Background: Caffeine regulates alternative splicing by increasing SRSF2, normally constrained by a negative feedback loop.
Results: Caffeine blocks nonsense-mediated decay, induces 3′ UTR alternative splicing, and down-regulates SRSF2-targeting microRNAs, thereby breaking the negative feedback loop to increase SRSF2.
Conclusion: Caffeine modulates multiple post-transcriptional processes to increase SRSF2.
Significance: This study expands our understanding of SRSF2 regulation, and may provide insight into SRSF2 dysregulation in disease.
Keywords: alternative splicing; gene regulation; homeostasis; microRNA (miRNA); translation regulation; caffeine, SRSF2, nonsense-mediated decay
Abstract
We have previously reported that the methylxanthine caffeine increases expression of the splicing factor SRSF2, the levels of which are normally controlled by a negative autoregulatory loop. In the present study we have investigated the mechanisms by which methylxanthines induce this aberrant overexpression. RT-PCR analyses suggested little impact of caffeine on SRSF2 total mRNA levels. Instead, caffeine induced changes in the levels of SRSF2 3′ UTR splice variants. Although some of these variants were substrates for nonsense-medicated decay (NMD), and could potentially have been stabilized by caffeine-mediated inhibition of NMD, down-regulation of NMD by a genetic approach was not sufficient to reproduce the phenotype. Furthermore, cell-based assays demonstrated that some of the caffeine-induced variants were intrinsically more efficiently translated than others; the addition of caffeine increased the translational efficiency of most SRSF2 transcripts. MicroRNA array analyses revealed a significant caffeine-mediated decrease in the expression of two SRSF2-targeting miRs, both of which were shown to repress translation of specific SRSF2 splice variants. These data support a complex model whereby caffeine down-regulates SRSF2-targeting microRNAs, leading to an increase in SRSF2 translation, which in turn induces SRSF2 splicing. SRSF2 splice variants are then stabilized by caffeine-mediated NMD inhibition, breaking the normal negative feedback loop and allowing the aberrant increase in SRSF2 protein levels. These findings highlight the complexity of SRSF2 gene regulation, and suggest ways in which SRSF2 expression may be dysregulated in disease.
Introduction
Alternative splicing (AS)2 of pre-mRNA is a fundamental cellular process that selectively joins alternate exons together to produce different mRNA variants from a single gene. More than 90% of human genes undergo alternative splicing, resulting in strikingly high levels of mRNA complexity (1–3). In some cases, AS leads to the selective inclusion or skipping of one or more protein-coding exons, resulting in functionally different protein isoforms with altered domains; this contributes significantly to proteomic diversity. In other cases, untranslated regions (UTRs) are altered or premature termination codons are introduced by AS, thereby generating complex mRNA populations that are substrates for post-transcriptional regulation at the level of translational efficiency, mRNA localization and/or mRNA stability (4–7).
The potential for AS to exert a complex and profound effect on gene expression requires that tightly controlled regulatory processes are in place. This control is primarily executed by positively or negatively acting splicing factors that bind to cis-elements within pre-mRNA near the regulated splice sites, modulating their recognition efficiency by the multiprotein spliceosome (8). Two major families of splicing factors have been characterized: the serine/arginine-rich (SR) protein family and the HnRNP protein family, whereas additional RNA-binding proteins have also been identified (9, 10). Splicing factors work antagonistically such that their relative concentration in a given cell is a critical determinant of the fate of targeted exons (11, 12). Accordingly, under steady-state conditions the relative levels of splicing factors are expected to remain constant, whereas intrinsic (i.e. during differentiation and development) or extrinsic (i.e. environmental cues, stressor) signals can alter this ratio, thereby altering AS choices and, potentially, cell fate.
An additional layer of control is exerted by nonsense-mediated decay (NMD), an RNA surveillance system that ensures the fidelity of gene expression by degrading non-productive mRNAs containing premature termination codons (13), including those produced by AS. Recent genome-scale studies revealed that many splicing regulators employ a dual mechanism, alternative splicing coupled with nonsense-mediated decay (AS-NMD), to limit their own expression and prevent excessive accumulation that can be deleterious to the cell (14, 15).
SRSF2 (SC35) is a ubiquitous splicing factor that plays a critical role in both constitutive and alternative splicing (16, 17), most often functioning as an activator to enhance the recognition of particular splice sites. Studies using SRSF2 conditional knock-out mice and SRSF2−/− mouse embryonic fibroblasts have suggested that this protein is essential for cell proliferation and the maintenance of genomic stability, at least during thymus and pituitary development (18). It was also suggested that SRSF2 plays a role in the regulation of transcription elongation (19, 20). Additionally, transcription of SRSF2 has been shown to be regulated by E2F1, and is required for E2F1-induced apoptosis (21). Alkylating agent-induced apoptosis was shown to be accompanied by a significant increase in both E2F1 and SRSF2, suggesting a possible role for SRSF2 in genotoxic stress response (21). Interestingly, a recent study suggested that SRSF2 is the most enriched splicing factor in human pluripotent stem cells, where SRSF2 is regulated by OCT4 and required for pluripotency via its ability to regulate the AS of MBD2, the methyl-CpG binding protein (22). Given the key role of SRSF2 in multiple regulatory pathways, its dysregulation is likely to be pathological; indeed, several studies have identified a strong correlation between SRSF2 mutations and subclassification and prognostication of myelodysplastic syndrome, a heterogeneous group of myeloid neoplasms that predispose to acute myeloid leukemia (23–26).
SRSF2 levels are carefully controlled. In addition to transcriptional regulatory controls, SRSF2 RNA is subjected to negative autoregulation via AS-NMD (27). In this scenario, when levels of SRSF2 protein exceed the tolerable cellular threshold, SRSF2 induces alternative splicing of its own transcript, resulting in the expression of two novel premature termination codon-containing splice variants at the expense of the primary SRSF2 transcript. These variants are destined for degradation by NMD; through this feedback loop, overall SRSF2 mRNA levels are reduced and SRSF2 protein expression returns to steady-state levels (27). Although a recent study suggested that both HnRNP H and TARDBP (TDP-43) act as antagonists to SRSF2 in regulating a retained intron in this unproductive alternative splicing at the 3′ UTR (28), the complexity of SRSF2 mRNA variant formation, its regulation, and the functional implications are poorly understood.
We have previously shown that caffeine can alter the AS of a subset of genes associated with the cancer phenotype. This is accomplished, at least in part, by the induction of high levels of SRSF2 (29). In the present report we extend our analyses by addressing the mechanism(s) that lead to increased levels of SRSF2 protein. We now show that this caffeine-mediated SRSF2 increase involves a complex series of events that includes down-regulation of SRSF2-targeting microRNAs, inhibition of NMD, and induction of novel SRSF2 splice variants with altered translational efficiency. As a consequence, the SRSF2 negative feedback loop is broken, allowing for a sustained increase of SRSF2 protein in the cell. This study advances our understanding of SRSF2 gene regulation, highlighting the multifaceted mechanisms at the post-transcriptional level that regulate SRSF2-mediated AS decisions, and provides a basis to investigate dysfunction of AS during pathogenesis.
Experimental Procedures
Cell Culture and Chemical Reagents
The human cervical carcinoma HeLa cells line (ATCC®, CCL-2) was maintained in DMEM supplemented with 10% (v/v) FBS and 2.0 mm glutamine. Caffeine and cycloheximide were purchased from Sigma and prepared immediately prior to use.
Cytotoxicity Assay
HeLa cells were seeded in 96-well plates at a density of 7,500 cells per well. Various concentrations (1.75 to ∼21 mm) of xanthine derivatives, including caffeine, pentoxifylline, theophylline, and isocaffeine, were added to wells with 8 repeats for each experimental condition. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays were performed 3 days after treatment following the manufacturer's instructions (Promega).
Semi-quantitative RT-PCR Assays
Total RNA was prepared with TRIzol reagent (Invitrogen) and analyzed using SS One-step RT-PCR reagents (Invitrogen) according to the manufacturer's recommendations. RNase-free DNase I treatment was performed when needed. The initial RNA input was 300 ng, and the number of amplification cycles was pre-determined to ensure a linear range amplification of targeted transcripts (28 cycles to assay total SRSF2 mRNA levels, 40 cycles to assay individual transcript groups including GA, GB, GC, and GD. Primers used for SRSF2 total mRNA: a: 5′-CTG AGG ACG CTA TGG ATG CCA-3′; b: 5′-GAC TTG GAC TTG GAC CTT CGT-3′; primers used for SRSF2 3′UTRs: c: 5′-CCA AGT CTC CTG AAG AGG AAG G-3′; d: 5′-CTG AGA AAA GCT AAC ACC AAG-3′; e: 5′-GAA AAT GGT AAT GTC TGG GAA TC-3′; and f: 5′-GTC AGG AGG CCA CAA ATT AGG-3′.
Real-time Quantitative RT-PCR
Purified RNA was first treated with RNase-free DNase I and then reverse-transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (AB Applied Biosystems/Life Technologies) following the manufacturer's instructions. Real-time PCR was performed using SYBR® Green PCR Master Mix (AB Applied Biosystems/Life Technologies) and individually designed primer sets. For total SRSF2 mRNA, sense: 5′-CTG AGG ACG CTA TGG ATG CCA-3′ and antisense: 5′-GAC TTG GAC TTG GAC CTT CGT-3′; for β2M, the same as previously reported (29); for FLAG-SRSF2, sense: 5′-CGA CTC ACT ATA GGG AGA CC-3′ and antisense: 5′-GAG GTG CGG TAG GTC AGG TT-3′; for GFP, sense: 5′-GGG TGA AGG TGA TGC AAC ATA C-3′ and antisense: 5′-CTC GCA AAG CAT TGA ACA CCA-3′. The final Ct value was an average of 3 repeats of each assay, repeated three times. The relative amount of mRNA in samples was determined using the 2−ΔΔCt method.
RNA Interference Assay
3 × 104 HeLa cells in 0.5-ml DMEM without antibiotics were seeded into each well of a 24-well plate and incubated overnight. 100 nm siRNA (SiGENOME SMARTpool reagent, DHARMACON) was mixed with Oligofectamine reagent (Invitrogen), incubated at room temperature for 20 min, and added dropwise to cells. 72 h after siRNA transfection, cells were harvested for either RNA isolation or whole cell lysates, and assayed by either RT-PCR or Western blot analysis.
Western Blot Analysis
Cells were washed twice with cold PBS and lysed in RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1.0% Triton X-100, 0.1% SDS, 1% deoxycholate plus protease inhibitor mixture (Roche Diagnostics), 1 mm sodium fluoride, 1 mm sodium orthovanadate, and 100 mg/ml of phenylmethylsulfonyl fluoride. The protein concentration of cell lysates was determined using the Pierce BCA protein assay. Equal amounts of total protein (15–25 μg) were analyzed by 10% SDS-PAGE followed by immunoblotting using the following antibodies: rabbit anti-TARBP (GeneTex, 1:1000), mouse anti-HnRNP F/H (Abcam, 1:1000), goat anti-hUpf1 (Abcam, 1:1000), mouse anti-SRSF2 (provided by Drs Cyril Bourgois and James Stevenin, 1:20), mouse anti-FLAG (Agilent, 1:2000) or mouse anti-tubulin (Santa Cruz Biotechnology, 1:1000). The secondary antibody was either HRP-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology, 1:2500), HRP-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, 1:2500), or HRP-conjugated goat anti-mouse IgG (Upstate Technology, 1:2500). Immunoreactive bands were visualized using the VisualizerTM Western blot Detection Kit (Upstate Technology) following the manufacturer's directions.
