Background: ApoE is a genetic risk factor for Alzheimer disease.
Results: As compared with apoE2/3, apoE4 failed to inhibit the conversion of Aβ protofibrils to fibrils in vitro. Intracerebral injection of Aβ protofibrils with apoE3 attenuated Aβ deposition, whereas apoE4 did not.
Conclusion: ApoE3, not apoE4, impedes β-amyloid formation.
Significance: Interaction between Aβ and apoE is a critical determinant of β-amyloid formation.
Keywords: Alzheimer disease, amyloid-β, apolipoprotein E, amyloid, protein stability
Abstract
Human APOE ϵ4 allele is a strong genetic risk factor of Alzheimer disease. Neuropathological and genetic studies suggested that apolipoprotein E4 (apoE4) protein facilitates deposition of amyloid β peptide (Aβ) in the brain, although the mechanism whereby apoE4 increases amyloid aggregates remains elusive. Here we show that injection of Aβ protofibrils induced Aβ deposition in the brain of APP transgenic mice, suggesting that Aβ protofibrils acted as a seed for aggregation and deposition of Aβ in vivo. Injection of Aβ protofibrils together with apoE3 significantly attenuated Aβ deposition, whereas apoE4 did not have this effect. In vitro assays revealed that the conversion of Aβ protofibrils to fibrils progressed more slowly upon coincubation with apoE2 or apoE3 compared with that with apoE4. Aβ protofibrils complexed with apoE4 were less stable than those with apoE2 or apoE3. These data suggest that the suppression effect of apoE2 or apoE3 on the structural conversion of Aβ protofibrils to fibrils is stronger than those of apoE4, thereby impeding β-amyloid deposition.
Introduction
Alzheimer disease (AD)3 is pathologically characterized by massive deposition of amyloid β peptides (Aβ) as senile plaques in brains. The amyloid hypothesis postulates the central role of Aβ aggregation as the major cause of neuronal degeneration in AD (1). A subset of AD is inherited as an autosomal dominant trait. Genetic analysis of familial AD (FAD) cases revealed three genes, Aβ precursor protein (APP), presenilin 1, and presenilin 2, that are causative for FAD (1). Subsequent studies revealed that these mutant genes increase the production of Aβ or accelerate fibrillization of Aβ, thereby leading to AD. However, it has not been clarified whether production or fibrillization of Aβ is up-regulated in the brains of patients with sporadic form of AD, which comprises the major population of AD in the elderly.
A number of non-Aβ proteinaceous components are deposited in senile plaques associated with Aβ (2). These proteins may interact with Aβ and modify its deposition in AD brains. The best characterized of these proteins is apolipoprotein E (apoE). ApoE is a 299-amino acid protein secreted from liver into blood plasma, which mediates lipoprotein metabolism. In the central nervous system, apoE is produced and secreted primarily from astrocytes and microglial cells (3–5). Three major polymorphisms in human apoE gene, i.e. ϵ2, ϵ3, and ϵ4, that alter the coding of residues 112 and 158 (E2: Cys-112/Cys-158, E3: Cys-112/Arg-158, E4: Arg-112/Arg-158) have been recognized, of which ϵ4 is associated with an increased risk for developing AD (6, 7). It has been reported that apoE is codeposited with Aβ and acquires insolubility along with Aβ in AD brains (8, 9). Moreover, the density of senile plaques in APOE ϵ4 homozygous AD patients is higher than those carrying ϵ3/ϵ3 or ϵ3/ϵ4 genotypes (10). These studies indicate that apoE proteins, especially apoE4, may be associated with the pathogenesis of AD through interaction with Aβ. However, the mechanism whereby apoE4 affects Aβ in the pathogenesis of AD has been unknown.
