mSPHK1, mouse SPHK1; mSPHK2, mouse SPHK2; zSphk1, zebrafish Sphk1; zSphk2, zebrafish Sphk2.
Maternal and Zygotic sphk2 Are Involved in Cardiac Progenitor Migration
We examined the zygotic sphk1 and sphk2 homozygous mutant phenotypes by crossing individual heterozygous males and females. Neither the zygotic sphk1 nor the sphk2 mutant exhibited any obvious developmental defects and grew to adults. Thereafter, we examined the developmental phenotypes of maternal and maternal-zygotic sphk1 or sphk2 gene mutants. As shown in Fig. 3, the maternal-zygotic sphk2 mutant embryos (MZsphk2) exhibited cardia bifida-like zygotic s1pr2 or spns2 mutants, whereas the maternal sphk2 mutant embryos (Msphk2) showed no obvious developmental defects similar to zygotic sphk2 mutant. In clear contrast, the maternal-zygotic sphk1 mutant embryos (MZsphk1) developed normally without cardia bifida and grew to adults (Fig. 3, E and L). Given that maternal and zygotic sphk2, but not maternal and zygotic sphk1, are required for cardiac development, we focused our study on the sphk2 gene.
FIGURE 3.
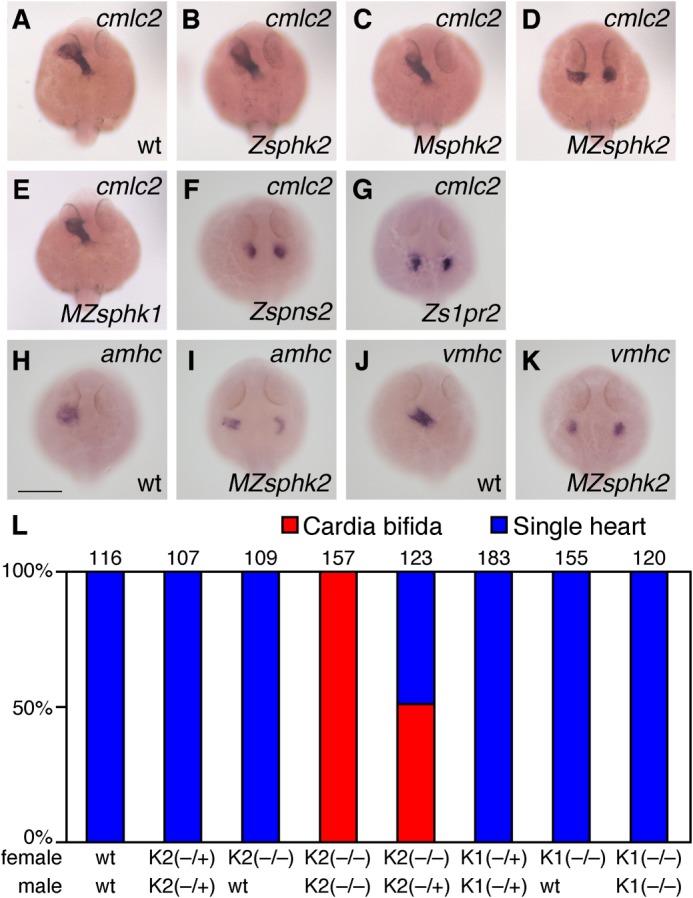
Maternally and zygotically supplied sphk2, but not sphk1, is indispensable for cardiac progenitor migrations. A–G, whole-mount in situ hybridization of cmlc2. Mating a homozygous sphk2 (−/−) mutant female with a heterozygous (−/+) sphk2 male produced 50% maternal-zygotic sphk2 mutants (MZsphk2) and maternal sphk2 mutants (Msphk2). Mating a heterozygous (−/+) sphk2 female with a heterozygous (−/+) sphk2 male produced 25% zygotic sphk2 mutants (Zsphk2). The expression of cmlc2 in wt embryos (A), Zsphk2 mutants (B), Msphk2 mutants (C), MZsphk2 mutants (D), MZsphk1 mutants (E), zygotic spns2 (Zspns2) (F), and s1pr2 (Zs1pr2) (G) at 24 h post-fertilization is shown. H–K, whole-mount in situ hybridization of the cardiac markers, atrial myosin heavy chain (amhc) and ventricular myosin heavy chain (vmhc). L, embryos exhibiting the two-heart phenotype, known as cardia bifida (red), or a normal single heart (blue) were quantified. The total number of embryos is shown at the top of each column. K1, sphk1; K2, sphk2. Mating was performed as indicated below the column (+, wild-type allele; −, mutant allele). Scale bar, 200 μm.
The expression of myocardial markers (cmlc2) and chamber-specific markers (amhc and vmhc) was detected in two separated domains (Fig. 3, D, I, and K). The separated hearts exhibited rhythmic beating (supplemental movies), suggesting that the cardiac progenitors differentiated into cardiomyocytes at bilateral positions.
sphk2 mRNA Expression during Embryogenesis
Whole-mount in situ hybridization analysis was performed to investigate sphk2 expression patterns during embryogenesis (Fig. 4). sphk2 expression was strongly detected at the one-cell stage (Fig. 4A), suggesting that sphk2 mRNA is maternally supplied and consistent with a previous observation analyzed by quantitative real time PCR (29). Furthermore, sphk2 transcripts in the YSL were detected from the dome stage to the 20-somite stage (Fig. 4, B–F and J). sphk2 was transiently expressed in developing somites at the 20-somite stage (Fig. 4G).