SRSF2 Transcript Expression Vectors
SRSF2 cDNAs with variations in their 3′ UTR were amplified by RT-PCR as described under “Results” and in the figures using primers with restriction enzyme linker EcoRI (attached to primers c and e) and XbaI (attached to primers d and f). Each of the RT-PCR products were gel-purified and digested with EcoRI and XbaI, then individually cloned into pcDNA3.1-Flag-SRSF2 downstream of the SRSF2 coding region. The resulting constructs (pcDNA3.1-Flag-SRSF2–3′UTRs: A1, A2, A3, D1, D2, D3, etc.) were verified by sequencing.
In Vitro Translation Assay
Equal amounts (125 ng) of each SRSF2 splice variant construct (A1, A2, A3, D1, D2, or D3) was assayed in the presence of EasyTagTM l-[35S]Methionine (PerkinElmer) using the TnT® Quick Coupled Transcription/translation System (Promega). Translated protein was analyzed by SDS-PAGE followed by radiography using the Bio-Rad PhosphorImager. Signals were quantified using Bio-Rad Quantity One software.
Cell-based Translational Assay
HeLa cells were seeded at 1.25 × 105 cells per well in 12-well plates. The next day 200 ng of plasmid DNA of each SRSF2 variant construct was individually co-transfected with 37.5 ng of plasmid DNA of GFP construct (pN3-GFP) in duplicate using Lipofectamine 2000 (Invitrogen). Seven hours post-transfection, cells were untreated or treated with caffeine; 3 h later, cells were collected from each well and divided in half. One-half was dissolved in RIPA buffer for Western blot analysis using anti-FLAG (Agilent, 1:2000) and anti-GFP antibodies (Santa Cruz Technologies, 1:500) in a single procedure. The other half was subjected to RNA purification using TRIzol (Invitrogen) for real-time RT-PCR analyses of FLAG-SRSF2 and GFP mRNAs. Translational efficiency was determined by normalizing FLAG-SRSF2 expression levels to corresponding GFP levels.
MicroRNA (miR) Array Analysis
HeLa cells were treated with caffeine (14 mm) for either 3 or 24 h. Treated samples and untreated controls were collected for total RNA using miRCURYTM RNA Isolation Kits (Exiqon), and labeled with appropriate dyes using miRCURY LNATM microRNA Array Hi-power Labeling Kit. The miRCURY LNATM microRNA Array, v11.0-human, was the platform utilized. Hybridization and processing of arrays were performed by the CINJ DNA Core Facility. Each experimental condition was repeated 6 times including three biological replicates and two technical replicates for each biological replicate. The average signal density was normalized by standard protocols (CINJ Bioinformatics Core Facility) as well as internal controls provided on the miRCURY LNATM microRNA Array platform using miRCURY LNATM microRNA Array Analysis software.
miR qRT-PCR
Total RNA was isolated using miRCURYTM RNA Isolation Kits (Exiqon), and cDNA was prepared using the Universal cDNA Synthesis Kit (Exiqon) following manufacturers' instructions. Individually pre-designed LNATM-enhanced microRNA qPCR primer sets were purchased from Exiqon. Quantitative PCR was performed using SYBR® Green Master Mix Kit (Exiqon) and a Stratagene MX3000p quantitative PCR system. RNA input was normalized by U1 snRNA and SNORD49a. RT-PCR efficiency was normalized using the RNA Spike-in kit (Exiqon). The final Ct value was an average of 5 repeats of each assay, repeated three times. The relative amount of mRNA in samples was determined using the 2−ΔΔCt method.
Anti-miR and miR Mimic Co-transfections
HeLa cells were seeded at ∼80,000 cells/well in 12-well plates and incubated overnight. Either mirVanaTM miRNA mimics or inhibitors (Ambion, Invitrogen), at 30–50 nm (determined empirically), were co-transfected with 150 ng of indicated SRSF2 3′ UTR plasmid DNA as well as 37.5 ng of a GFP expression construct (pN3-GFP). Lipofectamine 2000 reagent (Invitrogen) was used following the manufacturer's instructions. 18–22 h after transfection, whole cell lysates were collected for Western blot analyses of FLAG-SRSF2 expression. GFP was used to control for transfection efficiency.
To assay the effect of anti-miR and miR mimics on SRSF2 expression, HeLa cells were seeded at ∼80,000 cells/well in 12-well plates and incubated overnight. For miR mimics experiment, 45 nm mirVanaTM miRNA mimic (Ambion, Invitrogen) was co-transfected with the KLF6 minigene (29) as described. Caffeine was added 18 h after transfection, and 10 h later, cells were harvested for either Western blot analysis of SRSF2 expression or RT-PCR of KLF6. For miR inhibitor experiments, 45 nm mirVanaTM miRNA inhibitor (Ambion, Invitrogen) was co-transfected with hSMG-1 siRNA (100 nm, Dharmacon, GE Healthcare) and the KLF6 minigene as described. Following a 46-h incubation, cells were harvested for Western blot analysis and RT-PCR performed as above.
Statistical Analysis
All statistical analyses were performed using GraphPad PRISM version 6 software, nonparametric t tests. A difference with a p value < 0.05 was considered significant. Error bars represent the mean ± S.D.
Results
Methylxanthines Differentially Induce KLF6 Splice Variants and Increase Levels of SRSF2 Protein
We have previously reported that caffeine, a tri-methylxanthine derivative, can induce alternative splicing of a subset of cancer-associated genes, including the tumor suppressor gene KLF6. This induction is due, at least in part, to a caffeine-mediated increase (up to 6-fold) of the splicing factor SRSF2. In HeLa cells this increase can be observed as early as 1 h post-caffeine treatment and is sustained for at least 24 h (29). To evaluate the impact of other members of this class on splicing, we examined the ability of several xanthine derivatives, including pentoxifylline, caffeine, theophylline, and isocaffeine, on induction of the KLF6 splice variant, SpKLF6 (Fig. 1A). Within 18 h of treatment, pentoxifylline exhibited the strongest induction among the group, followed by caffeine and theophylline. Isocaffeine had minimal effect on SpKLF6 expression. This corresponded to the degree of induction of SRSF2 by these compounds (Fig. 1B), further supporting our observation that caffeine-induced SpKLF6 expression is mediated by SRSF2. Although pentoxifylline induced the highest level of SRSF2, it also caused severe cell death at the concentrations used (14 mm) (Fig. 1C). Therefore, caffeine was used as the model drug to investigate the mechanisms by which methylxanthines increase SRSF2.
FIGURE 1.
Methylxanthines induce alternative splicing of KLF6 and increase levels of SRSF2. A, RT-PCR analyses on samples treated with methylxanthines (14 mm), including pentoxifylline, caffeine, theophylline, and isocaffeine, revealed various degrees of SpKLF6 induction following 18 h of treatment. B, Western blotting (WB) analyses detected increased levels of SRSF2 in samples treated with pentoxifylline, caffeine, and theophylline; isocaffeine had a minimal effect. The order of magnitude of SRSF2 increase is pentoxifylline > caffeine > theophylline > isocaffeine, which coincided with the degree of SpKLF6 AS induction. C, cytotoxicity assays revealed considerable cell death following pentoxifylline treatment when compared with caffeine, at the effective concentration (14 mm) used to induce maximal alternative splicing of KLF6. UT, untreated.
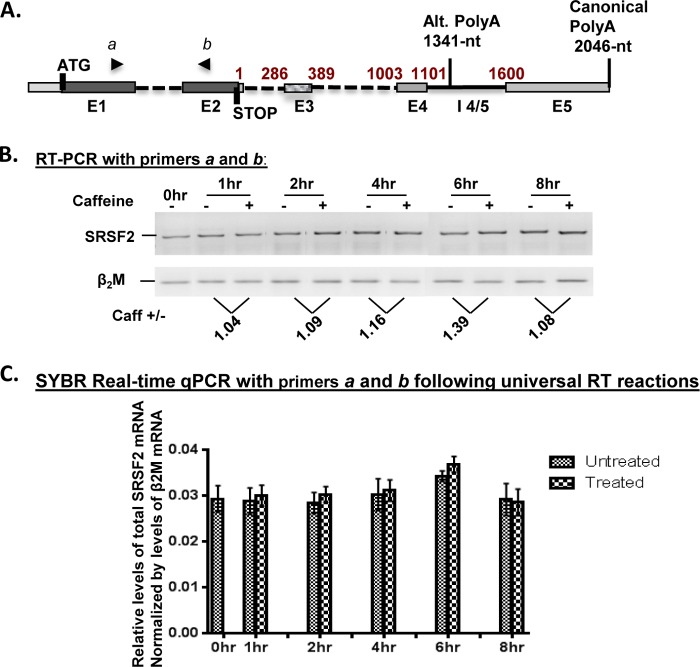
Caffeine Does Not Impact Total SRSF2 mRNA Levels
To investigate the molecular basis for the increase in SRSF2 protein, we first considered the possibility that caffeine was working at the level of SRSF2 transcription. To evaluate this, we employed both semi-quantitative and quantitative RT-PCR to examine the total SRSF2 mRNA following caffeine treatment. Previous studies have described three SRSF2 transcripts that differ in the splicing of exon 3 (E3) and/or intron 4/5 (I4/5) at the 3′ UTR (Fig. 2A) (27). Public databases (NCBI-Aceview and Ensembl) suggested the existence of additional splice variants of SRSF2 mRNA that differ in their 3′ UTR splicing choices and polyadenylation sites (Fig. 2A). Interestingly, there are no alternative splice sites documented within the SRSF2 coding region that spans Exon 1 (E1) and 2 (E2), indicating that both the known and predicted SRSF2 transcript variants would encode the same protein. To account for all known and predicted transcripts, primers located within the two protein coding exons, E1 and E2 (Fig. 2A, primers “a” and “b”), were chosen for analysis of RNAs isolated from HeLa cells either untreated or treated with caffeine for times indicated. After careful linear range optimization and quantitative analyses, both semi-quantitative RT-PCR (Fig. 2B) and quantitative RT-PCR (Fig. 2C) identified no significant change in total SRSF2 mRNA following caffeine treatment, suggesting that caffeine regulates SRSF2 protein levels post-transcriptionally.
FIGURE 2.
