Background: Down-regulation of Cl−/HCO3− exchanger SLC26A3 in gut inflammation results in diarrhea. Does all-trans-retinoic acid (ATRA), an anti-inflammatory agent, affect SLC26A3 (DRA)?
Results: ATRA increases DRA expression in enterocytes via transcriptional activation through RAR-β/HNF-1β signaling.
Conclusion: ATRA up-regulates DRA expression.
Significance: ATRA may act as a therapeutic agent for diarrhea by augmenting DRA expression.
Keywords: chloride transport, epithelial cell, inflammation, intestinal epithelial, transporter
Abstract
All-trans-retinoic acid (ATRA) is an active vitamin A derivative known to modulate a number of physiological processes, including growth and development, differentiation, and gene transcription. The protective effect of ATRA in gut inflammation and diarrheal diseases has been documented. In this regard, down-regulated in adenoma (DRA, a key luminal membrane Cl− transporter involved in NaCl absorption) has been shown to be suppressed in intestinal inflammation. This suppression of DRA is associated with diarrheal phenotype. Therefore, current studies were undertaken to examine the effects of ATRA on DRA expression. DRA mRNA levels were significantly elevated (∼4-fold) in response to ATRA with induction starting as early as 8 h of incubation. Similarly, ATRA increased DRA protein expression by ∼50%. Furthermore, DRA promoter activity was significantly increased in response to ATRA indicating transcriptional activation. ATRA effects on DRA expression appeared to be mediated via the RAR-β receptor subtype, as ATRA remarkably induced RAR-β mRNA levels, whereas RAR-β knockdown substantially attenuated the ability of ATRA to increase DRA expression. Results obtained from agonist (CH-55) and antagonist (LE-135) studies further confirmed that ATRA exerts its effects through RAR-β. Furthermore, ATRA treatment resulted in a significant increase in HNF-1β mRNA levels. The ability of ATRA to induce DRA expression was inhibited in the presence of HNF-1β siRNA indicative of its involvement in ATRA-induced effects on DRA expression. In conclusion, ATRA may act as an antidiarrheal agent by increasing DRA expression via the RAR-β/HNF-1β-dependent pathway.
Introduction
All-trans-retinoic acid (ATRA),2 a major active metabolite of vitamin A, is known to regulate several biological processes. For example, ATRA signaling has been shown to be important for inducing cellular differentiation, growth, and development of epithelial cells in various tissues including intestine (1–3). Also, a role of vitamin A and its metabolites in maintenance of intestinal epithelial integrity (4, 5) and immune homeostasis (6) has been established. Other beneficial effects of ATRA on intestinal mucosa include attenuation of intestinal inflammation and injury in neonatal rat model of necrotizing enterocolitis (7), 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis (8), and human ulcerative colitis (9). Effect of vitamin A supplementation in reducing severity of diarrheal episodes and diarrhea-associated infant mortality has been reported (10, 11). Furthermore, substantial contribution of vitamin A in preventing diarrhea in children with HIV infection or exposure to maternal HIV infection is also documented (12). Reduced incidences of respiratory infection and diarrhea-associated mortality are observed in children supplemented with weekly low doses of vitamin A (13) or iron and vitamin A co-supplementation (14). Repletion therapy of this vitamin in deficient individuals has been shown to reduce the risk of diarrhea and gut-barrier dysfunction emphasizing the anti-diarrheal nature of this micronutrient (15). However, the mechanisms underlying the anti-diarrheal effects of vitamin A are not understood.
Diarrhea is the most debilitating symptom associated with enteric infections or intestinal inflammation (16, 17). Although multifactorial in nature, diarrhea usually occurs when there is increased fluid and electrolyte secretion and/or reduced absorption. In this regard, electroneutral NaCl absorption in the ileum and colon predominantly occurs via the coupled operation of apical Na+/H+ exchanger (NHE3) and Cl−/HCO3− exchanger SLC26A3 or DRA (down-regulated in adenoma). Several lines of data implicate dysregulation of DRA in pathophysiology of diarrheal disorders. For example, mutations in the DRA gene cause congenital chloride diarrhea, characterized by a high volume of watery diarrhea with a massive loss of chloride (18, 19). Also, diarrheal phenotype due to loss of luminal membrane Cl−/base exchange activity is the predominant feature exhibited by DRA (but not PAT-1) knock-out mice (20, 21). The importance of DRA in diarrheal disorders is further evident from studies demonstrating reduction in DRA expression in animal models of inflammatory and infectious diarrhea and in inflammatory bowel disease patients (22–24). Thus, agents that increase DRA function and expression can be utilized as potential anti-diarrheals. Indeed, previous studies from our laboratory have shown that DRA activity and expression is up-regulated by various anti-diarrheal agents such as the probiotic Lactobacillus acidophilus (LA) and the bioactive lipid lysophosphatidic acid. L. acidophilus and lysophosphatidic acid-mediated increase in apical Cl−/HCO3− exchange activity occurred via an increase in surface DRA levels as well as via increasing total cellular levels of DRA by transcriptional mechanisms (25–28). The role of nuclear transcription factors HNF-1α and -1β in regulating DRA expression has also been recently reported (29).
ATRA is known to mediate its effects by binding to nuclear receptors: retinoic acid receptors (RARs-α, -β, and -γ) and retinoic X receptors (RXRs-α, -β, and -γ) (30, 31). ATRA binds RAR that dimerizes with RXR to activate gene transcription. In view of the beneficial effects of ATRA in reducing diarrheal episodes, it was of interest to systematically study the effects of ATRA on DRA expression in intestinal epithelial cells and to elucidate the underlying molecular mechanisms. Utilizing Caco-2 cells as an in vitro model, our results demonstrated that ATRA stimulated DRA expression and promoter activity via RAR-β. This increase in DRA expression in response to ATRA was mediated via the involvement of transcription factor HNF-1β. These findings indicate that ATRA may have potential antidiarrheal effects and may be of benefit as a therapeutic target in the treatment of diarrhea associated with inflammatory or infectious disorders of the gut.
Experimental Procedures
Materials
All-trans-retinoic acid, 9-cis-retinoic acid, and 13-cis-retinoic acid were purchased from Sigma. ATRA was dissolved in 100% alcohol to a 10 mm stock solution and stored in the dark at −80 °C. Agonist and antagonist for RAR-β, CH-55, and LE-135 and for RXR, DHA, and HX-531, respectively, were obtained from Tocris Bioscience (Bristol, UK). Goat anti-rabbit antibody conjugated to horseradish peroxidase was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The luciferase assay system was procured from Promega (Madison, WI) and β-galactosidase assay kit was obtained from Clontech (Palo Alto, CA). All other chemicals were at least reagent grade and were obtained from either Sigma or Fisher Scientific (Pittsburgh, PA).
Cell Culture
Caco-2 cells and Eagle's minimum essential medium were obtained from ATCC (American Type Culture Collection, Manassas, VA). Cells were grown routinely in Eagle's minimum essential medium supplemented with 100 units/ml of penicillin, 100 μg/ml of streptomycin, 2 mg/liter of gentamicin, and 20% fetal bovine serum in 5% CO2, 95% air environment at 37 °C in T-150-cm2 plastic flasks. Cells between passages 25 and 45 were used for the present study. Caco-2 cells were plated on 24-well plates (Costar, Corning, NY) at a density of 2 × 104 cells/well. Fully differentiated Caco-2 monolayers (10–14 days post-plating) were treated with ATRA for 8–24 h in serum-free cell culture medium for assessment of DRA mRNA and protein expression. For the promoter studies, 1.30 × 107 cells/well were plated on a 24-well plate and transiently transfected by electroporation utilizing the Amaxa nucleofactor system while still in suspension. Cell monolayers grown on transwell inserts at a density of 4 × 103 cells/well were used for immunofluorescence staining at 11 days post-plating.
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted from control and treated Caco-2 cells using RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Equal amounts of RNA from both treated and control samples was reverse transcribed and amplified in a one-step reaction using Brilliant SYBR Green qRT-PCR Master Mix Kit (Agilent Technologies, Santa Clara, CA). Gene-specific primers used are listed in Table 1.
TABLE 1.