FIGURE 4.
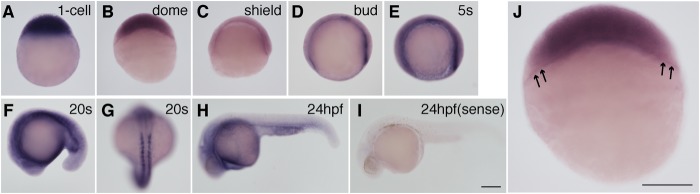
Expression of sphk2 during embryogenesis was examined by whole-mount in situ hybridization. A–J, pictures present lateral views (A–F and H–J) and dorsal views (G): from the bud to the 20-somite stages presented anterior to the left (D–F, H, and I). J, magnified image of a dome stage embryo. sphk2 transcripts were detected in the blastoderm and the YSL, as indicated by the arrows. Scale bar, 200 μm.
Requirement of Zygotic sphk2 in the YSL during Cardiac Progenitor Migration
We knocked down zygotic sphk2 by injecting a splice-blocking MO (sphk2-MO) into Msphk2 embryos obtained from mating sphk2ko822/ko822 females with wild-type males to delete maternal sphk2 and confirm the requirement for zygotic sphk2 during cardiac development (Fig. 5). When sphk2-MO was injected into Msphk2 embryos at the one-cell stage, cardia bifida was observed (Fig. 5, B and G). sphk2 expression in the YSL (Fig. 4) prompted us to perform a YSL-specific knockdown analysis. Cardia bifida was observed in Msphk2 embryos injected with sphk2-MO into the YSL around high stage embryos (Fig. 5, C and G). These results were consistent with the finding that both maternally and zygotically supplied sphk2 are involved in cardiac development, suggesting the importance of zygotically supplied sphk2 mRNA in the YSL.
FIGURE 5.
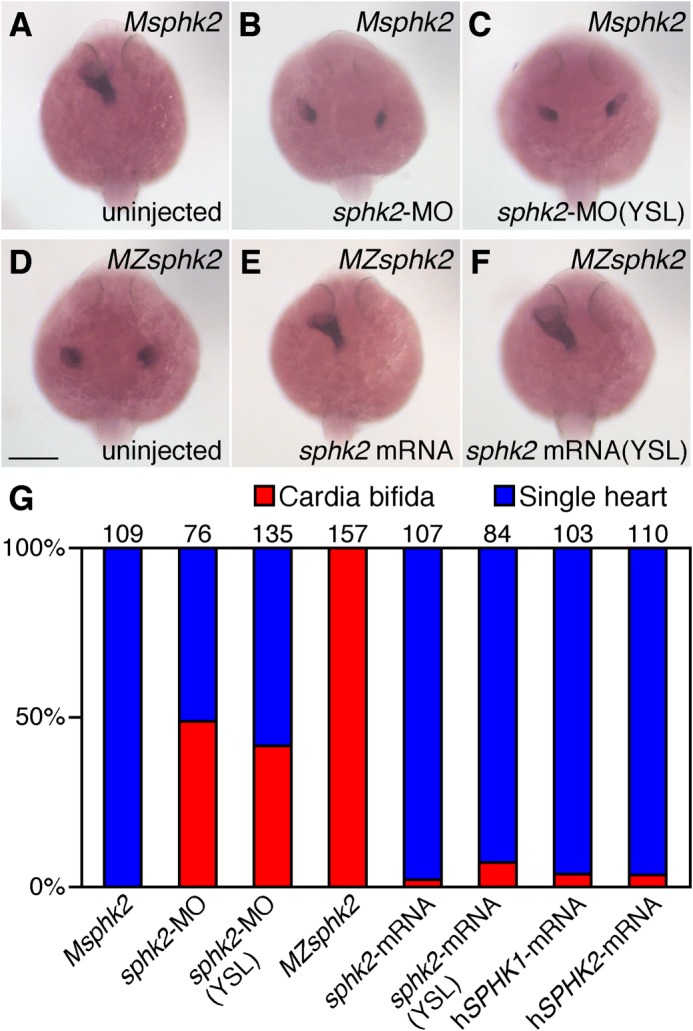
sphk2 knockdown and rescue experiments. A–F, whole-mount in situ hybridization of cmlc2. A–C, mating a homozygous sphk2 (−/−) mutant female with a wild-type male produced 100% Msphk2 mutants. Msphk2 one-cell stage embryos were injected with sphk2-morpholino oligomer (5 ng) into the yolk at one-cell stage embryos (B) or into the YSL around high stage embryos as confirmed by the distribution of co-injected rhodaminedextran (C). D–F, MZsphk2 embryos were injected with synthesized sphk2 mRNA (1 pg) into the yolk at one-cell stage embryos (E) or into the YSL around high stage embryos as confirmed by the distribution of co-injected rhodaminedextran (F). G, embryos exhibiting cardia bifida (red) or a normal single heart (blue) were quantified. Human SPHK1 or SPHK2 mRNA was injected into MZsphk2 embryos at the one-cell stage. The total number of embryos in each treatment is shown on the top of each column. Scale bar, 200 μm.