Caffeine does not change total SRSF2 mRNA levels. A, schematic illustration of SRSF2 gene structure. E1, exon 1; E2, exon 2; the canonical polyadenylation site is found 2046 nt downstream of the stop codon, whereas an alternative polyadenylation site is located at 1341 nt downstream of the stop codon. B, semi-quantitative RT-PCR analysis using primers “a” and “b” on RNA samples collected from HeLa cells with or without caffeine treatment at different time points. No change in SRSF2 total mRNA was observed following caffeine (14 mm) treatment. β2-microglobulin (β2M) RNA was used as a loading control. This assay was repeated at least three times with consistent results. Statistical analyses were performed using GraphPad Prism 6 software, and significance was determined by the non-parametric t tests. A p < 0.05 was considered significant. C, SYBR® real-time RT-PCR analyses confirmed the results from semi-quantitative RT-PCR, i.e. that no significant change in total SRSF2 mRNA was induced by caffeine. Error bars represent mean ± S.D.
Caffeine Influences Splicing Decisions at the SRSF2 3′ UTR
Because there was no apparent increase in total SRSF2 mRNA levels concomitant with the caffeine-induced increase in SRSF2 protein, and cycloheximide experiments did not indicate a caffeine-mediated change in SRSF2 protein stability (data not shown), we next considered the possibility that caffeine may impact SRSF2 splicing choices at the 3′ UTR, yielding transcripts with different stability and/or translatability.
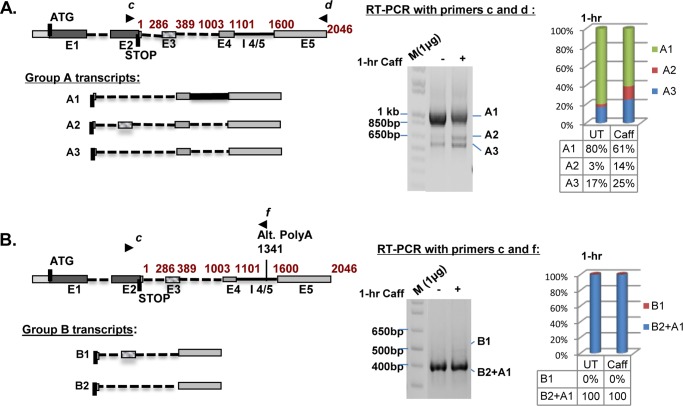
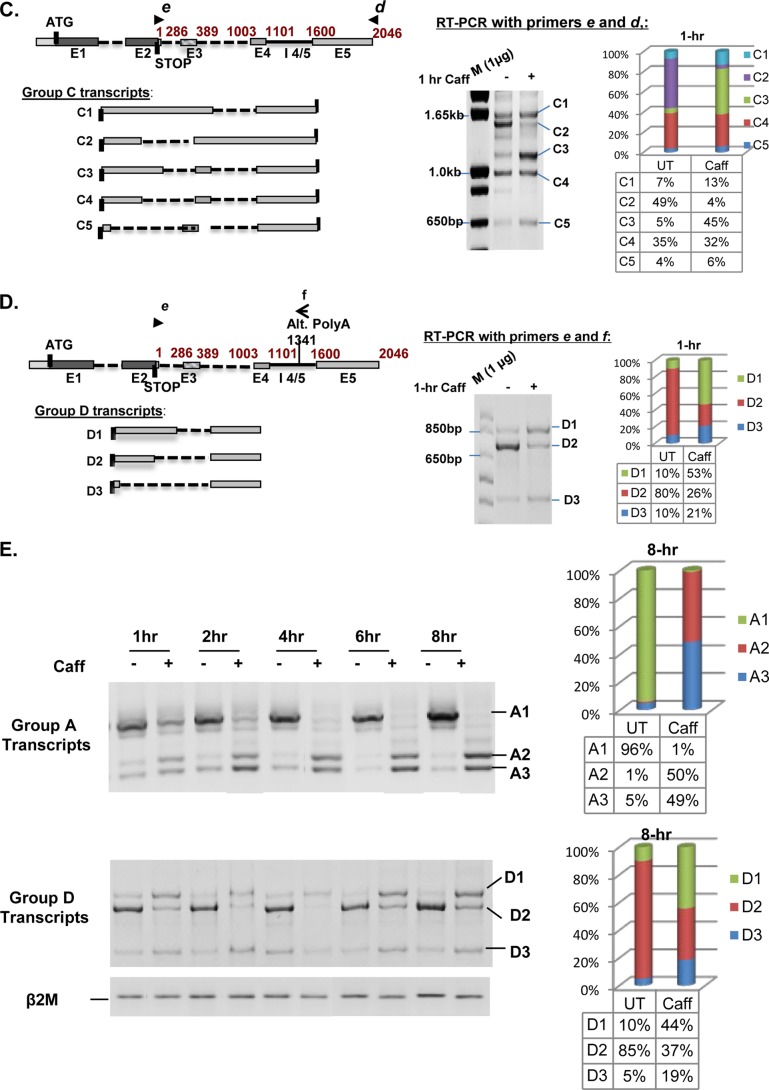
Four possible groups of SRSF2 transcripts have been either described before or predicted by the aforementioned databases. The major SRSF2 transcript (hereafter referred to as A1) includes the canonical E1, E2, and polyadenylation site, but skips E3 and retains intron 4/5 (Fig. 3A, diagram). Two additional SRSF2 transcripts, A2 and A3, can be induced by high levels of SRSF2 protein via AS at the 3′ UTR (Fig. 3A, diagrams). Because A2 and A3 each contain a premature termination codon, they are potential substrates for NMD (27, 30) and therefore can be quickly degraded to restore normal levels of SRSF2 protein. Together, we refer to these mRNAs as Group A (GA) transcripts. The predicted Group B (GB) transcripts are similar to GA transcripts in that they skip E3, but they utilize an alternate polyadenylation site (Fig. 3B, diagram). Group C (GC) transcripts are predicted to resemble GA transcripts in that they use the canonical polyadenylation site, but contain an elongated E2 due to differential utilization of the E2 5′ splice site (Fig. 3C, diagram). Finally, predicted Group D (GD) transcripts resemble GC transcripts in their E2 5′ splicing but use the alternative polyadenylation site (Fig. 3D, diagram).
FIGURE 3.
Caffeine induces rapid changes in the 3′ UTR of SRSF2 RNA (A–D). The known Group A (GA) transcripts as well as the putative Groups B, C, and D (GB, GC, GD) transcripts are schematically depicted in the left panels. Semi-quantitative RT-PCR was performed with different primers to query the existence of both known and putative SRSF2 transcripts in HeLa cells. A, analyses using primers c and d on samples with or without 1-h caffeine treatment revealed rapid changes in levels of the GA splice variants (right panel). B, analysis using primers c and f confirmed the existence of putative GB SRSF2 transcripts that utilize the alternative polyadenylation site instead of the canonical polyadenylation site. Caffeine treatment (14 mm) had minimal effect on the B1 transcript (inclusion of E3) (right panel). Note that primers c and f will also amplify A1. C, analysis using primers e and d confirmed the presence of GC transcripts that utilize the canonical polyadenylation site but select alternative E2 5′ splice sites at positions 1101 (C1), 389 (C2 and C4), 543 (C3), or 60 nt (C5) downstream of the stop codon. Caffeine induced changes in GC transcripts. GC transcripts are poorly expressed because they required 5 additional cycles of PCR to be comparably visualized. D, analysis using primers e and f confirmed novel GD transcripts that utilize the alternative polyadenylation site as well as alternative E2 5′ splice sites. Caffeine (1 h) induced D1 and D3 at the expense of D2. E, time course analyses of caffeine-induced alternative splicing changes within the 3′ UTR among GA and GD transcripts.
To systematically examine the impact of caffeine on all the identified and predicted variants of SRSF2 RNA, multiplex RT-PCR assays were developed to detect transcripts in the four distinct groups in untreated and caffeine-treated HeLa cells. Multiplex RT-PCR using primer “c” and “d” was used to detect the GA transcripts. Following a 1 h exposure to caffeine, a “switch” in the expression of splice variants was observed, with a significant increase in expression of A2 and, to a lesser extent A3, at the expense of A1 (Fig. 3A). Examination of putative GB transcripts using primers c and “f” detected B2 but not B1 in untreated samples, suggesting that SRSF2 transcripts that utilize the canonical E2 and the alternative polyadenylation site selectively skip E3; caffeine had a negligible effect on expression of this group (Fig. 3B). Multiplex RT-PCR using primers “e” and d detected a novel group of SRSF2 transcripts that contain an elongated E2 with alternative 5′ splice site choices at 1101 (C1), 389 (C2 and C4), 543 (C3), or 60 nt (C5) downstream of the stop codon (Fig. 3C). The impact of caffeine on GC transcripts was similar to what was observed for GA transcripts, in that alternative splicing of intron 4/5 was induced (C1) at the expense of C2 (Fig. 3C). In addition, utilization of novel 5′ splice sites 543 (C3) and 60 nt (C5) was promoted (Fig. 2C, note increased expression of C3 and C5). Notably, GC transcripts were not well represented in the population (visualization required 5 additional PCR amplification cycles). In contrast, GD transcripts D1, D2, and D3, also novel splice variants with similar alternative 5′ splice sites as GC transcripts, which utilize the alternative polyadenylation site, are well expressed. Caffeine increased the GD transcripts that utilize 5′ splice sites at 543 (D1) and 60 nt (D3), at the expense of D2.
To summarize, caffeine significantly changed the AS pattern of SRSF2 transcripts within the 3′ UTR. This effect was fairly rapid; as shown in Fig. 3E, upper panel, the effect of caffeine on A1 (decrease) and A2/A3 (increase) could be observed as early as 1 h, with maximum levels achieved by 8 h. Similar kinetics was obtained with GD transcripts (lower panel). As basal GC transcript expression was negligible, and caffeine had a minimal effect on the splicing of GB transcripts, we focused on GA and GD transcripts for the remainder of the study.
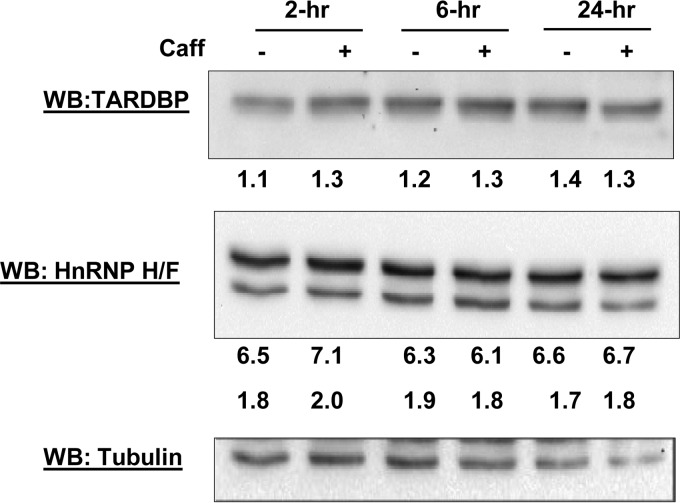
Caffeine Does Not Change the Levels of Known Repressors of SRSF2 3′ UTR Alternative Splicing
It has been previously reported that two negative splicing regulators, TARDBP and HnRNP H, can antagonize SRSF2-mediated splicing of intron 4/5 (28). We therefore considered the possibility that the expression of one or both of these negative regulators could be altered by caffeine, leading to the skipping of intron 4/5. To examine this possibility, TARDBP and HnRNP H levels were analyzed following treatment with or without caffeine; no significant change in levels of either factor was observed over a 24-h period (Fig. 4), suggesting that they are not responsible for caffeine-induced SRSF2 3′ UTR alternative splicing.