Primers used for real-time PCR
| Gene | Species | Accession No. | Primer sequence |
|---|---|---|---|
| SLC26A3 or DRA | Human | BC025671 | (F) 5′-TTCAGTTGCCAGCGTCTATTC-3′ |
| (R) 5′-GTGTTTTGCCTCCTGTGCTCT-3′ | |||
| SLC26A6 or PAT-1 | Human | NM_022911 | (F) 5′-AGATGCCCCACTACTCTGTCCT-3′ |
| (R) 5′-ATCCACACCACACCTCTGCTT-3′ | |||
| GAPDH | Human | NM_001101.3 | (F) 5′-GAAATCCCATCACCATCTTCC-3′ |
| (R) 5′-AAATGAGCCCCAGCCTTCT-3′ | |||
| HNF-1α | Human | NM_000545 | (F) 5′- TCCTGTATTTGTTCCCAAGAGCATC-3′ |
| (R) 5′-TCCCACAGGAGTAAGGACGACTTC-3′ | |||
| HNF-1β | Human | NM_000458 | (F) 5′-CCAAGCCGGTCTCCATACTC- 3′ |
| (R) 5′-TGGGAGGTGTGTCATAGTCGT-3′ | |||
| HNF-4α | Human | NM_175914 | (F) 5′-GTTCAAGGACGTGCTGCTCCTA-3′ |
| (R) 5′-ATGGACACCCGGCTCATCTC-3′ | |||
| RAR-α | Human | NM_001145301 | (F) 5′-GGGCAAATACACTACGAACAACA-3′ |
| (R) 5′-CTCCACAGTCTTAATGATGCACT-3′ | |||
| RAR-β | Human | NM_000965 | (F) 5′-TGGTGTCTTGTTCTGGGGTAT-3′ |
| (R) 5′-CGTGGAGTTTGCTAAACGTCT-3′ | |||
| RAR-γ | Human | NM_001243732 | (F) 5′-ATGCTGCGTATCTGCACAAG-3′ |
| (R) 5′-AGGCAAAGACAAGGTCTGTGA-3′ |
Cell Lysates and Western Blotting
Caco-2 cells were treated with 10 μm ATRA for different time periods (8, 16, and 24 h) or with RAR-β agonist, CH-55 (1 μm), and antagonist, LE-135 (1 μm) for 24 h. Control cells were treated with vehicle (100% alcohol) at 0.1% final concentration. After treatment, control or treated cells were washed with ice-cold 1× PBS to remove residual media. Total protein was extracted by suspending the cell pellet in cell lysis buffer (Cell Signaling, Danvers, MA) supplemented with protease inhibitor mixture from Roche Applied Science. The cells were lysed by sonication (three pulses for 20 s each) and the lysate was centrifuged at 13,000 rpm for 7 min at 4 °C to remove cell debris. The supernatant containing the total cell proteins was collected and the protein concentration was determined by the Bradford method (32). To examine the expression levels of DRA, equal amounts (75 μg/sample) of whole cell lysates were solubilized in SDS-gel loading buffer and boiled for 5 min. Proteins were loaded on a 7.5% SDS-polyacrylamide gel and transblotted to nitrocellulose membrane after electrophoretic separation. After 1 h of incubation in blocking buffer (1× PBS and 5% nonfat dry milk) the membrane was probed with affinity purified anti-DRA antibody (1:100 dilution). DRA antibody was raised against the C-terminal amino acid (745–764) sequence: INTNGGLRNRVYEPVETKF of SLC26A3 (accession number: BC025671) (at the Research Resource Centre, University of Illinois at Chicago), or GAPDH antibody (Sigma; 1:3,000 dilution) in 1× PBS and 2.5% nonfat dry milk overnight at 4 °C. The membrane was washed five times with wash buffer (1× PBS and 0.1% Tween 20) for 5 min and probed with HRP-conjugated goat anti-rabbit antibody (1:2,000 dilution) for 1 h followed by ECL (enhanced chemiluminescence, from Bio-Rad) detection. The expression of HNF-1β and RAR-β after siRNA transfection was probed using anti-HNF-1β antibody (Santa Cruz, 1:100 dilution) and RAR-β antibody (Abcam, 1:200 dilution).
Measurement of DRA Promoter Activity
Caco-2 cells were transiently transfected with full-length DRA promoter and different deletion constructs cloned upstream of the luciferase reporter gene and p-cytomegalovirus (CMV)-β, β-galactosidase mammalian expression vector (BD Biosciences, Clontech, Palo Alto, CA), by electroporation utilizing a Amaxa nucleofactor system as previously described (33). 24 h post-transfection, cells were treated with ATRA or RAR agonist/antagonist for different time periods. Control cells were treated with vehicle (100% alcohol) at 0.1% final concentration. After completion of the treatment, cells were washed with 1× PBS and lysed using passive lysis buffer (Promega, Madison, WI). Activities of luciferase and β-galactosidase were measured by a luminometer (Promega), utilizing kits from Promega and Clontech, respectively, according to the manufacturer's instructions. Promoter activity was calculated as a ratio of luciferase value to β-galactosidase value for each sample and expressed as % of control. All transfections were performed in triplicate and repeated at least three times with separate batches of cells.
siRNA-mediated Silencing
For small RNA interference studies, Caco-2 cells were plated on 6-well plates at a density of 1 × 105 cells/well, 24 h before transfection. After 24 h, Caco-2 cells were transfected with RAR-β (sense: 5′-GCGUGUAAUUACCUUGAAATT-3′, antisense: 5′-UUUCAAGGUAAUUACACGCTC-3′) or HNF-1β (sense: 5′-GCUCUGAGCCCACCAACAATT-3′, antisense: 5′-UUGUUGGUGGGCUCAGAGCAG-3′) specific siRNA (100 pmol) and scrambled (control) siRNA (100 pmol) (Qiagen, Valencia, CA) utilizing Lipofectamine 2000 transfection reagent (Invitrogen) as recommended by the manufacturer. Post-siRNA transfection (48 h), cells were treated with ATRA or vehicle for 24 h. Total RNA and protein was extracted as described before. Silencing was validated by real-time PCR utilizing RAR-β- or HNF-1β-specific primers and measuring the protein expression.
Immunofluorescence Staining and Confocal Microscopy
Caco-2 cells grown on transwell inserts were treated with ATRA for 24 h. After treatment the monolayers were washed twice in 1× PBS containing 1 mm CaCl2, pH 7.4, and then fixed with 2% paraformaldehyde at room temperature. Fixed cells were permeabilized using 0.08% saponin and blocked in 5% normal goat serum for 2 h. Monolayers were then incubated with rabbit anti-human DRA antibody (1:100) for 2 h followed by 3 washes for 5 min with 1× PBS containing CaCl2 and saponin. Cells were finally incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody at 1:100 dilution (Invitrogen) and rhodamine-phalloidin (1:60 dilution; Invitrogen) for 60 min at room temperature. Inserts were carefully removed from the transwell and mounted on glass slides using Slowfade gold antifade reagent (Invitrogen). Images were obtained on Carl Zeiss LSM 510 META laser scanning confocal microscope equipped with a ×63 water-immersion objective. Beams of 488 and 534 nm from an Ag/Kr laser and 361 nm from a UV laser were used for excitation. LP505 and -585 filters were used for detecting green and red fluorescence emissions, respectively. The multitracking function was used to sequentially scan two different fluorochromes to avoid any bleed-through among these fluorescent dyes. A series of sections were taken at z direction, and orthogonal sections were made in a z stack. The quantitative assessment of the apical amount of DRA was done using actin as an internal marker by Image J software.
Cell Surface Biotinylation Studies
Cell surface biotinylation was performed using sulfo-NHS-SS-biotin (1.5 mg/ml, Thermo Scientific, Rockford, IL) in borate buffer (in mm: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0) as previously described (24). After ATRA treatment, labeling of cell surface antigens was done for 60 min at 4 °C to prevent endocytosis and internalization of antigens. The biotinylated proteins were extracted from equal amounts of total protein by immunoprecipitation with neutravidin plus ultralink resin. The biotinylated proteins were released by boiling in Laemmli buffer containing dithiothreitol and subjected to SDS-PAGE, followed by transfer to nitrocellulose membrane. The blots were immunostained with anti-DRA antibody. The surface DRA was normalized with total cellular DRA (sum of biotinylated fraction and the amount of DRA not removed by the neutravidin precipitation method (intracellular pool)).