sphk mRNA Injection Rescues the MZsphk2 Cardiac Defect
We performed rescue experiments using synthetic sphk2 mRNA to verify that the cardia bifida in MZsphk2 embryos was caused by the sphk2 gene mutation. Cardia bifida in MZsphk2 embryos was rescued by the injecting sphk2 mRNA at the one-cell stage embryos (Fig. 5, E and G). Furthermore, YSL-specific injection of sphk2 mRNA rescued the cardiac defect (Fig. 5, F and G). These results suggest that injecting sphk2 mRNA into the YSL is sufficient to produce the S1P required for migration of the cardiac progenitors. Injection of both human SPHK2 mRNA and human SPHK1 mRNA into one-cell stage MZsphk2 embryos also rescued the cardiac defect (Fig. 5G), suggesting the importance of S1P production by sphingosine kinases in zebrafish cardiac development (Fig. 5G).
Sphk2 Is Genetically Located Upstream of the Spns2-S1pr2 Signaling
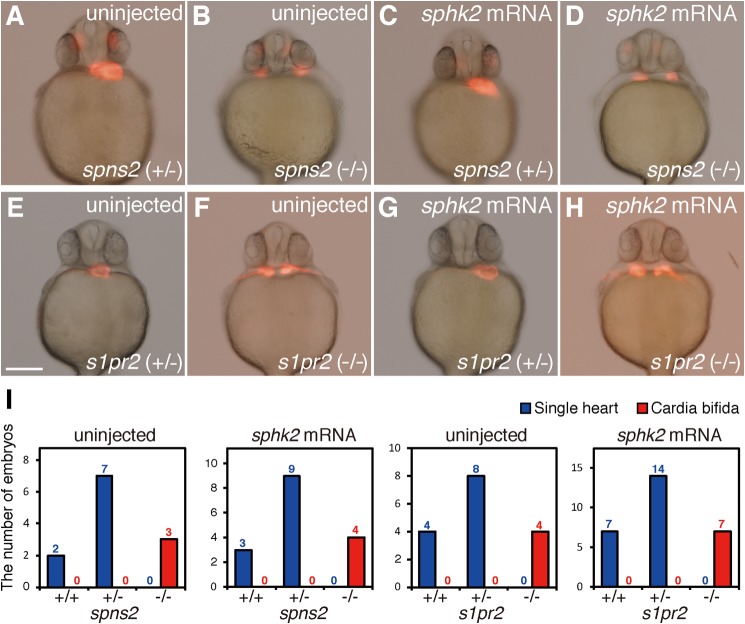
We examined whether zygotic spns2 and s1pr2 mutants were rescued or not by sphk2 mRNA injection. Zebrafish sphk2 mRNA was injected into one-cell stage embryos from heterozygous spns2 or s1pr2 mutants (23, 25). The cardia bifida phenotype in spns2 or s1pr2 mutants was not rescued by sphk2 mRNA injection (Fig. 6), suggesting that Sphk2 acts upstream of Spns2 and S1pr2 in the cardiac development as expected by their molecular functions.
FIGURE 6.
sphk2 mRNA injection into zygotic s1pr2 mutant or spns2 mutant embryos. A–D, embryos obtained from mating male and female s1pr2 (−/+) were injected with synthesized sphk2 mRNA (1 pg) into a blastomere at the one-cell stage. After taking pictures of phenotypic analysis, genomic DNAs were prepared from individual embryos, and genotyping of s1pr2 mutant was determined by heteroduplex mobility assay. E–H, embryos obtained from mating male and female spns2 (−/+) were injected with synthesized sphk2 mRNA (1 pg) into a blastomere at the one-cell stage. After taking pictures of phenotypic analysis, genomic DNAs were prepared from individual embryos, and genotyping of spns2 mutant was determined by direct sequence of spns2 locus. I, embryos exhibiting cardia bifida (two-heart) or a normal single heart were quantified. The total number of embryos is shown on the top of each column. Scale bar, 200 μm.