FIGURE 4.
Caffeine (14 mm) does not change the levels of TARDBP and HnRNP F/H to influence SRSF2 AS at the 3′ UTR. Western blotting (WB) analysis was performed on samples collected at the indicated time points. Tubulin was used as the internal control.
Inhibition of NMD Is Not Sufficient to Increase SRSF2
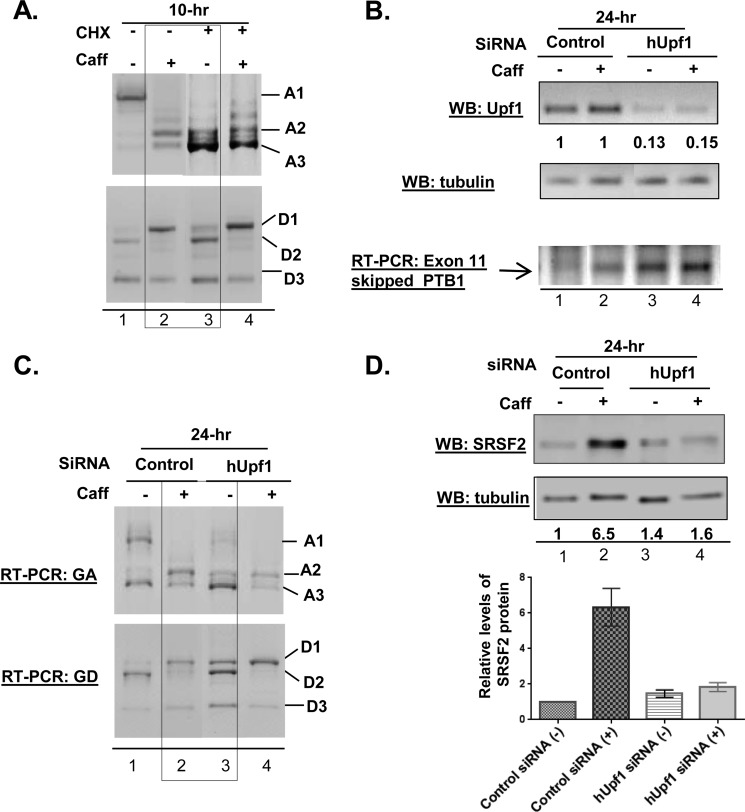
One of the well documented effects of caffeine is inhibition of NMD, due to the negative impact of caffeine on phosphorylation of the essential factor hUpf1 (31–34). Because several of the caffeine-induced SRSF2 transcripts (A2, A3, D1, D2, and D3) are potential substrates for NMD, we next considered the possibility that inhibition of NMD by caffeine was sufficient to allow NMD-sensitive isoforms to be stabilized and accumulated. First, cycloheximide was used to inhibit the pioneer round of protein translation that is required for NMD (33, 35). As shown in Fig. 5A, cycloheximide treatment alone partially altered the GA and GD expression profiles (compare lane 3 to lane 1); however, treatment with cycloheximide and caffeine resulted in a distinctive splicing pattern within the 3′ UTR (compare lane 3 to lane 2), indicating that cycloheximide-mediated NMD inhibition could not reproduce the effect of caffeine on SRSF2 variants expression. To directly determine whether NMD inhibition alone was sufficient to generate alternate SRSF2 transcripts as well as increased SRSF2 protein levels, an RNAi approach was used to knockdown hUpf1 (36–38). As shown in Fig. 5B, hUpf1 levels were decreased by at least ∼85% using a targeted siRNA smart pool (Dharmacon), resulting in inhibition of NMD as evidenced by the accumulation of a known NMD substrate, the alternatively spliced, exon 11 (34 nt)-skipped PTB1 (39). Further RT-PCR analyses on GA and GD transcripts revealed that, although hUpf1 knockdown induced some changes in SRSF2 splice variant expression (Fig. 5C, compare lane 3 to lane 1), it was not sufficient to induce the full spectrum of changes that is observed in the presence of caffeine. Importantly, NMD inhibition was not sufficient to induce an increase in SRSF2 protein (Fig. 5D, lane 3 versus lane 2). We have previously shown that caffeine had no effect on other SR proteins that employ AS-NMD as their autoregulatory mechanism (15, 29, 33), consistent with our hypothesis that NMD inhibition itself is not sufficient to induce SRSF2 protein and an additional, gene-specific mechanism must be in place.
FIGURE 5.
Inhibition of NMD is not sufficient to increase SRSF2 levels. A, cycloheximide-mediated NMD inhibition only resulted in partial mimics of the effect of caffeine on alternative splicing pattern in GA and GD transcripts. B, RNAi reduced the hUpf1 level by ∼85%, inhibiting NMD as evidenced by the accumulation of a PTB1 splice variant (exon 11-skipped PTB1), known to be an NMD target. Note that caffeine treatment alone (lane 2) also resulted in accumulation of exon 11-skipped PTB1, indicating some degree of inhibition of NMD by caffeine. C, the SRSF2 3′ UTR splicing pattern induced by caffeine was only partially reproduced by hUpf1 knockdown-mediated NMD inhibition. D, Western blot (WB) analysis revealed that hUpf1 RNAi-mediated NMD inhibition was not sufficient to increase levels of SRSF2 protein. Quantitation was normalized to input control tubulin. Statistical analysis was performed based on data from at least three experiments. Error bars represent mean ± S.D.
SRSF2 Transcripts with Varied 3′ UTRs Exhibit Different Translational Efficiencies
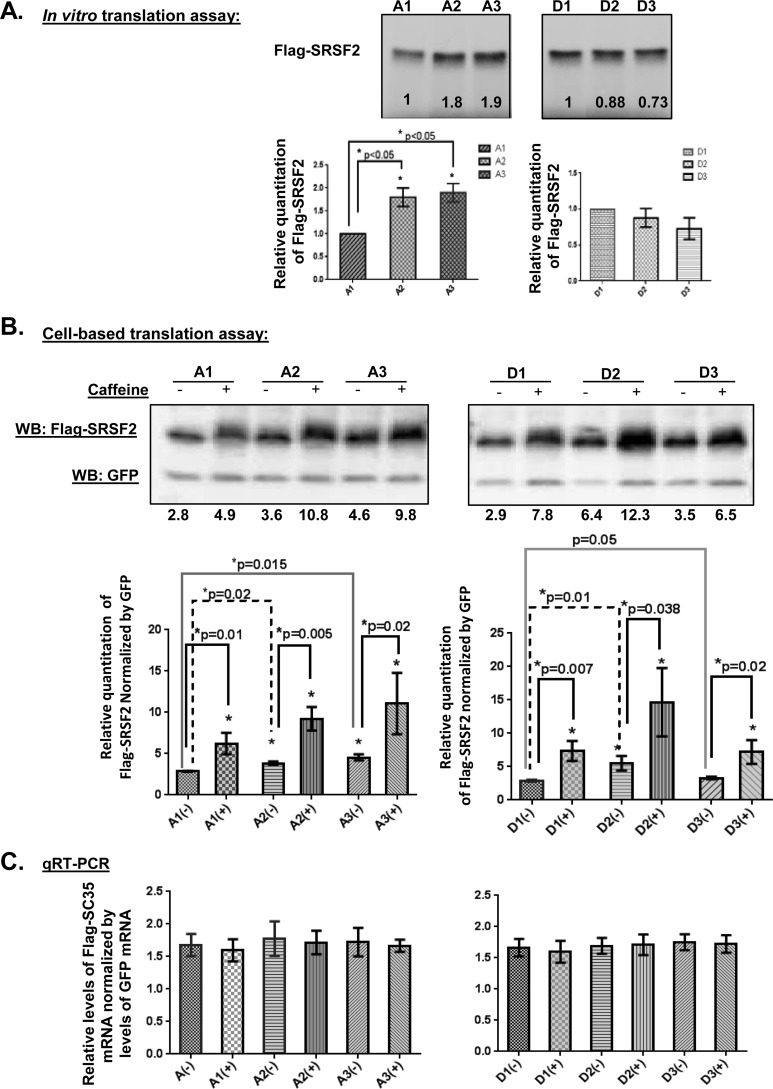
Given that caffeine altered the SRSF2 3′ UTR alternative splicing choices without changing the total mRNA levels and that the 3′ UTR is known to play a critical role in the regulation of translational efficiency (40–42), we considered the possibility that the caffeine-regulated AS transcripts had different translational efficiencies. Both in vitro translation assays and cell-based translational assays were employed to evaluate this possibility. Initially, the individual 3′ UTR of SRSF2 transcripts were inserted into a Renilla luciferase reporter vector to test for relative translational efficiency, whereas firefly luciferase vector served as a transfection control, as is standard in the field. Unfortunately, caffeine interfered with detection of luciferase activity in this assay. Therefore, FLAG-SRSF2 cDNA (created by fusing a FLAG tag to the N-terminal of each construct) was used instead of Renilla luciferase cDNA as a reporter and GFP replaced firefly luciferase as a control for transfection efficiency. Under these conditions, transcription of each variant (A1, A2, A3, and D1, D2, D3, and complete mRNA) is controlled by the same promoter, and products of each can be distinguished from endogenous SRSF2 using the anti-FLAG antibody.
Plasmid DNA was prepared and carefully quantitated for each construct. The same amount of input DNA was assayed with TnT® Quick Coupled Transcription/Translation System (Promega). As shown in Fig. 6A, transcripts A2 and A3 exhibited significantly higher intrinsic translational efficiency than A1 in this assay, whereas translation of GD group members were similar. Next, cell-based translation was assayed by co-transfecting each of the SRSF2 transcript constructs with a GFP expression construct to serve as a control for transfection efficiency (43). Approximately 7 h post-transfection, cells were either untreated or treated with caffeine. After 3 h, cells were harvested for Western blot analysis of both FLAG-SRSF2 and GFP. The translational efficiency of each SRSF2 transcript was determined by normalizing FLAG-SRSF2 expression to the expression of GFP. To assure that the expression/stability of mRNA did not differ among the transcripts, mRNA levels of each FLAG-SRSF2 and GFP were examined using real-time quantitative RT-PCR assay. When normalized to GFP, the levels of FLAG-SRSF2 mRNA remained constant for all transcripts tested (A1-A3, D1-D3) (Fig. 6C).
FIGURE 6.
GA and GD SRSF2 variants exhibit different translational efficiencies and caffeine (14 mm) increases their translation. A, in vitro translation assays detected a higher intrinsic translational efficiency of A2 and A3 when compared with A1 transcripts. No significant difference was detected among GD SRSF2 variants by this assay. B, cell-based translation assays also indicated that the caffeine-induced transcripts A2 and A3 were translated at a higher rate as compared with A1. Caffeine increased translational efficiency of all SRSF2 variants. SRSF2 cDNA in-frame with an N-terminal FLAG tag was tethered with individual SRSF2 3′ UTRs and inserted into a mammalian expression plasmid. Co-transfection assays introduced both SRSF2 and GFP constructs into HeLa cells. Western blot analysis was utilized to quantitate relative translation efficiency. GFP was used as a control for transfection efficiency. C, real-time RT-PCR analyses of FLAG-SRSF2 and GFP mRNA in the cell-based translational efficiency assay. The relative levels of FLAG-SRSF2 mRNA of each SRSF2 variants were normalized to levels of GFP mRNA from the same cell populations/experimental conditions. Statistical analysis was performed based on data from at least three repeats using GraphPad PRISM 6 software, non-parametric t tests. A difference with a p < 0.05 was considered significant. Error bars represent mean ± S.D.