Statistical Analysis
Results are expressed as mean ± S.E. of three to five independent experiments. Student's t test or one-way analysis of variance with Tukey's test was used for statistical analysis. Differences between control and treated groups were considered significant at p value of 0.05 or less.
Results
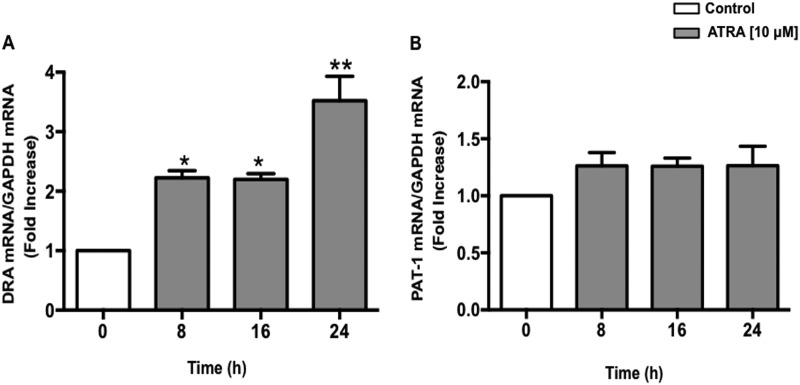
ATRA Up-regulates DRA but Not PAT-1 mRNA Levels in Caco-2 Cells
Two members of the SLC26 gene family, DRA (SLC26A3) and PAT-1 (SLC26A6) have been identified as candidate genes for apical Cl−/HCO3− exchange activity in the mammalian intestine (34). Therefore we examined whether ATRA treatment modulates DRA and/or PAT-1 mRNA expression. Caco-2 cells were treated with 10 μm ATRA for 8, 16, and 24 h and mRNA levels of DRA and PAT-1 were determined. As shown in Fig. 1A, mRNA levels of DRA were found to be significantly increased as early as 8 h with ∼3.5-fold increase at the 24 h time point. However, PAT-1 mRNA levels remained unaltered in response to ATRA treatment (Fig. 1B).
FIGURE 1.
ATRA stimulates DRA but not PAT-1 mRNA levels in Caco-2 cells. Caco-2 cells were treated with 10 μm ATRA for 8, 16, and 24 h in serum-free cell culture medium. RNA was amplified utilizing DRA (A) or PAT-1 (B) gene-specific primers for real-time PCR quantification. Data represent the relative expression of DRA or PAT-1 normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-changes in mRNA levels in treated cells as compared with control. Data represent mean ± S.E. of 4 separate experiments. *, p < 0.05; **, p < 0.001 compared with control.
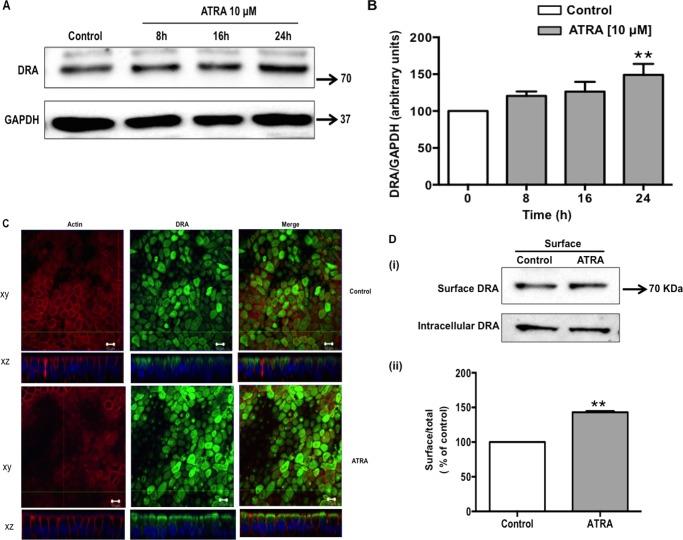
ATRA Increases DRA Protein Expression
Effect of ATRA treatment for 8, 16, and 24 h was next examined on DRA protein expression. Parallel to the effect of ATRA on DRA mRNA, Western blotting data demonstrated a significant increase in DRA protein expression (Fig. 2A). Densitometric analysis of the protein bands showed that ATRA treatment increased DRA protein levels by ∼50% as compared with control at 24 h. However, no significant change in protein expression was observed at 8- and 16-h time periods (Fig. 2B). These results were further supported by our confocal immunofluorescence studies. The vertical XY and horizontal XZ images in Fig. 2C show apical localization of DRA (green) with actin (red) in control cells. ATRA treatment resulted in a significant increase in DRA protein expression (138.3 ± 23.5%) as compared with the vehicle-treated cells taken as 100%. Additionally, cell surface biotinylation studies were utilized to quantify the increase in surface DRA (defined as the biotin accessible fraction of the total cellular DRA level) levels in response to ATRA treatment (Fig. 2D, i). Densitometric analysis of the protein bands (surface DRA/total DRA (surface + intracellular) ratio) suggested that ATRA increased the surface DRA level by 43% compared with untreated control (Fig. 2D, ii).
FIGURE 2.
ATRA induces DRA protein expression. Caco-2 cells were treated with 10 μm ATRA for 8, 16, and 24 h in serum-free cell culture medium. Control and ATRA-treated cell lysates were subjected to 7.5% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. A, the blot was probed with rabbit anti-DRA or anti-GAPDH antibody. A representative blot of 3 separate experiments is shown. B, results of densitometric analysis are expressed as DRA/GAPDH levels. C, confocal microscopic localization of DRA (green) and Alexa Fluor 568-conjugated phalloidin (actin; red) and slow fade gold antifade reagent with DAPI labeled the nuclei (blue). ATRA (10 μm for 24 h) treated cells show an enhanced expression of the DRA on the apical surface compared with control. Representative image from 3 separate experiments is shown. D, Caco-2 monolayers were treated with ATRA (10 μm) in serum-free cell culture medium for 24 h and subjected to biotinylation at 4 °C using sulfo-NHS-SS-biotin. Surface and intracellular fractions were run on 7.5% SDS-PAGE followed by transfer to nitrocellulose membrane. The blot was immunostained with a rabbit anti-DRA antibody. (i) Representative blot of 3 different experiments is shown. (ii) Data were quantified by densitometric analysis and expressed as surface/total DRA (surface plus intracellular DRA). Values represent mean ± S.E. of 3 different experiments. **, p < 0.001 compared with control.
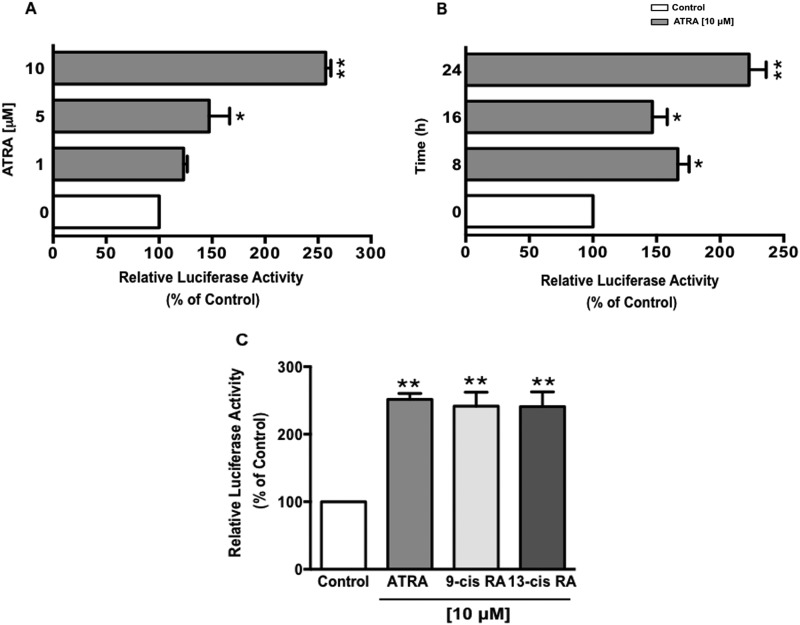
ATRA Increases DRA Promoter Activity in Caco-2 Cells
As ATRA increased DRA mRNA levels; we next investigated whether this increase was through a transcriptional mechanism. Caco-2 cells were transiently transfected with DRA promoter construct (p-1183/+114) along with pCMV-β, β-galactosidase expression vector. Post-transfection, cells were treated for 24 h with different concentrations of ATRA (1, 5, and 10 μm), and DRA promoter activity was determined by firefly luciferase assay and the measurement of β-galactosidase as an internal control to correct for transfection efficiency. As shown in Fig. 3A, ATRA treatment significantly stimulated DRA promoter activity with 2.5-fold induction at 10 μm concentration. The time course ATRA treatment demonstrated an increase in DRA promoter activity as early as 8 h with a significantly higher increase at the 24 h time point (Fig. 3B). Most of the effects of vitamin A are attributable to its active metabolites such as ATRA, 9-cis-, and 13-cis-retinoic acid. Not only ATRA, but its 9-cis and 13-cis stereoisomers (10 μm, 24 h) also significantly increased the DRA promoter activity (Fig. 3C). These data demonstrate that transcription of DRA is up-regulated by treatment of ATRA and its 9-cis and 13-cis isomers. However, for all other studies, we used ATRA, as it is the predominant and most active form under most physiological situations (35).