Lysosphingolipid Levels in Zebrafish Embryos
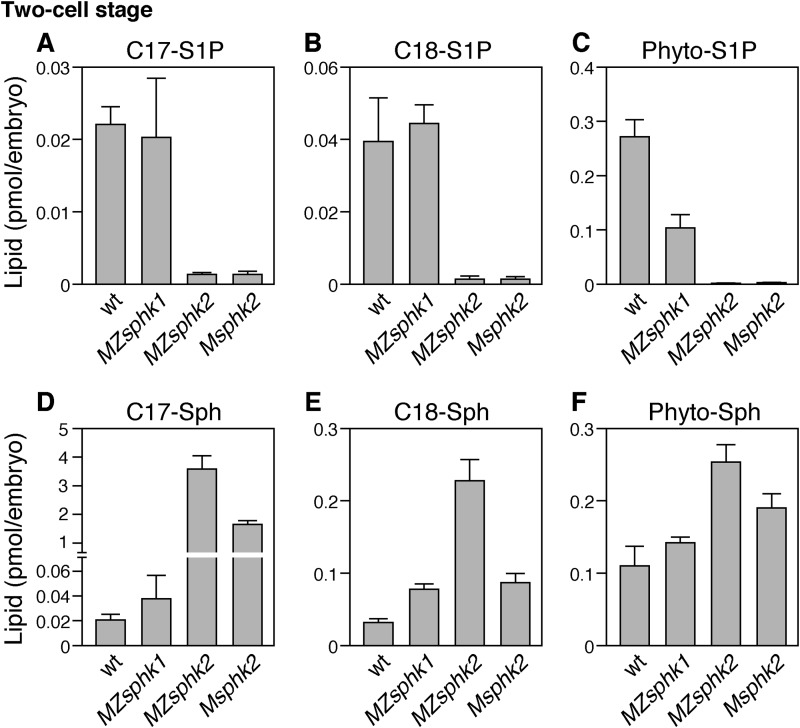
Two-cell stage embryos were collected to eliminate unfertilized eggs, and lysosphingolipid levels in the embryos were measured by LC-MS/MS. As shown in Fig. 7, about 0.02 pmol of C17-S1P, 0.04 pmol of C18-S1P, and 0.27 pmol of phyto-S1P exist in a fertilized wild-type egg. The quantities of these lysosphingolipid in MZsphk2 were considerably lower when compared with those in the wild type, whereas precursors, such as C17-sphingosine, C18-sphingosine, and phyto-sphingosine, increased. In Msphk2 embryos, the quantities of C17-S1P, C18-S1P, and phyto-S1P were also decreased to levels comparable with those in MZsphk2, whereas that of C18-sphingosine did not increase. Furthermore, the quantities of C17-S1P and C18-S1P remained unchanged in MZsphk1, whereas that of phyto-S1P decreased to 40% of that in the wild type, suggesting a functional requirement of sphk1 for phyto-S1P production. These data suggest that the S1P accumulating in the two-cell stage embryos is mainly dependent on maternal sphk2.
FIGURE 7.
Lysosphingolipid contents in fertilized eggs. Two-cell stage embryos were collected from wt, MZsphk1, MZsphk2, and Msphk2. A–F, the quantities of C17-S1P (A), C18-S1P (B), phyto-S1P (C), C17-sphingosine (C17-Sph) (D), C18-sphingosine (C18-Sph) (E), and phyto-sphingosine (Phyto-Sph) (F) were quantified by liquid chromatography-tandem mass spectrometry. Bars and error bars represent means ± S.D., respectively, from five experiments.
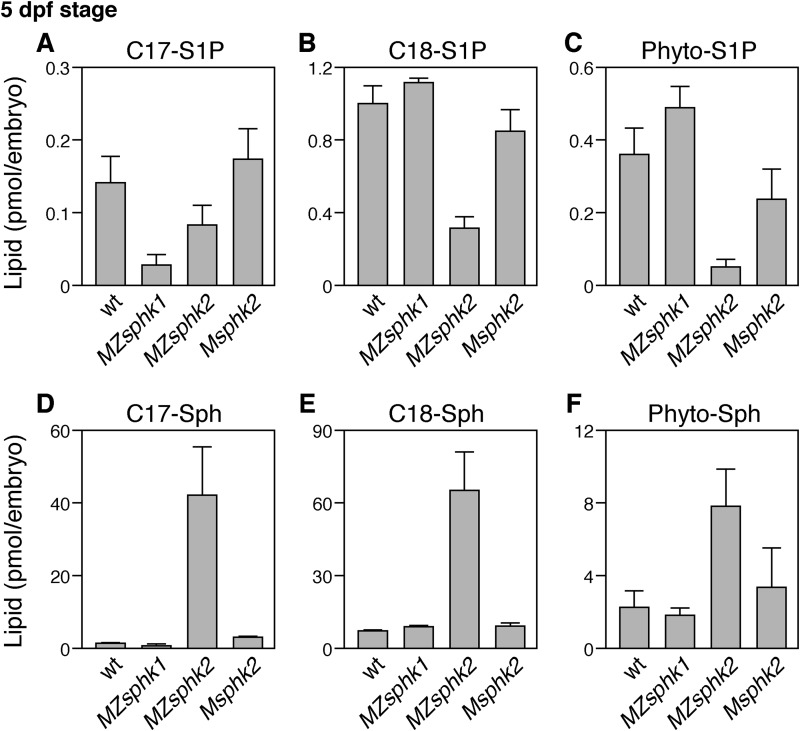
Furthermore, sphingolipid levels at 5 dpf embryos were measured to examine the roles of zygotic sphingosine kinases. About 0.15 pmol of C17-S1P, 1 pmol of C18-S1P, and 0.4 pmol of phyto-S1P exist in a 5 dpf wild-type embryo (Fig. 8). The quantity of C17-S1P was decreased in MZsphk1. The quantities of C18-S1P and phyto-S1P were decreased in MZsphk2 but not in Msphk2, suggesting that S1P synthesis at 5 dpf embryos was mainly dependent on zygotic sphks.
FIGURE 8.
Lysosphingolipid contents at 5 dpf embryos. Embryos were collected from wt, MZsphk1, MZsphk2, and Msphk2. A–F, the quantities of C17-S1P (A), C18-S1P (B), phyto-S1P (C), C17-sphingosine (C17-Sph) (D), C18-sphingosine (C18-Sph) (E), and phyto-sphingosine (Phyto-Sph) (F) were quantified by liquid chromatography-tandem mass spectrometry. Bars and error bars represent means + S.D., respectively, from five experiments.