As shown in Fig. 6B, GA transcripts with shorter 3′ UTRs (A2, A3) exhibited higher translation efficiency than A1 (left panel), whereas the GD transcript D2 was translated with the highest efficiency among the GD members (right panel). Importantly, caffeine significantly increased translational efficiency of every variant tested by ∼2–3-fold. This moderate regulation of translation was reminiscent of the impact of microRNAs (miRs) on translation efficiency (44).
Caffeine-mediated Down-regulation of miR-183-5p and miR-33a-5p Alters Protein Translation Efficiency of Specific SRSF2 Transcripts
MicroRNAs are small non-coding RNAs that regulate gene expression by base-pairing with specific mRNA molecules, usually resulting in altered protein translation or mRNA stability (45, 46). MicroRNA-mediated translation repression has recently been suggested as a mechanism for SRSF2 regulation in amygdala during acute stress (miR-183-5p) (47) and in hepatocellular carcinoma during the acquisition of drug resistance (miR-193a-5p) (48).
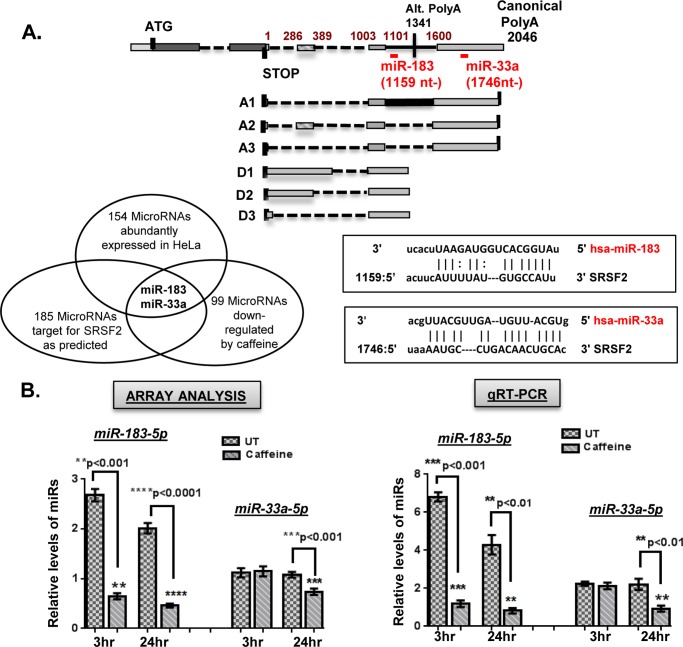
To determine whether caffeine regulates the expression of miRs that could in turn impact SRSF2 expression, microarray analyses were carried out using the miRCURY LNATM microRNA Array platform and total RNA isolated from either untreated HeLa cells or cells treated for 3 or 24 h with caffeine. Each condition was repeated three times and data were normalized to internal controls present on the array. After careful data mining using common criteria, ∼8% of the miRs examined (99 of ∼1200) were found to be down-regulated by caffeine (supplemental Tables S1 and S2). This group of miRs was aligned with computationally predicted SRSF2-targeting miRs (185 miRs, based on the miRscan database), then further limited to miRs that were abundantly expressed in untreated control cells. Two microRNAs, miR-183-5p and miR-33a-5p, were found to be significantly down-regulated by caffeine; miR-183-5p has previously been suggested to repress SRSF2 translation (47) by interacting with the SRSF2 transcript at position 1159 nt within the 3′ UTR. miR-33a-5p was suggested to target the SRSF2 3′ UTR at position 1746 nt (Fig. 7A). Therefore, miR-183-5p would likely target A1, D1, D2, and D3 and miR-33a-5p could impact translation of A1, A2, and A3 (Fig. 7A). Quantitative RT-PCR analysis of RNAs isolated from untreated and treated HeLa cells was performed to validate the down-regulation of these miRs by caffeine. As shown in Fig. 7B, following 3-h of caffeine treatment, miR-183-5p was decreased dramatically (5∼6-fold), whereas miR-33q-5p levels remain largely unchanged. However, following 24 h of caffeine treatment, expression of miR-183-5p was still decreased and miR-33a-5p was decreased by almost half, similar to what was observed in the microarray analysis.
FIGURE 7.
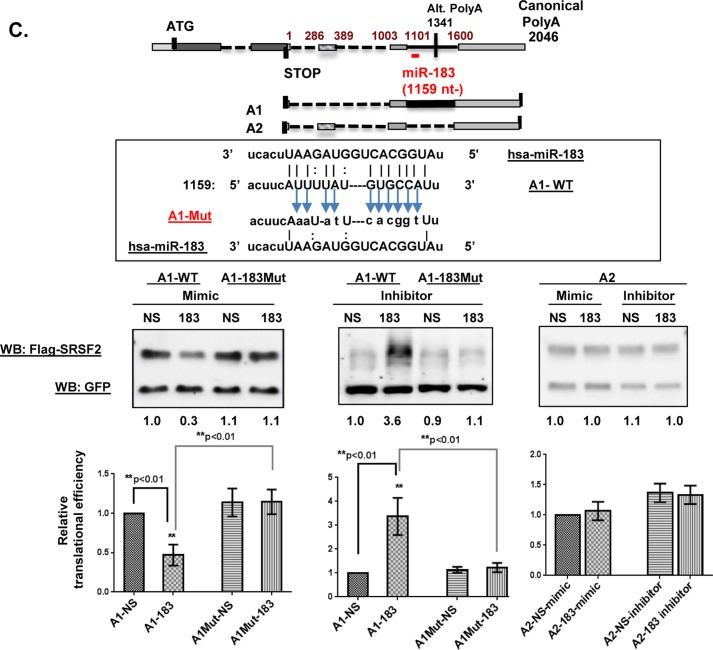
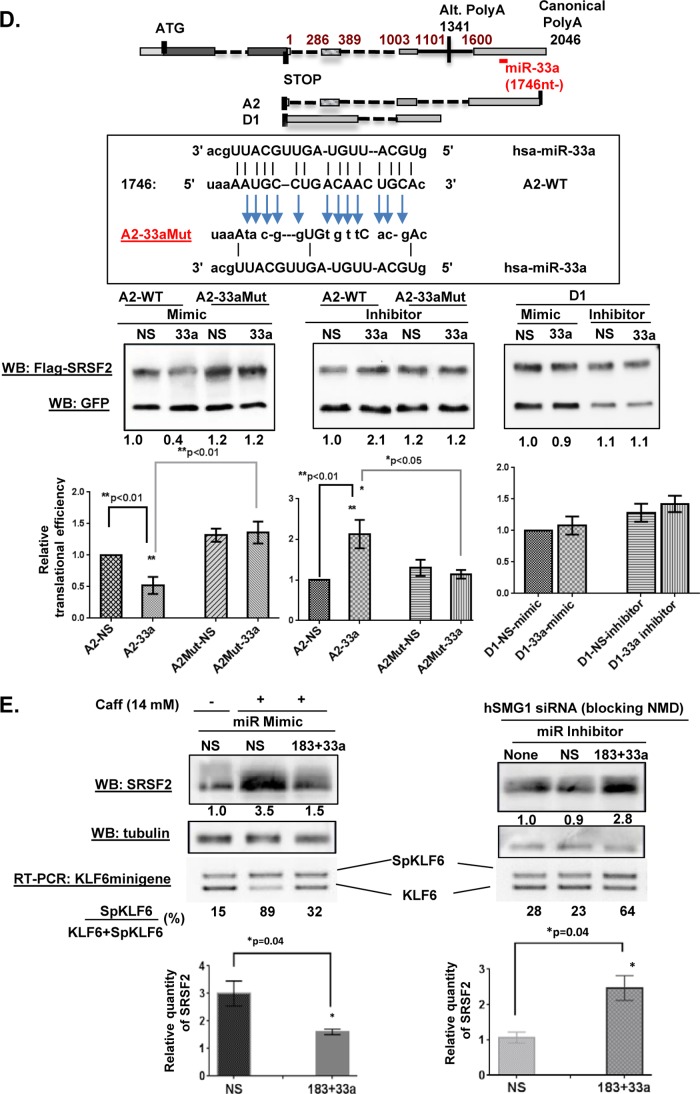
Caffeine decreases levels of SRSF2-targeting miRs to increase translational efficiency of certain SRSF2 transcripts. A, strategy for identifying putative caffeine-decreased SRSF2-targeting miRs in HeLa cells (lower, left) and schematic of miR binding sites within the SRSF2 3′ UTR (top). Two candidates were selected (miR-183-5p and miR-33a-5p) and mapped to GA and GD transcripts. B, microRNA array analyses revealed caffeine-mediated decreases in the levels of miR-183-5p and miR-33a-5p (left). This decrease was validated by quantitative RT-PCR (right). The final Ct value was an average of 5 repeats of each assay, and each assay was repeated three times. A difference with a p < 0.05 was considered significant. C, the miR-183-5p binding site in the FLAG-SRSF2 A1 transcript was disabled by site-directed mutagenesis (top). Either A-1WT or A1–183Mut was co-transfected with either miR-183-5p mimics or inhibitor. GFP was included in the transfection mixture as transfection efficiency control. Each assay was repeated three times. A difference at p < 0.05 was considered significant. D, the miR-33a-5p binding site in FLAG-SRSF2 A2 transcript was disabled by site-directed mutagenesis (top). Either A-2WT or A2–33aMut was co-transfected together with miR-33a-5p mimics or inhibitors. GFP was included as the transfection efficiency control. E, levels of endogenous SRSF2 and alternative splicing of KLF6 minigene were analyzed after cells were transfected with miR mimics in the presence of caffeine (left panel) or miR inhibitors in the presence of hSMG1 siRNA to block NMD (right panel). Nonspecific miRs served as controls. Each assay was repeated three times. Statistical analyses were performed using GraphPad Prism version 6 software, and significance was determined by the non-parametric t test. A difference with a p < 0.05 was considered significant. Error bars represent mean ± S.D. WB, Western blot.