FIGURE 3.
ATRA stimulates DRA promoter activity in a dose-dependent manner. Caco-2 cells were transiently transfected with DRA luciferase promoter construct (p-1183/+114) along with the mammalian expression vector for β-galactosidase (pCMVβgal). 24 h post-transfection, Caco-2 cells were treated with: A, different doses of ATRA ranging from 1 to 10 μm for 24 h; or B, 10 μm ATRA for different time points; or C, 10 μm, ATRA, 9-cis- or 13-cis-retinoic acid. Promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results represent mean ± S.E. of 4 separate experiments performed in triplicate and expressed as % of control comparing transfected cells treated with ATRA to vehicle-treated cells (control). *, p < 0.05; **, p < 0.001 compared with control.
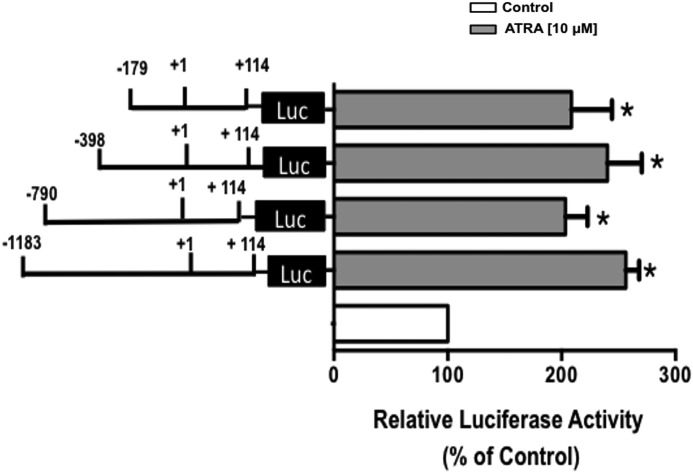
Effect of ATRA on DRA Promoter Deletion Constructs
We next aimed to identify the ATRA responsive region mediating the stimulatory effect of ATRA on DRA promoter activity. Caco-2 cells were transiently transfected with various constructs representing progressive 5′ deletions in the DRA promoter. Cells were then treated with ATRA (10 μm) for 24 h, and promoter activity was measured by luciferase assay. As shown in Fig. 4, ATRA treatment resulted in a significant increase in the relative luciferase activity of each deletion construct compared with their respective controls taken as 100%. The stimulatory effect of ATRA was retained until the smallest construct flanking the region between −179 and +114 of the DRA promoter was reached. These results indicate that ATRA-mediated activation required only the shortest fragment of the DRA promoter and ATRA response elements are located in this region of DRA gene.
FIGURE 4.
Functional analysis of DRA promoter deletion constructs in response to ATRA treatment. Caco-2 cells were transiently transfected with full-length DRA promoter (p-1183/+114) and its progressive 5′ deletion constructs along with pCMVβgal vector. At 24 h post-transfection, cells were treated with 10 μm ATRA for 24 h. Cells were then harvested 48 h post-transfection and the promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results are expressed as % of respective control for each promoter construct and represent mean ± S.E. of 3 separate experiments performed in triplicate. *, p < 0.05 compared with respective control taken as 100%.
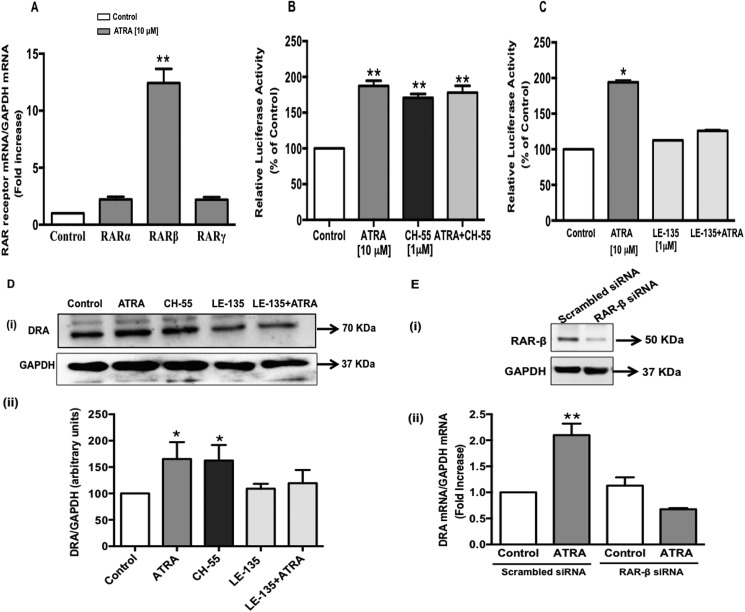
RAR-β Receptor Is Involved in the Induction of DRA Promoter Activity
Effects of ATRA are receptor mediated. It is known that ATRA regulates the expression of its own receptors. Keeping this in view, we first examined the expression of RAR isoforms in response to ATRA treatment. As shown in Fig. 5A, ATRA treatment resulted in a 12-fold increase in RAR-β mRNA expression as compared with control, with a modest change in RAR-α or -γ. Suggesting that RAR-β might play a role in the observed effects of ATRA. To confirm the involvement of RAR-β, Caco-2 cells transiently transfected with the DRA promoter were treated with the RAR-β agonist (CH-55, 1 μm) alone or in combination with ATRA for 24 h. As shown in Fig. 5B, treatment with the RAR-β agonist significantly increased the DRA promoter activity similar to the effect of ATRA. However, the combined effects of ATRA and CH-55 on DRA promoter activity were not additive indicating that both ATRA and CH-55 increase DRA promoter activity by the same pathway via RAR-β. The role of RAR-β was further confirmed by treatment of the DRA promoter-transfected Caco-2 cells with RAR-β antagonist (LE-135). As shown in Fig. 5C, an ATRA-mediated increase in DRA promoter activity was completely abrogated in the presence of LE-135 (1 μm). These results indicate that RAR-β is involved in stimulating DRA promoter activity. Effects of RAR-β agonist and antagonist observed on the DRA promoter were further validated at the protein level. Parallel to ATRA, CH-55 treatment of Caco-2 cells for 24 h also resulted in a significant increase (∼50%) in DRA protein expression. Whereas, the ATRA-induced increase in DRA protein levels were abrogated in the presence of LE-135 (Fig. 5D, i and ii).
FIGURE 5.