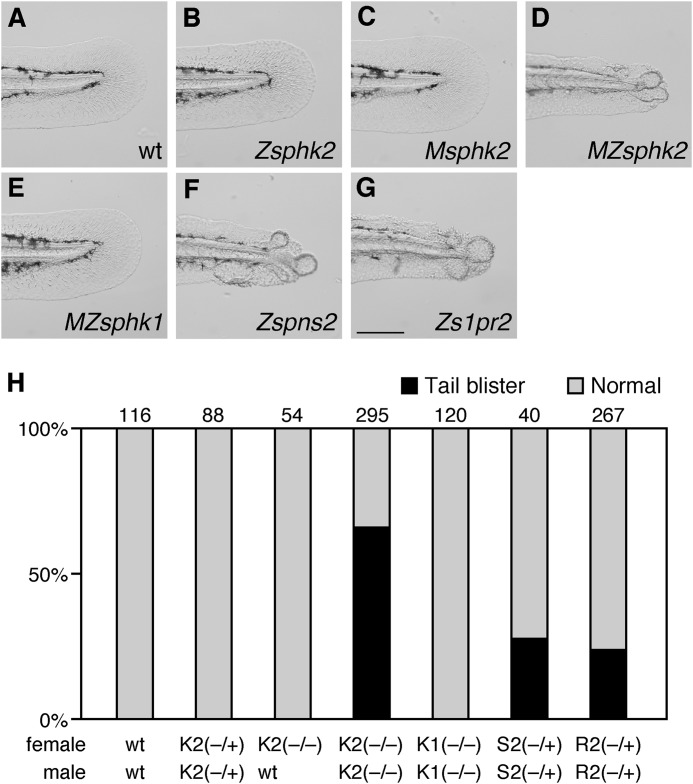
Tail Blister and Disorganized Jaw Phenotypes in MZsphk2
In addition to cardiac defect, both s1pr2 and spns2 mutants exhibited a tail blister phenotype that has not been observed in other characterized cardia bifida mutants (23–25). Cardia bifida and tail blister phenotypes were simultaneously exhibited in about 25% of the progeny from a spns2ko157/+ or s1pr2ko322/+ incross, which is equivalent to a Mendelian ratio of a homozygous mutant (Fig. 9, F–H), suggesting that all zygotic spns2 or s1pr2 mutants are present as the tail blister phenotype. MZsphk2 embryos, but not MZsphk1, Msphk2, or zygotic sphk2 mutant embryos, exhibited tail blisters (Fig. 9), suggesting that both maternal and zygotic sphk2 are also involved in fin morphology. However, unlike the spns2 or s1pr2 mutants, ∼66% of MZsphk2 embryos exhibited tail blisters, whereas cardia bifida was observed in all MZsphk2 embryos (Figs. 3L and 9H).
FIGURE 9.
MZsphk2 embryos exhibit the tail blister phenotype. A–G, lateral bright field images of the medial fin at 2 dpf embryos. MZsphk2, zygotic spns2 (Zspns2), and zygotic s1pr2 (Zs1pr2) embryos show the tail blister phenotype, whereas wt, MZsphk1, zygotic sphk2 (Zsphk2), and Msphk2 embryos show normal fins. H, embryos exhibiting the tail blister (black) or normal fin (gray) phenotypes were quantified. The total number of embryos is shown on the top of each column. K1, sphk1; K2, sphk2; S2, spns2; R2, s1pr2. Mating was performed as indicated below the column (+, wild-type allele; −, mutant allele). Scale bar, 200 μm.
Spns2-S1pr2 signaling also promotes the formation of the ventral pharyngeal cartilage, and zygotic spns2 or s1pr2 mutants exhibit lower jaw developmental defect (21, 22). We analyzed the pharyngeal arch structure of MZsphk1 and MZsphk2 embryos with Alcian blue staining. Lower jaw morphology was disorganized in MZsphk2 embryos, but not in MZsphk1 embryos (Fig. 10), indicating that Sphk2 produces the S1P required for lower jaw development.
FIGURE 10.
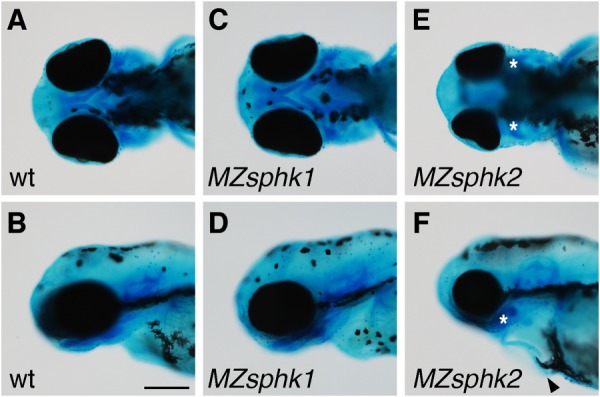
Lower jaw morphology. A–F, lower jaw morphology of wt (A and B), MZsphk1 (C and D), and MZsphk2 embryos (E and F) at 3 dpf was visualized with Alcian blue staining (A, C, and E, ventral view; B, D, and F, lateral view). The ventral pharyngeal arches of MZsphk2, but not MZsphk1 embryos, were disorganized; vestiges are shown as asterisks. Cardiac edema was also observed in MZsphk2 embryos, as shown by the black arrowhead (F). Scale bar, 200 μm.