To test the hypothesis that these miRs impact translation of specific SRSF2 transcripts, the levels of miR-183-5p and miR-33a-5p were manipulated using either specific miR mimics or specific miR inhibitors, which were co-transfected into HeLa cells along with one of the putative target SRSF2 transcripts. We reasoned that if the miR indeed regulated the translation of the co-transfected SRSF2 transcripts, differences should be observed in their translational efficiency when compared with controls. Similar to what was employed for the cell-based transfection-translation assays in Fig. 6, a GFP expression plasmid was used to control for transfection efficiency. 18–20 h post-transfection, both FLAG-SRSF2 and GFP levels were assessed by Western blotting, and translation efficiency was determined by normalizing FLAG-SRSF2 levels to GFP levels. As shown in Fig. 7C, the translation efficiency of the wild-type A1 transcript (A1-WT), predicted to be repressed by miR-183-5p, was further decreased by miR-183-5p mimics and increased by miR-183-5p inhibitors. Importantly, when the putative miR-183-5p binding site within A1 was mutated (A1–183Mut), neither the miR-183-5p mimic nor the inhibitor had a significant impact on A1–183 mutant translation. Moreover, neither the miR-183-5p mimic nor inhibitor had an effect on translation of A2, which lacks the miR-183-5p binding site. Taken together, these results identify miR-183-5p as an SRSF2 (A1)-targeting miR that binds to the 3′ UTR at 1159 nt downstream of the stop codon to repress A1 translation. Similar assays using A2 WT and A2–33aMut also identified miR-33a-5p as an SRSF2-targeting miR imposing translational regulation (Fig. 7D), with D1 serving as a negative control.
To further confirm the effect of miR-183-5p and miR-33a-5p on caffeine-mediated SRSF2 translation, miR-183-5p and miR-33a-5p mimics were co-transfected with the KLF6 minigene, followed by caffeine treatment. As shown in Fig. 7E (left panel, top), whereas caffeine increased endogenous SRSF2 by almost 4-fold in the presence of nonspecific miR mimics, this effect was largely reduced in the presence of the specific miR mimics with a concomitant decrease in KLF6 alternative splicing (∼3-fold, from 89 to 32%). We next investigated the effect that down-regulation of miR-183-5p and miR-33a-5p had on SRSF2 and KLF6, reasoning that this should mimic at least some of the effects of caffeine. Because our data suggested that caffeine has two activities that regulate SRSF2/KLF6, down-regulation of the SRSF2-targeting miRs and inhibition of NMD, we inhibited NMD by siRNA-mediated down-regulation of the key NMD protein, hSMG1 (37), which is also the direct target of caffeine (31, 32), in this experiment. As shown in Fig. 7E, right panel, when NMD is inhibited, down-regulation of the miRs increased SRSF2 levels (∼3-fold) and KLF6 alternative splicing (∼3-fold, from 23 to 68%). Taken together, our results reveal a novel effect of caffeine on miR regulation that in turn impacts SRSF2 translation and KLF6 alternative splicing, supporting the hypothesis that caffeine-induced SRSF2 translational regulation contributes significantly to caffeine-induced increases in SRSF2 protein levels and alternative splicing decisions.
Discussion
We have previously reported that caffeine can impact AS of a subset of cancer-related genes. Using KLF6 as a model, we demonstrated that the change in the KLF6 splicing pattern could not be mimicked by inhibition of NMD, even though the caffeine-induced KLF6 splice variant was a potential target for this RNA surveillance mechanism. Instead, the altered splicing of KLF6 was a result, at least in part, of a caffeine-induced increase (∼6-fold) in levels of the splicing factor SRSF2 (29). In this report, we show that additional methylxanthines can also induce AS of KLF6, with a concomitant increase in SRSF2 levels. SRSF2 gene expression is known to be guarded by a negative feedback loop, and a low steady level of SRSF2 is important for homeostasis of isoform expression as well as cell proliferation and genome stability (18). Thus, it was important to understand how methylxanthines, using caffeine as a prototype, had affected this normally unsustainable increase of SRSF2. We demonstrate that, in addition to its ability to inhibit NMD, caffeine can also regulate the expression of specific microRNAs, resulting in changes in translation efficiency of SRSF2 transcripts. Together, these modifications result in increased translation of certain SRSF2 variants and stabilization of NMD-sensitive SRSF2 transcripts with higher intrinsic translational efficiency; together these caffeine-induced changes lead to a surge of SRSF2 protein levels and an increase in SRSF2 splice variants designated for degradation by NMD, and the cycle continued. Thus, caffeine breaks the negative feedback loop controlling SRSF2 gene expression and further enforces a positive feed-forward loop that fuels the increased production of SRSF2 (see model in Fig. 8). Although we have not yet determined whether caffeine plays a similar role in vivo, it is likely that it is mimicking an endogenous process or processes that are responsible for fine-tuning SRSF2 gene expression in response to developmental or environmental signals, or during pathogenesis. Thus, caffeine represents a valuable tool to dissect this mechanism.
FIGURE 8.
Proposed model illustrating the mechanisms by which caffeine increases SRSF2 protein expression. Under normal conditions, SRSF2 homeostasis is maintained by a complex interplay of post-transcriptional mechanisms including microRNA-mediated translation repression (left panel) and an alternative splicing associated NMD (AS-NMD), the autoregulatory feedback loop (middle panel). Translation of the major SRSF2 transcripts A1 and D2 are suppressed by specific miRs binding to the 3′ UTR. When SRSF2 levels are increased due to intrinsic or environmental signals, the increased SRSF2 promotes alternative splicing at the 3′ UTR, resulting in multiple splice variants such as A2, A3, D1, and D3. These transcripts are destined for NMD, thereby decreasing the level of SRSF2 mRNA and the production of SRSF2 protein. Caffeine has two key effects on SRSF2 homeostasis regulation. First, caffeine decreases the levels of SRSF2-targeting miRs, releasing translational repression of the major SRSF2 transcripts, allowing a surge of SRSF2 protein synthesis. This increased SRSF2 triggers the synthesis of splice variants A2, A3, D1, and D3, normally substrates for NMD. However, caffeine also inhibits NMD, blocking the degradation of SRSF2 transcript variants. Thus, caffeine affects multiple regulatory mechanisms breaking the negative feedback loop resulting in a sustained increase of SRSF2 protein.
Previous studies have identified two SRSF2 3′ UTR variants induced by increased SRSF2 levels or depletion of the splicing repressor HnRNP H (27, 28, 30). These SRSF2 variants of the major transcript, A1, correspond to A2 and A3 in this study. In this study, we identified additional groups of transcripts, GB, GC, and GD. Most of these novel variants are potential targets of NMD, indicating that they are probably part of the AS-NMD circuit regulating SRSF2 protein levels (3, 49). For the major GA transcripts, translation of the variants in cultured cells fit the general dogma, variants with shorter 3′ UTRs were translated with the highest efficiency (50). However, translation of GD variants was less conventional, in that the variant with the intermediate size 3′ UTR (D2) was translated with the highest efficiency (Fig. 6). Interestingly, this difference among GD transcripts was not obvious in cell-free transcription/translation assays (Fig. 6A), suggesting that other cellular processes/signals, most likely interacting with the 3′ UTR sequence between 60 and 543 nt downstream of the stop codon, are involved. The GD transcripts account for a relatively small portion of the total SRSF2 mRNAs in HeLa cells, but appear to make a marked contribution to SRSF2 gene regulation, which may be more critical in tissue types or cell lines that preferentially utilize the alternative polyadenylation site (51). Our study is the most thorough examination of SRSF2 transcripts and their translational efficiency to date. Although the expression and role of these variants in different cells/tissues, and under different physiological and pathological conditions, has yet to be determined, this study, together with the comprehensive analysis of SRSF1 by the Krainer group (52), contributes to our understanding of the complex regulation of SR proteins gene expression.
One unexpected result of these studies was that depletion of hUpf1, best known as an essential factor in the NMD pathway, had little effect on SRSF2 levels alone, but prevented induction of SRSF2 by caffeine (Fig. 5C). Interestingly, recent studies suggest additional functions of hUpf1 other than in NMD, including a role in the regulation of protein translation. Yoneda and colleagues (53) recently reported that hUpf1 was required for Stau2 overexpression-mediated induction of translation, whereas Moore and colleagues (54) suggested that hUpf1, when associated with Exon junction complex, may contribute to the translation of spliced mRNAs. Indeed, NMD factors may be involved in both RNA degradation and enhanced translation when splicing efficiency is improved (55). Given that our model indicates a substantial contribution from caffeine-stabilized/NMD-targeted SRSF2 transcripts to caffeine-mediated SRSF2 increase, we speculate that hUpf1 may be required in translation of these transcripts. Further studies will be needed to validate this hypothesis.
While searching for a second mechanism for the caffeine induction of SRSF2, we identified a novel action of this methylxanthine, the regulation of miR expression. miR-mediated gene expression regulation is a well recognized post-transcriptional mechanism that either represses translation and/or destabilizes mRNA transcripts to control protein production (44, 56–58). Nearly 60% of protein-coding genes are predicted to be targeted by microRNAs and most microRNAs act by base pairing with the 3′ UTR of the targeted mRNA (59). One gene could potentially be targeted by multiple microRNAs; however, it is unlikely that all of them would be functional at the same time because miRs are expressed in a tissue- and development-specific manner (44, 56–58, 60). For SRSF2, algorithms including microRNA (microrna.org) and miRScan predict ∼185 putative miR binding sites within the 3′ UTR. Only two of these have been previously validated: miR-183-5p in stress response (47) and miR-193a-3p in cancer drug resistance (48). We now show that caffeine can regulate a subset of miRs, including miR-183-5p, which targets A1 and possibly all GD members (Fig. 7). The down-regulation of miR-183-5p by caffeine was rapid, dramatic, and persistent (Fig. 7B and supplemental Tables S1 and S2), supporting a significant role of this down-regulation in caffeine-mediated SRSF2 protein induction. In addition, we have for the first time validated miR-33a-5p as an SRSF2-targeting miR, and shown that this miR is also down-regulated by caffeine. Because the putative binding site for miR-33a-5p is located 1746 nt downstream of the stop codon, this miR primarily impacted protein production of transcripts using the canonical polyadenylation site such as the GA group, the major group of SRSF2 transcripts.
It should be noted that the miR-183-5p binding site, shared by A1 and D1–D3, overlaps a putative ARE (A-U rich element) (56). AREs are found in the 3′ UTR of many mRNAs and can interact with a number of proteins to either stabilize (the Hu family of proteins) or destabilize (AUF1, TTP, BRF1, TIA-1, TIAR, and KSRP) the mRNA (57–60), a common determinant of RNA stability. To date, the putative AREs within SRSF2 3′ UTR have not been functionally tested, nor have their cognate binding proteins, either stabilizing or destabilizing, been identified. Nevertheless, it is feasible that their proximity to the miR binding site may impact miR activity under certain conditions. The function of these AREs and their potential impact on the miR activity and SFSF2 RNA stability (or vice versa) is currently under investigation.
Our results suggest that aberrant expression of either miR-183-5p or miR-33a-5p would have a marked impact on SRSF2 protein production, which in turn could influence alternative splicing choices in SRSF2-targeted genes, including many cancer-related genes. Indeed, abnormal levels of miR-183-5p have been observed in various tumors (61), where it appears to play a role in tumor progression due to its impact on proliferation, apoptosis, and metastasis (62–65). Altered regulation of miR-183-5p has also made it an attractive biomarker candidate (66–72). miR-33a-5p has also been suggested to play a role in tumorigenesis (73–75), as well as in controlling cholesterol and lipid metabolism (76, 77). However, to date there has been no evidence to link aberrant expression of these miRs to AS in cancers. Therefore, our observation that alteration of these miRs can have a profound effect on the expression of SRSF2, a critical splicing factor regulating genes involved in cell proliferation, cell death and metastasis, warrants further studies.