RAR-β receptor is involved in the induction of DRA promoter activity. A, Caco-2 cells were treated with 10 μm ATRA for 24 h in serum-free cell culture medium. RNA was amplified utilizing RAR-α, -β, or -γ gene-specific primers for real-time PCR quantification. Data represent the relative expression of RAR-α, -β, or -γ normalized to the respective GAPDH mRNA (internal control) levels and are expressed as fold-changes compared with vehicle-treated controls. Values represent mean ± S.E. of 3 separate experiments performed. **, p < 0.001 compared with respective control. B and C, Caco-2 cells were transiently transfected with DRA luciferase promoter construct (p-1183/+114) along with pCMVβgal vector. B, after 24 h, cells were treated with RAR-β receptor agonist CH-55 (1 μm) or ATRA (10 μm) or both for 24 h. C, transiently transfected cells were pretreated with RAR-β receptor antagonist LE-135 (1 μm) for 60 min and then coincubated with ATRA (10 μm) for 24 h. Promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results are expressed as % of control and represent mean ± S.E. of 3 separate experiments. *, p < 0.05; **, p < 0.001 compared with control. D, Caco-2 cells were treated with ATRA (10 μm) or CH-55 (1 μm) or LE-135 (1 μm) alone or in combination with ATRA for 24 h in serum-free media. Cell lysates prepared from the above treatment groups were subjected to 7.5% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. (i) The blot was probed with rabbit anti-DRA or anti-GAPDH antibody. A representative blot of 3 separate experiments is shown. (ii) Results of densitometric analysis are expressed as DRA/GAPDH levels. Values represent mean ± S.E. of 3 different experiments. *, p < 0.05 compared with control. E, Caco-2 cells were transfected with scrambled or RAR-β-specific small interfering RNA (siRNA) for 48 h. (i) siRNA-mediated knockdown of RAR-β was confirmed by immunoblots with anti-RAR-β antibody. (ii) in a separate set of experiments, the cells were treated with 10 μm ATRA for an additional 24 h. Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing primers specific for DRA. Data represent the relative expression of DRA normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-changes in mRNA levels compared with control. Values represent mean ± S.E. of 3 separate experiments. **, p < 0.001 compared with control.
As a complementary approach, we also examined the effect of RAR-β knockdown on ATRA-induced DRA expression. Treatment of Caco-2 cells with RAR-β-specific siRNA duplex for 48 h significantly decreased the RAR-β mRNA expression (∼70% decrease) compared with scrambled siRNA controls, demonstrating efficient knockdown. This was also validated at the protein level (Fig. 5E, i). As shown in Fig. 5E, ii, ATRA treatment to cells transfected with scrambled siRNA (control) resulted in a significant increase in DRA mRNA expression. However, similar to the inhibition by LE-135 (RAR-β antagonist), transfection of RAR-β-specific siRNA substantially attenuated the ability of ATRA to increase DRA expression. These findings clearly indicate the involvement of RAR-β in the ATRA-induced increase in DRA expression.
HNF-1β Mediates the Effect of ATRA on DRA
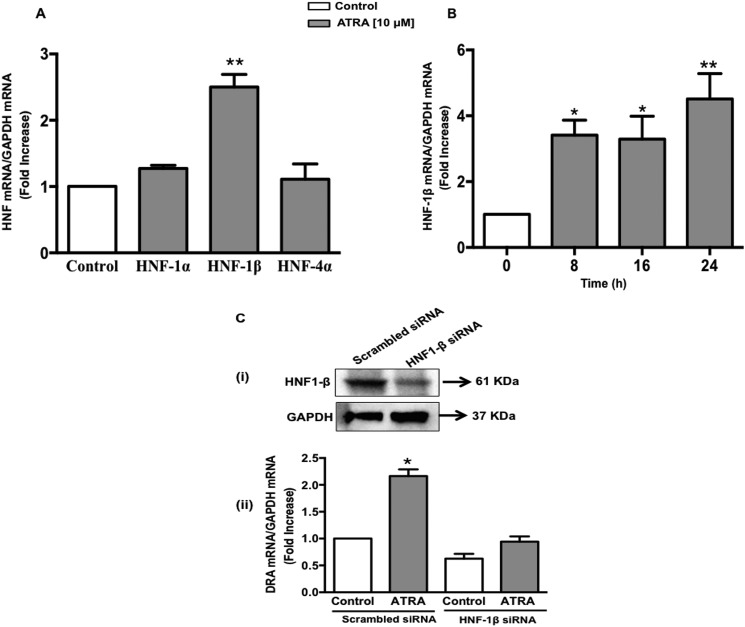
Results obtained from the transfection studies with progressive 5′-deletion constructs of the DRA promoter demonstrated that the minimal promoter region has cis-elements mediating the ATRA-induced effects on DRA promoter. Interestingly, the sequence analysis of this region of the DRA promoter revealed the binding sites for hepatocyte nuclear factors (HNFs). In this regard a previous study has suggested transcriptional regulation of DRA expression by HNFs (36). Complete loss of DRA expression has been shown in the small intestine and colon of intestine-specific HNF-1α and -1β double knock-out mice (29). Thus the potential involvement of HNFs in the ATRA-induced increase in DRA expression was evaluated. Caco-2 monolayers were treated with ATRA (10 μm) for 24 h and the expression levels of HNFs (1α/1β/4α) were examined. ATRA markedly increased HNF-1β mRNA expression as compared with HNF-1α and HNF-4α (Fig. 6A). The time-dependent increase in HNF-1β mRNA expression in response to ATRA (Fig. 6B) showed a similar pattern as observed for DRA mRNA. These data suggest that HNF-1β may be involved in mediating the effects of ATRA on DRA. To confirm this, HNF-1β expression was attenuated in Caco-2 cells using siRNA. Treatment of Caco-2 cells with the HNF-1β-specific siRNA duplex for 48 h significantly decreased the HNF-1β mRNA expression (∼50% decrease) compared with scrambled siRNA controls, demonstrating efficient knockdown. This was also validated at the protein level (Fig. 6C, i). As shown in Fig. 6C, ii, ATRA significantly increased DRA mRNA levels in the cells transfected with scrambled siRNA. siRNA-mediated silencing of HNF-1β resulted in ∼35% decrease in basal DRA mRNA expression. However, the ATRA-induced increase in DRA mRNA levels was significantly decreased by HNF-1β knockdown. These data suggest that ATRA modulates DRA expression by increasing HNF-1β in intestinal Caco-2 cells.
FIGURE 6.
ATRA-mediated stimulation of DRA promoter activity is HNF-1β dependent. A, Caco-2 cells were treated with 10 μm ATRA for 24 h in serum-free cell culture medium. RNA was amplified utilizing HNF-1α, -1β, or -4α gene-specific primers. Data represent the relative expression of HNF-1α, -1β, or -4α normalized to the respective GAPDH mRNA (internal control) levels. B, Caco-2 cells were treated with 10 μm ATRA for 8, 16, and 24 h in serum-free cell culture medium and HNF-1β mRNA expression was determined using gene-specific primers. Results are expressed as fold-change in mRNA levels in ATRA treated as compared with vehicle-treated control cells (n = 3, *, p < 0.05; **, p < 0.001); C, Caco-2 cells were transfected with scrambled or HNF-1β-specific small interfering RNA (siRNA) for 48 h. (i) siRNA-mediated knockdown of HNF-1β was confirmed by immunoblotting with anti-HNF1-β antibody. (ii) In a separate set of experiments, the cells were treated with 10 μm ATRA for an additional 24 h. Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing DRA gene-specific primers. Data represent the relative expression of DRA normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-change in mRNA levels of treated as compared with control cells. Data represent mean ± S.E. of 3 separate experiments. *, p < 0.05 compared with control.
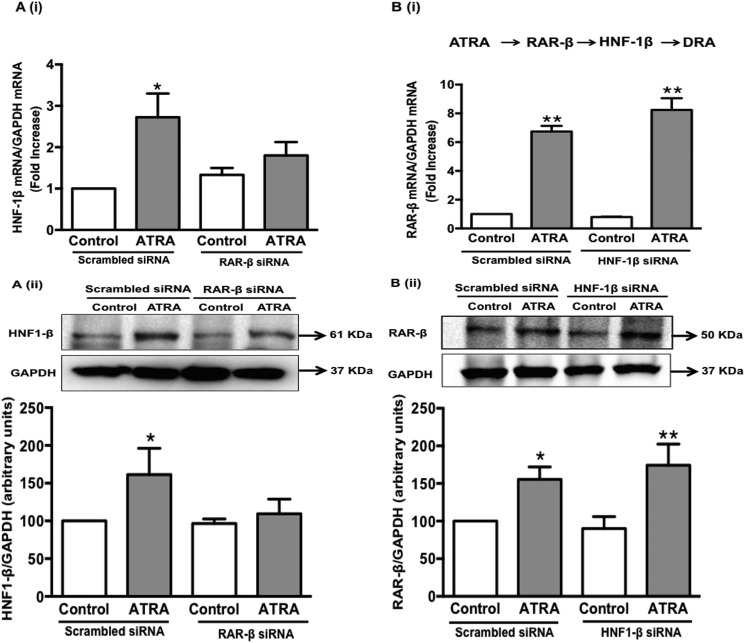
RAR-β Is Upstream of HNF-1β
As the siRNA-mediated silencing of both RAR-β and HNF-1β blocked the effects of ATRA on DRA mRNA expression; we next sought to determine the sequence of events involved. As shown in Fig. 7A, i and ii, ATRA treatment to Caco-2 cells transfected with scrambled siRNA resulted in a significant increase in HNF-1β mRNA and protein levels and this increase was abolished by RAR-β knockdown. However, the levels of RAR-β mRNA and protein, in response to ATRA treatment remained elevated with HNF-1β silencing (Fig. 7B, i and ii) indicating that in the sequence of events RAR-β is upstream of HNF-1β.