Discussion
In this study, we present genetic evidence that both maternal and zygotic Sphk2 regulate the cardiac progenitor migration after analyzing sphk zebrafish mutants generated with TALENs. Sphk1/2 double KO mice, but not Sphk1 or Sphk2 single KO mice, exhibited severe defects in the vascular and neural development (18, 19); however, the physiological roles specific to individual SPHKs remain unresolved. MZsphk2 embryos, but not MZsphk1 embryos, exhibited the impaired cardiac progenitor migration and disorganized tail and lower jaw morphologies, presenting the Sphk2-specific roles during early zebrafish embryogenesis. The tail blister phenotype was observed in ∼66% of MZsphk2 embryos (Fig. 9H), whereas the homozygous spns2 or s1pr2 mutant simultaneously exhibited cardia bifida and tail blister phenotypes, and all MZsphk2 exhibited cardia bifida, suggesting an auxiliary role for Sphk1 in fin morphogenesis. Unlike MZsphk2, MZsphk1 fish were viable and fertile without any apparent developmental defects. Sphk1 may play physiological roles during later developmental stages cooperatively with Sphk2. We may have observed Sphk1-specific phenotypes when we performed some stress tests or perturbations. There are possibilities that the zebrafish genome may have other sphks from genome duplication and that S1P could be produced by an uncharacterized Sphk-independent pathway(s).
Previous studies have shown that S1P signaling through Spns2 and S1pr2 regulates the cardiac progenitor migration (23–25). Although s1pr2 transcripts were detected in the endoderm and cardiac progenitors, Gα13/RhoGEF activation in the endoderm downstream of S1pr2 is essential for cardiac progenitor migration (26). spns2 transcripts were expressed in the YSL, which is a transient extra-embryonic syncytial tissue formed by ventrally located blastomeres around the 1,000-cell stage. Subsequently, the yolk and blastoderm are physically separated by the YSL, and yolk contents (mRNAs, proteins, etc.) cannot easily diffuse into the blastoderm after the YSL formation (40). We found that YSL-specific knockdown of spns2 by spns2-specific MO induced cardia bifida (23), suggesting that Spns2 supplies the S1P from the YSL to S1pr2 in the endoderm, resulting in the activation of Gα13/RhoGEF to regulate cardiac progenitor migration. In this study, we demonstrated that sphk2 required for cardiac progenitor migration is supplied maternally and zygotically, whereas the loss of zygotically supplied s1pr2 or spns2 induced cardia bifida. Zygotic sphk2 mutant did not exhibit cardia bifida, probably because maternally supplied S1P is accumulated in the yolk and can recover the loss of S1P derived from zygotic sphk2. Conversely, the depletion of maternally derived S1P could have been recovered by sphk2 expressed in the YSL during cardiac development.
Fertilized eggs contain significant quantities of S1P (Fig. 7, A and B), which dramatically decreased in MZsphk2 and Msphk2 embryos, suggesting that most of the S1P is not produced by zygotic Sphk2 but by maternal Sphk2 at the two-cell stage. At the 5 dpf stage, however, quantity of the abundant S1P species, C18-S1P, is reduced in MZsphk2 embryos, but almost unchanged in Msphk2 or MZsphk1 embryos (Fig. 8B), showing that zygotic Sphk2 is a major S1P supplier at this stage. The reduced S1P level was reflected in concomitant accumulation of a metabolic precursor, sphingosine (Fig. 8E). Thus, the biochemical data clearly demonstrate that both maternal Sphk2 and zygotic Sphk2 can supply S1P in embryos (or fertilized eggs), which is consistent with the genetic evidence that both maternal and zygotic sphk2 are required for the cardiac progenitor migration.
We detected sphk2 transcripts in the YSL at the dome stage (Fig. 4J), and YSL-specific knockdown of sphk2 in Msphk2 embryos induced cardia bifida (Fig. 5C), suggesting that some of the zygotically supplied sphk2 was located in the YSL to produce S1P, which can be efficiently transported by Spns2. In addition, injecting not only human SPHK2 but also human SPHK1 mRNA rescued the cardiac defect of MZsphk2 (Fig. 5G), suggesting that human SPHK1 and SPHK2 have an ability to phosphorylate sphingosine in zebrafish and that the resultant increase in S1P level activated the signaling pathway essential for the cardiac progenitor migration in the MZsphk2 mutant.