It is not yet known how caffeine modulates levels of miR-183-5p, miR-33a-5p, or other miRs. Because transcriptional regulation can be influenced by caffeine (78, 79, 80), we compared the effects of caffeine on miR-183-5p levels to that of two additional miRs within the miR-183-96-182 cluster, miR-96 or miR-182, because they share the same promoter (81, 82). No significant effect was observed (supplemental Tables S1 and S2), suggesting that transcriptional regulation is unlikely to be the underlying mechanism. Given the pleiotropic effect of caffeine on cells (83–85), it is possible that multiple mechanisms may be involved in caffeine-mediated miR regulation.
In summary, we have shown that caffeine regulates expression of SRSF2 through a complex set of post-transcriptional mechanisms that work together to break the AS-NMD feedback loop and foster a feed-forward loop that increases SRSF2 protein levels. Our studies to date underscore the complicated interplay of multiple mechanisms needed to control SRSF2 expression that in turn controls the expression of a large subset of target genes. This knowledge may provide a foundation for exploring the aberrant regulation of SRSF2 and target genes in pathogenesis.
Supplementary Material
Acknowledgments
We thank Drs. Cyril F. Bourgeois and James Stevenin at Institut de Je′ne′tique et de Biologie Mole′culaire et Cellulaire, France, for generously providing SRSF2(SC35) monoclonal antibody.
Note Added in Proof
Supplemental Tables S1 and S2 were missing in the version of this article that was published as a Paper in Press on March 28, 2015. The supplemental tables are now available.
This work was supported, in whole or in part, by National Institutes of Health Grants NCI-R01 CA 122573 (to K. W. S.) and NCI-P30CA072720 to the Rutgers Cancer Institute of New Jersey and Microarray Core facility and Bioinformatics Core facility of the Rutgers Cancer Institute of New Jersey Grant P30CA072720.

This article contains supplemental Tables S1 and S2.
- AS
- alternative splicing
- NMD
- nonsense-mediated decay
- nt
- nucleotide
- TARDBP
- TAR DNA-binding protein.
References
- 1. Takeda J., Suzuki Y., Sakate R., Sato Y., Gojobori T., Imanishi T., Sugano S. (2010) H-DBAS: human-transcriptome database for alternative splicing: update 2010. Nucleic Acids Res. 38, D86-D90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauman J. A., Kole R. (2011) Modulation of RNA splicing as a potential treatment for cancer. Bioeng. Bugs 2, 125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGlincy N. J., Smith C. W. (2008) Alternative splicing resulting in nonsense-mediated mRNA decay: what is the meaning of nonsense? Trends Biochem. Sci. 33, 385–393 [DOI] [PubMed] [Google Scholar]
- 4. Glisovic T., Bachorik J. L., Yong J., Dreyfuss G. (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 582, 1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ajay S. S., Athey B. D., Lee I. (2010) Unified translation repression mechanism for microRNAs and upstream AUGs. BMC Genomics 11, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galbán S., Kuwano Y., Pullmann R., Jr., Martindale J. L., Kim H. H., Lal A., Abdelmohsen K., Yang X., Dang Y., Liu J. O., Lewis S. M., Holcik M., Gorospe M. (2008) RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1α. Mol. Cell. Biol. 28, 93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato Y., Nakamura A. (2009) Mechanisms underlying maternal RNA translation and localization during Drosophila oogenesis. Tanpakushitsu Kakusan Koso 54, 2159–2164 [PubMed] [Google Scholar]
- 8. Kalsotra A., Cooper T. A. (2011) Functional consequences of developmentally regulated alternative splicing. Nat. Rev. Genet. 12, 715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braeutigam C., Rago L., Rolke A., Waldmeier L., Christofori G., Winter J. (2014) The RNA-binding protein Rbfox2: an essential regulator of EMT-driven alternative splicing and a mediator of cellular invasion. Oncogene 33, 1082–1092 [DOI] [PubMed] [Google Scholar]
- 10. Coelho M. B., Smith C. W. (2014) Regulation of alternative pre-mRNA splicing. Methods Mol. Biol. 1126, 55–82 [DOI] [PubMed] [Google Scholar]
- 11. Smith C. W., Valcárcel J. (2000) Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 25, 381–388 [DOI] [PubMed] [Google Scholar]
- 12. Cáceres J. F., Kornblihtt A. R. (2002) Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 18, 186–193 [DOI] [PubMed] [Google Scholar]
- 13. Mendell J. T., Sharifi N. A., Meyers J. L., Martinez-Murillo F., Dietz H. C. (2004) Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 36, 1073–1078 [DOI] [PubMed] [Google Scholar]
- 14. McIlwain D. R., Pan Q., Reilly P. T., Elia A. J., McCracken S., Wakeham A. C., Itie-Youten A., Blencowe B. J., Mak T. W. (2010) Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc. Natl. Acad. Sci. U.S.A. 107, 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saltzman A. L., Kim Y. K., Pan Q., Fagnani M. M., Maquat L. E., Blencowe B. J. (2008) Regulation of multiple core spliceosomal proteins by alternative splicing-coupled nonsense-mediated mRNA decay. Mol. Cell Biol. 28, 4320–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu X. D., Mayeda A., Maniatis T., Krainer A. R. (1992) General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative 5′ and 3′ splice site selection. Proc. Natl. Acad. Sci. U.S.A. 89, 11224–11228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacke R., Manley J. L. (1995) The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 14, 3540–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao R., Sun Y., Ding J. H., Lin S., Rose D. W., Rosenfeld M. G., Fu X. D., Li X. (2007) Splicing regulator SC35 is essential for genomic stability and cell proliferation during mammalian organogenesis. Mol. Cell. Biol. 27, 5393–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji X., Zhou Y., Pandit S., Huang J., Li H., Lin C. Y., Xiao R., Burge C. B., Fu X. D. (2013) SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 153, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin S., Coutinho-Mansfield G., Wang D., Pandit S., Fu X. D. (2008) The splicing factor SC35 has an active role in transcriptional elongation. Nat. Struct. Mol. Biol. 15, 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merdzhanova G., Edmond V., De Seranno S., Van den Broeck A., Corcos L., Brambilla C., Brambilla E., Gazzeri S., Eymin B. (2008) E2F1 controls alternative splicing pattern of genes involved in apoptosis through upregulation of the splicing factor SC35. Cell Death Differ. 15, 1815–1823 [DOI] [PubMed] [Google Scholar]
- 22. Lu Y., Loh Y. H., Li H., Cesana M., Ficarro S. B., Parikh J. R., Salomonis N., Toh C. X., Andreadis S. T., Luckey C. J., Collins J. J., Daley G. Q., Marto J. A. (2014) Alternative splicing of MBD2 supports self-renewal in human pluripotent stem cells. Cell Stem Cell 15, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Müller-Thomas C., Rudelius M., Rondak I. C., Haferlach T., Schanz J., Huberle C., Schmidt B., Blaser R., Kremer M., Peschel C., Germing U., Platzbecker U., Götze K. (2014) Response to azacitidine is independent of p53 expression in higher-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica 99, e179–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsson C. A., Cote G., Quintás-Cardama A. (2013) The changing mutational landscape of acute myeloid leukemia and myelodysplastic syndrome. Mol. Cancer Res. 11, 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lasho T. L., Jimma T., Finke C. M., Patnaik M., Hanson C. A., Ketterling R. P., Pardanani A., Tefferi A. (2012) SRSF2 mutations in primary myelofibrosis: significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood 120, 4168–4171 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida K., Sanada M., Shiraishi Y., Nowak D., Nagata Y., Yamamoto R., Sato Y., Sato-Otsubo A., Kon A., Nagasaki M., Chalkidis G., Suzuki Y., Shiosaka M., Kawahata R., Yamaguchi T., Otsu M., Obara N., Sakata-Yanagimoto M., Ishiyama K., Mori H., Nolte F., Hofmann W. K., Miyawaki S., Sugano S., Haferlach C., Koeffler H. P., Shih L. Y., Haferlach T., Chiba S., Nakauchi H., Miyano S., Ogawa S. (2011) Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 [DOI] [PubMed] [Google Scholar]
- 27. Sureau A., Gattoni R., Dooghe Y., Stévenin J., Soret J. (2001) SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. EMBO J. 20, 1785–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dreumont N., Hardy S., Behm-Ansmant I., Kister L., Branlant C., Stévenin J., Bourgeois C. F. (2010) Antagonistic factors control the unproductive splicing of SC35 terminal intron. Nucleic Acids Res. 38, 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi J., Hu Z., Pabon K., Scotto K. W. (2008) Caffeine regulates alternative splicing in a subset of cancer-associated genes: a role for SC35. Mol. Cell. Biol. 28, 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lareau L. F., Brooks A. N., Soergel D. A., Meng Q., Brenner S. E. (2007) The coupling of alternative splicing and nonsense-mediated mRNA decay. Adv. Exp. Med. Biol. 623, 190–211 [DOI] [PubMed] [Google Scholar]
- 31. Usuki F., Yamashita A., Higuchi I., Ohnishi T., Shiraishi T., Osame M., Ohno S. (2004) Inhibition of nonsense-mediated mRNA decay rescues the phenotype in Ullrich's disease. Ann. Neurol. 55, 740–744 [DOI] [PubMed] [Google Scholar]
- 32. Ivanov I., Lo K. C., Hawthorn L., Cowell J. K., Ionov Y. (2007) Identifying candidate colon cancer tumor suppressor genes using inhibition of nonsense-mediated mRNA decay in colon cancer cells. Oncogene 26, 2873–2884 [DOI] [PubMed] [Google Scholar]
- 33. Morinière M., Delhommeau F., Calender A., Ribeiro L., Delaunay J., Baklouti F. (2010) Nonsense-mediated mRNA decay (NMD) blockage promotes nonsense mRNA stabilization in protein 4.1R deficient cells carrying the 4.1R Coimbra variant of hereditary elliptocytosis. Blood Cells Mol. Dis. 45, 284–288 [DOI] [PubMed] [Google Scholar]
- 34. Johnson J. K., Waddell N., kConFab Investigators, and Chenevix-Trench G. (2012) The application of nonsense-mediated mRNA decay inhibition to the identification of breast cancer susceptibility genes. BMC Cancer 12, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dang Y., Low W. K., Xu J., Gehring N. H., Dietz H. C., Romo D., Liu J. O. (2009) Inhibition of nonsense-mediated mRNA decay by the natural product pateamine A through eukaryotic initiation factor 4AIII. J. Biol. Chem. 284, 23613–23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mendell J. T., ap Rhys C. M., Dietz H. C. (2002) Separable roles for rent1/hUpf1 in altered splicing and decay of nonsense transcripts. Science 298, 419–422 [DOI] [PubMed] [Google Scholar]