FIGURE 7.
RAR-β is upstream of HNF-1β in ATRA-mediated stimulation of DRA expression. Caco-2 cells were transfected with scrambled, HNF-1β-specific and RAR-β-specific small interfering RNA (siRNA) for 48 h. The cells were then treated with 10 μm ATRA for an additional 24 h. Total RNA and protein was extracted. A, (i) quantitative real-time RT-PCR was performed utilizing HNF-1β gene specific primers and (ii) protein expression of HNF-1β was examined using anti-HNF1-β antibody in cells where RAR-β was silenced. B, (i) quantitative real-time RT-PCR was performed utilizing RAR-β gene-specific primers and (ii) protein expression of RAR-β was examined using anti-RAR-β antibody in the cells where HNF-1β was silenced. Data represent the relative expression of HNF-1β or RAR-β normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-changes in mRNA levels compared with control. For protein expression, a representative blot of 3 separate experiments is shown. Results of densitometric analysis are expressed as HNF-1β or RAR-β/GAPDH levels. Values represent mean ± S.E. of 3 different experiments. *, p < 0.05; **, p < 0.001 compared with control.
Discussion
DRA functions as the key mediator of apical Cl−/HCO3− exchange in the electroneutral NaCl absorption process in the intestinal epithelial cells. Disturbances in NaCl absorption accompany diarrhea associated with inflammatory bowel diseases and pathogenic bacterial infections (23). Therefore, the maintenance of optimal expression of DRA is critical for normal electrolyte homeostasis in the intestine and mechanisms regulating DRA expression assume added importance in understanding the pathophysiology of diarrheal diseases. Results from the current study demonstrate that ATRA increases DRA gene expression and promoter activity in intestinal epithelial cells. Furthermore, ATRA-mediated stimulation of DRA promoter through RAR-β appears to be indirect, involving the increased gene expression of another transcription factor HNF-1β. This novel mechanism of up-regulating DRA expression in intestinal epithelial cells may have therapeutic importance in the treatment of diarrheal diseases.
ATRA is required for growth, maintenance, and differentiation of intestinal epithelial cells. Notably, the epithelial cells lining the gastrointestinal tract are exposed to varying concentrations of this dietary nutrient (37, 38). In addition, the absorbed dietary retinol is metabolized directly to ATRA in the enterocytes (1), and ATRA is the primary retinoid that affects the cellular process at the level of transcription. Our results showed that the promoter activity of DRA is significantly increased parallel to an increase in the mRNA expression of DRA by ATRA, suggesting the involvement of transcriptional regulation. The effects on DRA were specific as PAT-1 mRNA remained unchanged in response to ATRA treatment. DRA co-exists with NHE-3 in distal ileum and mid-colon, and the coupled operation favors electroneutral NaCl absorption. The expression profiles of both DRA and NHE-3 exhibit regional variations along the length of the intestine as well as vertically along the crypt-villus axis (39, 40). NHE-3 expression is more in ileum as compared with colon and the pattern is inverse for DRA. An increase in expression of both DRA and NHE-3 has been reported by lysophosphatidic acid (27, 28, 41) and the probiotic L. acidophilus (25, 26, 42). Thus, in future studies it will be interesting to examine the changes in the expression pattern of NHE-3 and DRA in vivo in response to ATRA treatment.
ATRA signaling is mediated by its binding to RARs, which form heterodimers with RXRs. It is established that ATRA exerts its effects by differentially regulating gene expression of its receptor isotypes such as RAR-α and RAR-β (43), which also determines the sensitivity of the individual cell lines to the effect of ATRA (44, 45). The present study utilized the well established human intestinal epithelial cell line Caco-2 as an in vitro model to understand mechanisms underlying ATRA-mediated effects on DRA. Caco-2 cells represented the excellent in vitro model for our studies, as on differentiation these cells manifest many anatomic and functional similarities to absorptive enterocytes. Previous studies have demonstrated that retinoic acid receptors are expressed in Caco-2 cells (1, 37, 38). In our study, ATRA treatment of Caco-2 cells caused a highly significant (∼12-fold) increase in RAR-β mRNA. In this regard, previous studies in hepatic epithelial cells have also shown a similar up-regulation of RAR-β in response to ATRA, mediated by binding the RXR/RAR heterodimer to the promoter region of the RAR-β gene (43). A question arises whether RXR is also involved in the observed effects of ATRA on DRA. However, in the current study, DRA promoter activity and RNA expression both remained unaffected in the presence of RXR agonist (DHA) and antagonist (HX531) (data not shown) suggesting that transcriptional activation of the DRA promoter depends on binding of ATRA to the RAR partner of RAR/RXR heterodimer. Interestingly, our results show that DRA promoter activity was stimulated by pharmacological activation of RAR-β and the effects were blocked in the presence of RAR-β antagonist (LE-135). Also, the increase in DRA mRNA expression by ATRA was abrogated by RAR-β knockdown in Caco-2 cells. Collectively, these results suggest that ATRA effects on DRA gene expression are dependent on RAR-β. Our studies, however, do not rule out the involvement of RAR-α in the ATRA-mediated effects on DRA. It is important to note that RAR-α has been previously identified as a critical activator of ATRA-mediated RAR-β gene expression in human mammary epithelial cells (46) and cervical cells (47). A similar involvement of RAR-α can be expected in the ATRA-induced increase in DRA gene expression via RAR-β, and future investigations will address this possibility.
ATRA and its derivatives are dietary factors, which regulate cellular differentiation. As expression of DRA increases with enterocyte maturation, it was of interest to examine whether the ATRA-induced DRA expression is through the regulation of DRA promoter activity or is secondary to alterations in the cellular differentiation/proliferation status of the Caco-2 cells. To test this, the effect of ATRA on alkaline phosphatase and proliferating cell nuclear antigen, markers of differentiation and proliferation, respectively, was assessed. ATRA (10 μm) treatment to pre-confluent (24 h post-plating) and post-confluent (14 days post-plating) Caco-2 cells for 24 h had no significant effect on alkaline phosphatase and proliferating cell nuclear antigen expression (data not shown). This indicates that it is unlikely that the observed increase in DRA expression is secondary to cellular differentiation.