LC-MS/MS analysis revealed that the depletion of maternal sphk2 caused the decrease in C17-S1P and a considerable increase in its precursor, C17-sphingosine (Fig. 7, A and D). Although the quantity of C18-S1P also decreased in MZsphk2 and Msphk2 embryos, that of C18-sphingosine, a precursor of C18-S1P, increased to a lesser degree in MZsphk2 embryos, but not in Msphk2 embryos at two-cell stage (Fig. 7, B and E). We speculate that the metabolic pathways between C17- and C18-sphingosine may be different in zebrafish or that C18-sphingosine may be degraded to biosynthesize other lipids. In mammals, phyto-sphingosine is phosphorylated by SPHK2, but not by SPHK1 (17). However, unlike C17- and C18-S1P, the quantity of phyto-S1P decreased 60% in MZsphk1 at two-cell stage, suggesting that zebrafish Sphk1 is involved in phyto-S1P production. Therefore, endogenous substrates of Sphk1/2 may be slightly different between mammals and zebrafish. Phyto-sphingosine and phyto-S1P are predominant in plants and fungi and exist only in certain tissues, including the skin, small intestine, and kidney in mammals (2). In zebrafish-fertilized eggs, phyto-S1P was stored at about a 10-fold higher concentration than S1P (Fig. 7, A and C), although phyto-S1P levels became lower than C18-S1P at 5 dpf (Fig. 8, A and C), suggesting that phyto-S1P, in addition to S1P, may play physiological roles during zebrafish early embryogenesis.
Loss-of-function analyses of S1PRs in mice revealed that S1PR-mediated signaling is involved in physiological events, such as lymphocyte egress, angiogenesis, and bone homeostasis. In addition, it has been proposed that S1P functions as a second messenger in the intracellular compartment. In mammals, tumor necrosis factor receptor-associated factor 2 and histone deacetylases have been identified as intracellular S1P targets (7, 8). Recently, it was reported that SPHK2-derived S1P in megakaryocytes controls the expression of Src family kinases and is not mediated by the S1P receptor (41). We found that cardia bifida, tail blister, and disorganized jaw morphology phenotypes observed in MZsphk2 were identical to those observed in zygotic spns2 or s1pr2 mutants (21–25), suggesting that Sphk2-derived S1P functions as an intercellular signaling molecule and that the Sphk2-Spns2-S1pr2 signaling axis plays essential roles during cardiac and lower jaw development. In addition to S1P, dihydro-S1P, which is produced by SPHKs, can be an endogenous ligand for S1P receptors (42, 43), raising the possibility that this signaling pathway might be through dihydro-S1P.
In summary, we revealed that Sphk2, but not Sphk1, acts as an upstream molecule in the Spns2-mediated intercellular S1P signaling pathway, regulating cardiac progenitor migration, suggesting that Sphk2-derived S1P in the YSL is upstream of the Spns2-S1pr2 axis.
Supplementary Material
Acknowledgment
We thank Hitoshi Okamoto for support and for providing the opportunity to conduct this work.
Note Added in Proof
The supplemental movies were missing from the version of this article that was published as a Paper in Press on April 23, 2015. The movies are now available.
This work was supported by the Japan Society for the Promotion of Science and the Program for Next Generation World-Leading Researchers (NEXT Program) and by Takeda Science Foundation. This work was also supported by the Special Postdoctoral Researcher Program (to Y. H.) from RIKEN and by PRESTO (Precursory Research for Embryonic Science and Technology; to A. I.) and CREST (to J. A.), from Japan Science and Technology Agency (JST).

This article contains supplemental movies.
- S1P
- sphingosine 1-phosphate
- S1PR
- S1P receptor
- SPHK
- sphingosine kinase
- Sph
- sphingosine
- MO
- morpholino oligomer
- YSL
- yolk syncytial layer
- TALEN
- transcription activator-like effector nuclease
- dpf
- days post-fertilization
- LC-MS/MS
- lipid chromatography-tandem mass spectrometry
- GEF
- guanine nucleotide exchange factor.
References
- 1. Neubauer H. A., Pitson S. M. (2013) Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 280, 5317–5336 [DOI] [PubMed] [Google Scholar]
- 2. Kihara A., Mitsutake S., Mizutani Y., Igarashi Y. (2007) Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 46, 126–144 [DOI] [PubMed] [Google Scholar]
- 3. Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 4. Hisano Y., Kobayashi N., Kawahara A., Yamaguchi A., Nishi T. (2011) The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 286, 1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orr Gandy K. A., Obeid L. M. (2013) Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim. Biophys. Acta 1831, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strub G. M., Maceyka M., Hait N. C., Milstien S., Spiegel S. (2010) Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 688, 141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishi T., Kobayashi N., Hisano Y., Kawahara A., Yamaguchi A. (2014) Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta 1841, 759–765 [DOI] [PubMed] [Google Scholar]
- 10. Obinata H., Hla T. (2012) Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 34, 73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hisano Y., Nishi T., Kawahara A. (2012) The functional roles of S1P in immunity. J. Biochem. 152, 305–311 [DOI] [PubMed] [Google Scholar]
- 12. Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. (2012) Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitson S. M., Moretti P. A., Zebol J. R., Zareie R., Derian C. K., Darrow A. L., Qi J., D'Andrea R. J., Bagley C. J., Vadas M. A., Wattenberg B. W. (2002) The nucleotide-binding site of human sphingosine kinase 1. J. Biol. Chem. 277, 49545–49553 [DOI] [PubMed] [Google Scholar]