- 37. Usuki F., Yamashita A., Kashima I., Higuchi I., Osame M., Ohno S. (2006) Specific inhibition of nonsense-mediated mRNA decay components, SMG-1 or Upf1, rescues the phenotype of Ullrich disease fibroblasts. Mol. Ther. 14, 351–360 [DOI] [PubMed] [Google Scholar]
- 38. Akaike Y., Kurokawa K., Kajita K., Kuwano Y., Masuda K., Nishida K., Kang S. W., Tanahashi T., Rokutan K. (2011) Skipping of an alternative intron in the srsf1 3′ untranslated region increases transcript stability. J. Med. Invest. 58, 180–187 [DOI] [PubMed] [Google Scholar]
- 39. Wollerton M. C., Gooding C., Wagner E. J., Garcia-Blanco M. A., Smith C. W. (2004) Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol. Cell 13, 91–100 [DOI] [PubMed] [Google Scholar]
- 40. Jia J., Yao P., Arif A., Fox P. L. (2013) Regulation and dysregulation of 3′UTR-mediated translational control. Curr. Opin. Genet. Dev. 23, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ratti A., Fallini C., Colombrita C., Pascale A., Laforenza U., Quattrone A., Silani V. (2008) Post-transcriptional regulation of neuro-oncological ventral antigen 1 by the neuronal RNA-binding proteins ELAV. J. Biol. Chem. 283, 7531–7541 [DOI] [PubMed] [Google Scholar]
- 42. Khabar K. S. (2010) Post-transcriptional control during chronic inflammation and cancer: a focus on AU-rich elements. Cell. Mol. Life Sci. 67, 2937–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muroski M. E., Kogot J. M., Strouse G. F. (2012) Bimodal gold nanoparticle therapeutics for manipulating exogenous and endogenous protein levels in mammalian cells. J. Am. Chem. Soc. 134, 19722–19730 [DOI] [PubMed] [Google Scholar]
- 44. Baek D., Villén J., Shin C., Camargo F. D., Gygi S. P., Bartel D. P. (2008) The impact of microRNAs on protein output. Nature 455, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farazi T. A., Hoell J. I., Morozov P., Tuschl T. (2013) MicroRNAs in human cancer. Adv. Exp. Med. Biol. 774, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Risso G., Pelisch F., Quaglino A., Pozzi B., Srebrow A. (2012) Regulating the regulators: serine/arginine-rich proteins under scrutiny. IUBMB Life 64, 809–816 [DOI] [PubMed] [Google Scholar]
- 47. Meerson A., Cacheaux L., Goosens K. A., Sapolsky R. M., Soreq H., Kaufer D. (2010) Changes in brain MicroRNAs contribute to cholinergic stress reactions. J. Mol. Neurosci. 40, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma K., He Y., Zhang H., Fei Q., Niu D., Wang D., Ding X., Xu H., Chen X., Zhu J. (2012) DNA methylation-regulated miR-193a-3p dictates resistance of hepatocellular carcinoma to 5-fluorouracil via repression of SRSF2 expression. J. Biol. Chem. 287, 5639–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ni J. Z., Grate L., Donohue J. P., Preston C., Nobida N., O'Brien G., Shiue L., Clark T. A., Blume J. E., Ares M., Jr. (2007) Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 21, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandberg R., Neilson J. R., Sarma A., Sharp P. A., Burge C. B. (2008) Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang H., Lee J. Y., Tian B. (2005) Biased alternative polyadenylation in human tissues. Genome Biol. 6, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun S., Zhang Z., Sinha R., Karni R., Krainer A. R. (2010) SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nat. Struct. Mol. Biol. 17, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miki T., Kamikawa Y., Kurono S., Kaneko Y., Katahira J., Yoneda Y. (2011) Cell type-dependent gene regulation by Staufen2 in conjunction with Upf1. BMC Mol. Biol. 12, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nott A., Le Hir H., Moore M. J. (2004) Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev. 18, 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gudikote J. P., Imam J. S., Garcia R. F., Wilkinson M. F. (2005) RNA splicing promotes translation and RNA surveillance. Nat. Struct. Mol. Biol. 12, 801–809 [DOI] [PubMed] [Google Scholar]
- 56. Bhattacharya S., Giordano T., Brewer G., Malter J. S. (1999) Identification of AUF-1 ligands reveals vast diversity of early response gene mRNAs. Nucleic Acids Res. 27, 1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. von Roretz C., Di Marco S., Mazroui R., Gallouzi I. E. (2011) Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip. Rev. RNA 2, 336–347 [DOI] [PubMed] [Google Scholar]
- 58. Zhuang R., Rao J. N., Zou T., Liu L., Xiao L., Cao S., Hansraj N. Z., Gorospe M., Wang J. Y. (2013) miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration. Nucleic Acids Res. 41, 7905–7919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee H. H., Kim W. T., Kim D. H., Park J. W., Kang T. H., Chung J. W., Leem S. H. (2013) Tristetraprolin suppresses AHRR expression through mRNA destabilization. FEBS Lett. 587, 1518–1523 [DOI] [PubMed] [Google Scholar]
- 60. Maitra S., Chou C. F., Luber C. A., Lee K. Y., Mann M., Chen C. Y. (2008) The AU-rich element mRNA decay-promoting activity of BRF1 is regulated by mitogen-activated protein kinase-activated protein kinase 2. RNA 14, 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Q. H., Sun H. M., Zheng R. Z., Li Y. C., Zhang Q., Cheng P., Tang Z. H., Huang F. (2013) Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene 527, 26–32 [DOI] [PubMed] [Google Scholar]
- 62. Zhu J., Feng Y., Ke Z., Yang Z., Zhou J., Huang X., Wang L. (2012) Down-regulation of miR-183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am. J. Pathol. 180, 2440–2451 [DOI] [PubMed] [Google Scholar]
- 63. Zhang Z., Li S., Cheng S. Y. (2013) The miR-183 approximately 96 approximately 182 cluster promotes tumorigenesis in a mouse model of medulloblastoma. J. Biomed. Res. 27, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shi X. Y., Gu L., Chen J., Guo X. R., Shi Y. L. (2014) Downregulation of miR-183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. Int. J. Mol. Med. 33, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoshino H., Seki N., Itesako T., Chiyomaru T., Nakagawa M., Enokida H. (2013) Aberrant expression of microRNAs in bladder cancer. Nat. Rev. Urol. 10, 396–404 [DOI] [PubMed] [Google Scholar]
- 66. Silva-Santos R. M., Costa-Pinheiro P., Luis A., Antunes L., Lobo F., Oliveira J., Henrique R., Jerónimo C. (2013) MicroRNA profile: a promising ancillary tool for accurate renal cell tumour diagnosis. Br. J. Cancer 109, 2646–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Võsa U., Vooder T., Kolde R., Vilo J., Metspalu A., Annilo T. (2013) Meta-analysis of microRNA expression in lung cancer. Int. J. Cancer 132, 2884–2893 [DOI] [PubMed] [Google Scholar]
- 68. Rizos E., Siafakas N., Koumarianou A., Katsantoni E., Filippopoulou A., Ntounas P., Touloumis Ch., Kastania A., Zoumpourlis V. (2012) miR-183 as a molecular and protective biomarker for cancer in schizophrenic subjects. Oncol. Rep. 28, 2200–2204 [DOI] [PubMed] [Google Scholar]
- 69. Lin Q., Mao W., Shu Y., Lin F., Liu S., Shen H., Gao W., Li S., Shen D. (2012) A cluster of specified microRNAs in peripheral blood as biomarkers for metastatic non-small-cell lung cancer by stem-loop RT-PCR. J. Cancer Res. Clin. Oncol. 138, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu W., Liu X., He J., Chen D., Hunag Y., Zhang Y. K. (2011) Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: a case control study. BMC Cancer 11, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamada Y., Enokida H., Kojima S., Kawakami K., Chiyomaru T., Tatarano S., Yoshino H., Kawahara K., Nishiyama K., Seki N., Nakagawa M. (2011) MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 102, 522–529 [DOI] [PubMed] [Google Scholar]
- 72. Schaefer A., Jung M., Mollenkopf H. J., Wagner I., Stephan C., Jentzmik F., Miller K., Lein M., Kristiansen G., Jung K. (2010) Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int. J. Cancer 126, 1166–1176 [DOI] [PubMed] [Google Scholar]
- 73. Kuo P. L., Liao S. H., Hung J. Y., Huang M. S., Hsu Y. L. (2013) MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim. Biophys. Acta 1830, 3756–3766 [DOI] [PubMed] [Google Scholar]
- 74. Cirera-Salinas D., Pauta M., Allen R. M., Salerno A. G., Ramírez C. M., Chamorro-Jorganes A., Wanschel A. C., Lasuncion M. A., Morales-Ruiz M., Suarez Y., Baldan Á., Esplugues E., Fernández-Hernando C. (2012) Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle 11, 922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thomas M., Lange-Grünweller K., Weirauch U., Gutsch D., Aigner A., Grünweller A., Hartmann R. K. (2012) The proto-oncogene Pim-1 is a target of miR-33a. Oncogene 31, 918–928 [DOI] [PubMed] [Google Scholar]
- 76. Wijesekara N., Zhang L. H., Kang M. H., Abraham T., Bhattacharjee A., Warnock G. L., Verchere C. B., Hayden M. R. (2012) miR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes 61, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Najafi-Shoushtari S. H., Kristo F., Li Y., Shioda T., Cohen D. E., Gerszten R. E., Näär A. M. (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hardingham G. E., Cruzalegui F. H., Chawla S., Bading H. (1998) Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium 23, 131–134 [DOI] [PubMed] [Google Scholar]
- 79. Svenningsson P., Nomikos G. G., Fredholm B. B. (1999) The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J. Neurosci. 19, 4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dassesse D., Vanderwinden J. M., Goldberg I., Vanderhaeghen J. J., Schiffmann S. N. (1999) Caffeine-mediated induction of c-fos, zif-268 and arc expression through A1 receptors in the striatum: different interactions with the dopaminergic system. Eur. J. Neurosci. 11, 3101–3114 [DOI] [PubMed] [Google Scholar]
- 81. Tang X., Zheng D., Hu P., Zeng Z., Li M., Tucker L., Monahan R., Resnick M. B., Liu M., Ramratnam B. (2014) Glycogen synthase kinase 3β inhibits microRNA-183-96-182 cluster via the β-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 42, 2988–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mihelich B. L., Khramtsova E. A., Arva N., Vaishnav A., Johnson D. N., Giangreco A. A., Martens-Uzunova E., Bagasra O., Kajdacsy-Balla A., Nonn L. (2011) miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells. J. Biol. Chem. 286, 44503–44511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuranda K., Leberre V., Sokol S., Palamarczyk G., François J. (2006) Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 61, 1147–1166 [DOI] [PubMed] [Google Scholar]
- 84. Rao F. V., Andersen O. A., Vora K. A., Demartino J. A., van Aalten D. M. (2005) Methylxanthine drugs are chitinase inhibitors: investigation of inhibition and binding modes. Chem. Biol. 12, 973–980 [DOI] [PubMed] [Google Scholar]
- 85. Tornaletti S., Russo P., Parodi S., Pedrini A. M. (1989) Studies on DNA binding of caffeine and derivatives: evidence of intercalation by DNA-unwinding experiments. Biochim. Biophys. Acta 1007, 112–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.