Basic sequence pivotal for the classical ATRA signaling pathway involves ligand binding, receptor dimerization, DNA binding, and transcriptional modulation of the target gene. However, there are possibilities where ATRA regulates an intermediary factor (usually another transcription factor), which in turn regulates the target gene (indirect target) (48). Sequence analysis of the DRA promoter revealed the potential binding sites for RAR-β. However, it should be noted that deletion of DRA promoter regions harboring the RAR-β binding sites retained significant responsiveness to ATRA. These data indicated that other potential cis-elements present in the p-179/+114 region of DRA promoter might have contributed to ATRA-mediated stimulation of DRA promoter activity. Sequence analysis of this region revealed a potential HNF-1β recognition site located within the −94/−76 region from the transcription start site (−94AGTTAATGAGAGTTAATTA−76). ATRA treatment resulted in a significant increase in HNF-1β mRNA expression (as compared with HNF-1α and -4α), which was abrogated by RAR-β knockdown in Caco-2 cells. On the other hand, HNF-1β silencing resulted in blocking the stimulatory effects of ATRA on DRA mRNA expression, whereas RAR-β mRNA and protein levels remained up-regulated. These data indicated that the effects of ATRA on DRA expression are mediated by transcriptional up-regulation of HNF-1β. In this regard, indirect regulation of α-fetoprotein by ATRA via HNF-1α and HNF-4α in the hepatic cell line has also been reported earlier (49). In the intestine, HNFs play a key role in regulating the expression of genes involved in diverse physiological processes such as those related to differentiation, cell fate, barrier function, transport, and metabolism (29). With respect to the regulation of transporter genes, HNF-1α has been shown to regulate CFTR gene expression in Caco-2 cells (50). On the other hand, a recent study has shown that the expression of ileal and colonic DRA were substantially reduced in mice deficient in either intestinal HNF-1α or HNF-1β, and were completely abolished in mice deficient in both factors in the intestine (29). These mice also exhibited diarrheal phenotype. Additionally, our previous report on regulation of the basal DRA promoter by HNF-4α (36) suggest that HNFs might play critical roles in modulating DRA expression in the normal intestine and its alterations in inflammation. Our current findings further indicate a novel role for HNF-1β in mediating the effects of ATRA on DRA expression. It is intriguing that DRA mRNA and promoter activity showed an equal amount of increase at 8 and 16 h, which further increased at the 24-h time point. The time-dependent increase in HNF-1β expression in response to ATRA also showed a similar pattern as observed for DRA mRNA. We speculate that the ATRA-induced increase in HNF-1β mRNA expression may reflect the involvement of two mechanisms including an increase in HNF-1β promoter activity maximally as early as 8 h and a secondary mechanism involving chromatin remodeling at later time points in response to ATRA treatment, which is subsequently reflected in the expression pattern of the target gene (DRA). In this regard, a recent study has shown that ATRA can induce chromatin remodeling (51).
In conclusion, our present studies provide novel data on the up-regulation of DRA expression by ATRA via transcriptional mechanisms. We propose a model (Fig. 8) that ATRA through RAR-β stimulates the promoter activity of DRA via HNF-1β leading to induction of transcription and subsequent increase in DRA message and protein expression. This study highlights mechanisms that underlie potential antidiarrheal effects of ATRA. These findings are clinically relevant, as induction of DRA expression may be beneficial in conditions like diarrhea associated with inflammatory bowel diseases where DRA expression is down-regulated. However, future studies are needed to verify whether ATRA has an impact on DRA gene expression in vivo in normal and disease models.
FIGURE 8.
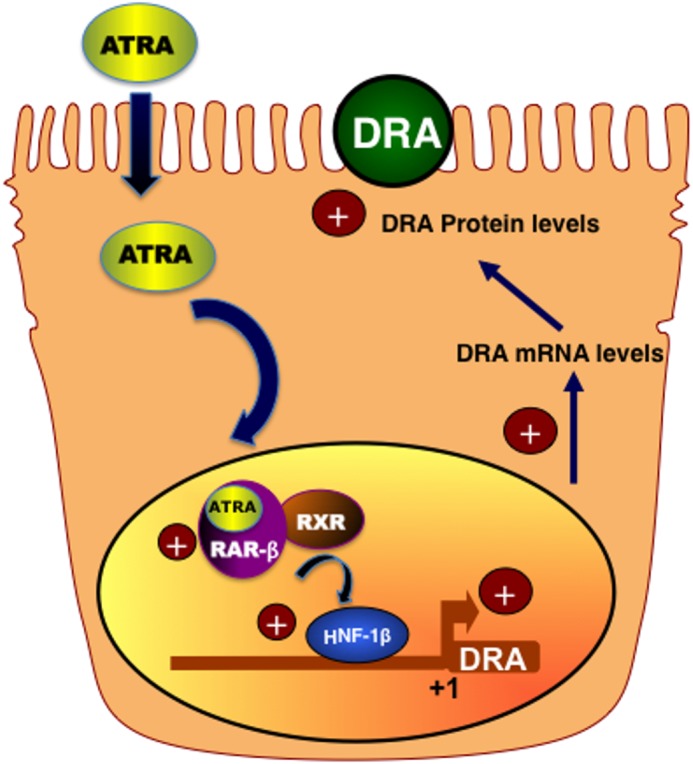
Proposed model for the effects of ATRA on DRA via HNF-1β.
This work was supported, in whole or in part, by National Institutes of Health Grants DK54016, DK81858, and DK92441 (to P. K. D.), DK71596 (to W. A. A.), DK96254 (to S. S.), and RO3 DK 096258 (to R. K. G.) from the NIDDK, Department of Veteran Affairs Grants BX002011 and a senior research career scientist award (to P. K. D.) and Grant BX000152 (to W. A. A.).
- ATRA
- all-trans-retinoic acid
- RXR
- retinoic X receptor
- DRA
- down-regulated in adenoma
- RAR
- retinoic acid receptor
- HNF
- hepatocyte nuclear factor.
References
- 1. Lampen A., Meyer S., Arnhold T., Nau H. (2000) Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J. Pharmacol. Exp. Ther. 295, 979–985 [PubMed] [Google Scholar]
- 2. Sporn M. B., Roberts A. B., Goodman D. S. (1994) The Retinoids, Raven Press, NY [Google Scholar]
- 3. Sun S. Y., Lotan R. (2002) Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 41, 41–55 [DOI] [PubMed] [Google Scholar]
- 4. Filteau S. M., Rollins N. C., Coutsoudis A., Sullivan K. R., Willumsen J. F., Tomkins A. M. (2001) The effect of antenatal vitamin A and β-carotene supplementation on gut integrity of infants of HIV-infected South African women. J. Pediatr. Gastroenterol. Nutr. 32, 464–470 [DOI] [PubMed] [Google Scholar]
- 5. Thurnham D. I., Northrop-Clewes C. A., McCullough F. S., Das B. S., Lunn P. G. (2000) Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J. Infect. Dis. 182, S23–S28 [DOI] [PubMed] [Google Scholar]
- 6. Veldhoen M., Brucklacher-Waldert V. (2012) Dietary influences on intestinal immunity. Nat. Rev. Immunol. 12, 696–708 [DOI] [PubMed] [Google Scholar]
- 7. Ozdemir R., Yurttutan S., Sari F. N., Oncel M. Y., Erdeve O., Unverdi H. G., Uysal B., Dilmen U. (2013) All-trans-retinoic acid attenuates intestinal injury in a neonatal rat model of necrotizing enterocolitis. Neonatology 104, 22–27 [DOI] [PubMed] [Google Scholar]
- 8. Bai A., Lu N., Zeng H., Li Z., Zhou X., Chen J., Liu P., Peng Z., Guo Y. (2010) All-trans retinoic acid ameliorates trinitrobenzene sulfonic acid-induced colitis by shifting Th1 to Th2 profile. J. Interferon Cytokine Res. 30, 399–406 [DOI] [PubMed] [Google Scholar]
- 9. Bai A., Lu N., Guo Y., Liu Z., Chen J., Peng Z. (2009) All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J. Leukocyte Biol. 86, 959–969 [DOI] [PubMed] [Google Scholar]
- 10. Ghana VAST study Team. (1993) Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 342, 7–12 [PubMed] [Google Scholar]
- 11. Villamor E., Fawzi W. W. (2000) Vitamin A supplementation: implications for morbidity and mortality in children. J. Infect. Dis. 182, S122–S133 [DOI] [PubMed] [Google Scholar]
- 12. Humphreys E. H., Smith N. A., Azman H., McLeod D., Rutherford G. W. (2010) Prevention of diarrhoea in children with HIV infection or exposure to maternal HIV infection. Cochrane Database of Systematic Reviews 10.1002/14651858.CD008563 [DOI] [PubMed] [Google Scholar]
- 13. Sempértegui F., Estrella B., Camaniero V., Betancourt V., Izurieta R., Ortiz W., Fiallo E., Troya S., Rodríguez A., Griffiths J. K. (1999) The beneficial effects of weekly low-dose vitamin A supplementation on acute lower respiratory infections and diarrhea in Ecuadorian children. Pediatrics 104, e1. [DOI] [PubMed] [Google Scholar]
- 14. Chen K., Chen X. R., Zhang L., Luo H. Y., Gao N., Wang J., Fu G. Y., Mao M. (2013) Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition 29, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 15. Duggan C., Gannon J., Walker W. A. (2002) Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 75, 789–808 [DOI] [PubMed] [Google Scholar]
- 16. Dudeja P. K., Gill R. K., Ramaswamy K. (2003) Absorption-secretion and epithelial cell function: colonic diseases. in Colonic Diseases, pp. 3–24, Humana Press, Totowa, NJ [Google Scholar]