- 14. Yokota S., Taniguchi Y., Kihara A., Mitsutake S., Igarashi Y. (2004) Asp177 in C4 domain of mouse sphingosine kinase 1a is important for the sphingosine recognition. FEBS Lett. 578, 106–110 [DOI] [PubMed] [Google Scholar]
- 15. Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug. Discov. 9, 883–897 [DOI] [PubMed] [Google Scholar]
- 16. Kihara A., Igarashi Y. (2008) Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim. Biophys. Acta 1781, 496–502 [DOI] [PubMed] [Google Scholar]
- 17. Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275, 19513–19520 [DOI] [PubMed] [Google Scholar]
- 18. Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 19. Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 25, 11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizugishi K., Li C., Olivera A., Bielawski J., Bielawska A., Deng C. X., Proia R. L. (2007) Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 117, 2993–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hisano Y., Ota S., Takada S., Kawahara A. (2013) Functional cooperation of spns2 and fibronectin in cardiac and lower jaw development. Biol. Open 2, 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balczerski B., Matsutani M., Castillo P., Osborne N., Stainier D. Y. R., Crump J. G. (2012) Analysis of sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors. Dev. Biol. 362, 230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 24. Osborne N., Brand-Arzamendi K., Ober E. A., Jin S.-W., Verkade H., Holtzman N. G., Yelon D., Stainier D. Y. R. (2008) The Spinster homolog, Two of Hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 18, 1882–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kupperman E., An S., Osborne N., Waldron S., Stainier D. Y. (2000) A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406, 192–195 [DOI] [PubMed] [Google Scholar]
- 26. Ye D., Lin F. (2013) S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence. Development 140, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langdon Y. G., Mullins M. C. (2011) Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu. Rev. Genet. 45, 357–377 [DOI] [PubMed] [Google Scholar]
- 28. Li L., Zheng P., Dean J. (2010) Maternal control of early mouse development. Development 137, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendelson K., Zygmunt T., Torres-Vázquez J., Evans T., Hla T. (2013) Sphingosine 1-phosphate receptor signaling regulates proper embryonic vascular patterning. J. Biol. Chem. 288, 2143–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim H., Kim J. S. (2014) A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 15, 321–334 [DOI] [PubMed] [Google Scholar]
- 31. Hisano Y., Ota S., Kawahara A. (2014) Genome editing using artificial site-specific nucleases in zebrafish. Dev. Growth Differ. 56, 26–33 [DOI] [PubMed] [Google Scholar]
- 32. Hisano Y., Ota S., Arakawa K., Muraki M., Kono N., Oshita K., Sakuma T., Tomita M., Yamamoto T., Okada Y., Kawahara A. (2013) Quantitative assay for TALEN activity at endogenous genomic loci. Biol. Open 2, 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cermak T., Doyle E. L., Christian M., Wang L., Zhang Y., Schmidt C., Baller J. A., Somia N. V., Bogdanove A. J., Voytas D. F. (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakuma T., Hosoi S., Woltjen K., Suzuki K., Kashiwagi K., Wada H., Ochiai H., Miyamoto T., Kawai N., Sasakura Y., Matsuura S., Okada Y., Kawahara A., Hayashi S., Yamamoto T. (2013) Efficient TALEN construction and evaluation methods for human cell and animal applications. Genes. Cells 18, 315–326 [DOI] [PubMed] [Google Scholar]
- 35. Dahlem T. J., Hoshijima K., Jurynec M. J., Gunther D., Starker C. G., Locke A. S., Weis A. M., Voytas D. F., Grunwald D. J. (2012) Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8, e1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanaoka R., Katayama S., Dawid I. B., Kawahara A. (2006) Characterization of the heme synthesis enzyme coproporphyrinogen oxidase (CPO) in zebrafish erythrogenesis. Genes Cells 11, 293–303 [DOI] [PubMed] [Google Scholar]
- 37. Ota S., Hisano Y., Muraki M., Hoshijima K., Dahlem T. J., Grunwald D. J., Okada Y., Kawahara A. (2013) Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells 18, 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saigusa D., Shiba K., Inoue A., Hama K., Okutani M., Iida N., Saito M., Suzuki K., Kaneko T., Suzuki N., Yamaguchi H., Mano N., Goto J., Hishinuma T., Aoki J., Tomioka Y. (2012) Simultaneous quantitation of sphingoid bases and their phosphates in biological samples by liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Bioanal. Chem. 403, 1897–1905 [DOI] [PubMed] [Google Scholar]
- 39. Okudaira M., Inoue A., Shuto A., Nakanaga K., Kano K., Makide K., Saigusa D., Tomioka Y., Aoki J. (2014) Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 55, 2178–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carvalho L., Heisenberg C. P. (2010) The yolk syncytial layer in early zebrafish development. Trends Cell Biol. 20, 586–592 [DOI] [PubMed] [Google Scholar]
- 41. Zhang L., Urtz N., Gaertner F., Legate K. R., Petzold T., Lorenz M., Mazharian A., Watson S. P., Massberg S. (2013) Sphingosine kinase 2 (Sphk2) regulates platelet biogenesis by providing intracellular sphingosine 1-phosphate (S1P). Blood 122, 791–802 [DOI] [PubMed] [Google Scholar]
- 42. Spiegel S., Milstien S. (2000) Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta 1484, 107–116 [DOI] [PubMed] [Google Scholar]
- 43. Kimura T., Watanabe T., Sato K., Kon J., Tomura H., Tamama K., Kuwabara A., Kanda T., Kobayashi I., Ohta H., Ui M., Okajima F. (2000) Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 348, 71–76 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.