- 17. Gill R. K., Alrefai W. A., Ramasamy A., Dudeja P. K. (2003) Mechanisms and regulation of NaCl absorption in the human intestine. in Recent Research Developments in Physiology, Vol. I, Part II, pp. 643–647, Research Signpost, Trivandrum, India [Google Scholar]
- 18. Bieberdorf F. A., Gorden P., Fordtran J. S. (1972) Pathogenesis of congenital alkalosis with diarrhea: implications for the physiology of normal ileal electrolyte absorption and secretion. J. Clin. Investig. 51, 1958–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mäkelä S., Kere J., Holmberg C., Höglund P. (2002) SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 20, 425–438 [DOI] [PubMed] [Google Scholar]
- 20. Schweinfest C. W., Spyropoulos D. D., Henderson K. W., Kim J. H., Chapman J. M., Barone S., Worrell R. T., Wang Z., Soleimani M. (2006) slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 281, 37962–37971 [DOI] [PubMed] [Google Scholar]
- 21. Wang Z., Wang T., Petrovic S., Tuo B., Riederer B., Barone S., Lorenz J. N., Seidler U., Aronson P. S., Soleimani M. (2005) Renal and intestinal transport defects in Slc26a6-null mice. Am. J. Physiol. Cell Physiol. 288, C957–C965 [DOI] [PubMed] [Google Scholar]
- 22. Borenshtein D., Schlieper K. A., Rickman B. H., Chapman J. M., Schweinfest C. W., Fox J. G., Schauer D. B. (2009) Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect. Immun. 77, 3639–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gill R. K., Borthakur A., Hodges K., Turner J. R., Clayburgh D. R., Saksena S., Zaheer A., Ramaswamy K., Hecht G., Dudeja P. K. (2007) Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J. Clin. Investig. 117, 428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang H., Jiang W., Furth E. E., Wen X., Katz J. P., Sellon R. K., Silberg D. G., Antalis T. M., Schweinfest C. W., Wu G. D. (1998) Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. 275, G1445–G1453 [DOI] [PubMed] [Google Scholar]
- 25. Borthakur A., Gill R. K., Tyagi S., Koutsouris A., Alrefai W. A., Hecht G. A., Ramaswamy K., Dudeja P. K. (2008) The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J. Nutr. 138, 1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raheja G., Singh V., Ma K., Boumendjel R., Borthakur A., Gill R. K., Saksena S., Alrefai W. A., Ramaswamy K., Dudeja P. K. (2010) Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G395–G401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singla A., Dwivedi A., Saksena S., Gill R. K., Alrefai W. A., Ramaswamy K., Dudeja P. K. (2010) Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl−/OH− exchange. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G182–G189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singla A., Kumar A., Priyamvada S., Tahniyath M., Saksena S., Gill R. K., Alrefai W. A., Dudeja P. K. (2012) LPA stimulates intestinal DRA gene transcription via LPA2 receptor, PI3K/AKT, and c-Fos-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G618–G627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Angelo A., Bluteau O., Garcia-Gonzalez M. A., Gresh L., Doyen A., Garbay S., Robine S., Pontoglio M. (2010) Hepatocyte nuclear factor 1α and β control terminal differentiation and cell fate commitment in the gut epithelium. Development 137, 1573–1582 [DOI] [PubMed] [Google Scholar]
- 30. Altucci L., Leibowitz M. D., Ogilvie K. M., de Lera A. R., Gronemeyer H. (2007) RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 6, 793–810 [DOI] [PubMed] [Google Scholar]
- 31. Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. (2006) International Union of Pharmacology: LX. retinoic acid receptors. Pharmacol. Rev. 58, 712–725 [DOI] [PubMed] [Google Scholar]
- 32. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33. Saksena S., Singla A., Goyal S., Katyal S., Bansal N., Gill R. K., Alrefai W. A., Ramaswamy K., Dudeja P. K. (2010) Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G159–G166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dudeja P. K., Ramaswamy K. (2006) Intestinal anion absorption. in Physiology of the Gastrointestinal Tract, pp. 1881–1916, Elsevier Academic, Oxford, UK [Google Scholar]
- 35. Miano J. M., Berk B. C. (2000) Retinoids: versatile biological response modifiers of vascular smooth muscle phenotype. Circ. Res. 87, 355–362 [DOI] [PubMed] [Google Scholar]
- 36. Alrefai W. A., Wen X., Jiang W., Katz J. P., Steinbrecher K. A., Cohen M. B., Williams I. R., Dudeja P. K., Wu G. D. (2007) Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am. J. Physiol. Gastrointest. Liver Physiol. 293, G923–G934 [DOI] [PubMed] [Google Scholar]
- 37. Baltes S., Nau H., Lampen A. (2004) All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev. Growth Differ. 46, 503–514 [DOI] [PubMed] [Google Scholar]
- 38. McCormack S. A., Viar M. J., Tague L., Johnson L. R. (1996) Altered distribution of the nuclear receptor RAR beta accompanies proliferation and differentiation changes caused by retinoic acid in Caco-2 cells. In Vitro Cell. Dev. Biol. Anim. 32, 53–61 [DOI] [PubMed] [Google Scholar]
- 39. Kiela P. R., LeSueur J., Collins J. F., Ghishan F. K. (2003) Transcriptional regulation of the rat NHE3 gene: functional interactions between GATA-5 and Sp family transcription factors. J. Biol. Chem. 278, 5659–5668 [DOI] [PubMed] [Google Scholar]
- 40. Talbot C., Lytle C. (2010) Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G358–G367 [DOI] [PubMed] [Google Scholar]
- 41. Lee-Kwon W., Kawano K., Choi J. W., Kim J. H., Donowitz M. (2003) Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J. Biol. Chem. 278, 16494–16501 [DOI] [PubMed] [Google Scholar]
- 42. Singh V., Raheja G., Borthakur A., Kumar A., Gill R. K., Alakkam A., Malakooti J., Dudeja P. K. (2012) Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1393–G1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan Y. J., Cai Y., Magee T. R. (1998) Retinoic acid differentially regulates retinoic acid receptor-mediated pathways in the Hep3B cell line. Exp. Cell Res. 238, 241–247 [DOI] [PubMed] [Google Scholar]
- 44. Davis K. D., Lazar M. A. (1993) Induction of retinoic acid receptor-β by retinoic acid is cell specific. Endocrinology 132, 1469–1474 [DOI] [PubMed] [Google Scholar]
- 45. Lee M. O., Han S. Y., Jiang S., Park J. H., Kim S. J. (2000) Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor β. Biochem. Pharmacol. 59, 485–496 [DOI] [PubMed] [Google Scholar]
- 46. Liu Y., Lee M. O., Wang H. G., Li Y., Hashimoto Y., Klaus M., Reed J. C., Zhang X. (1996) Retinoic acid receptor β mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol. Cell. Biol. 16, 1138–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Geisen C., Denk C., Gremm B., Baust C., Karger A., Bollag W., Schwarz E. (1997) High-level expression of the retinoic acid receptor β gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor α and is abnormally down-regulated in cervical carcinoma cells. Cancer Res. 57, 1460–1467 [PubMed] [Google Scholar]
- 48. Balmer J. E., Blomhoff R. (2002) Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808 [DOI] [PubMed] [Google Scholar]
- 49. Magee T. R., Cai Y., El-Houseini M. E., Locker J., Wan Y. J. (1998) Retinoic acid mediates down-regulation of the α-fetoprotein gene through decreased expression of hepatocyte nuclear factors. J. Biol. Chem. 273, 30024–30032 [DOI] [PubMed] [Google Scholar]
- 50. Mouchel N., Henstra S. A., McCarthy V. A., Williams S. H., Phylactides M., Harris A. (2004) HNF1α is involved in tissue-specific regulation of CFTR gene expression. Biochem. J. 378, 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wan Y., Yang S., Sun F., Wang J., Chen Q., Hong A. (2012) All-trans retinoic acid induces chromatin remodeling at the promoter of the mouse liver, bone, and kidney alkaline phosphatase gene in C3H10T 1/2 cells. Biochem. Genet. 50, 495–507 [DOI] [PubMed] [Google Scholar]