In vitro studies have revealed that formation of Aβ amyloid fibrils is a complex process, comprised of nucleation and elongation phases (11, 12). In the nucleation phase, seeds are formed from monomer Aβ through conformational changes. Following the nucleation phase, Aβ forms fibrils by binding to preformed seeds in the elongation phase. It has been shown that apoE inhibits the nucleation phase of Aβ fibrillization, although this effect is independent of the apoE isoforms (13, 14). Furthermore, it has not been well understood how apoE affects the Aβ seeds in the inhibition of nucleation. Along with the classical fibrillization process of Aβ, a variety of metastable intermediates (e.g. paranuclei, protofibrils, Aβ-derived diffusible ligands) have been reported (15–19). Aβ protofibrils, separated in the high molecular weight fraction (>200 kDa) by size exclusion chromatography (SEC), are flexible short fibrils of ∼5 nm in diameter and in lengths not exceeding ∼150 nm (15). Because an intra-Aβ E22G (Arctic) FAD mutation accelerates the Aβ protofibril formation (20, 21) and the Aβ protofibrils are shown to be potently neurotoxic (22, 23), Aβ protofibrils have been deemed as a causative species leading to neurodegeneration in AD brains. However, it remains unclear how Aβ protofibrils impact the pathogenesis of AD.
In this study, we found that intrabrain injection of Aβ protofibrils promoted Aβ deposition in APP transgenic mice, demonstrating that Aβ protofibrils act as aggregation seeds in brains in vivo. We further showed that injection of mixture of Aβ protofibrils and apoE3 attenuated Aβ deposition compared with Aβ protofibrils alone or with mixture of Aβ protofibrils and apoE4. Based on these data, we hypothesized that apoE affects Aβ fibrillization, especially during the process of conversion from protofibrils to fibrils, in an isoform-dependent manner. To test this hypothesis, we carried out in vitro experiments using SEC analysis and thioflavin T (ThT) binding assay (21) and showed that apoE2 or apoE3 induced the slower conversion of Aβ protofibrils to fibrils than apoE4. We also found that Aβ protofibril-apoE2 or -apoE3 complex was more stable than Aβ protofibril-apoE4 complex. These results support the notion that apoE2 or apoE3 inhibits Aβ fibrillization and attenuates Aβ deposition through suppression of the conversion of Aβ protofibrils to fibrils, whereas apoE4 had a weaker effect.
Experimental Procedures
Peptide and Reagents
Synthetic Aβ(1–42) peptides were purchased from Peptide Institute, Inc.. Peptides were solubilized in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; Kanto Chemical) at a concentration of 1 mg/ml, dried, resolubilized in PBS containing 2% (v/v) DMSO (Kanto Chemical) and filtrated through a 0.2-μm filter immediately prior to use, as described (21). Human recombinant apoE (Wako Pure Chemical), human α1-microglobulin (Dako), and human α2-macroglobulin (α2M)(Sigma) proteins were purchased from indicated vendors. Lipidated apoE particles were purified from culture media of immortalized astrocytes overexpressing human apoE3 or apoE4 using an affinity column as described (24). Briefly, astrocytes were cultured in advanced DMEM (Life Technologies) with 10% FBS. After reaching at 80–90% confluency, cells were washed by PBS and further incubated in advanced DMEM with N-2 supplement (Life Technologies) and 3 μm of 25-hydroxycholesterol (Sigma) during days 2 and 3. Collected culture media were applied onto a column conjugated with a mouse monoclonal antibody against human apoE (WUE-4). Lipid apoE particles were eluted from the column with 3 m sodium thiocyanate, concentrated using Apollo centrifugal quantitative concentrators (QMWL: 150 kDa, Orbital Biosciences) and dialyzed against PBS containing 0.02% sodium azide.
Animals
A7 mice are transgenic mice that overexpress human APP695 harboring K670N, M671L, and T714I FAD mutations in neurons under the control of Thy1.2 promoter (25). C57Bl/6N mice were used as wild-type animals. All animals were maintained on food and water with a 12-h light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences of the University of Tokyo.
In Vivo Aβ Seeding Assay
In vivo Aβ seeding assays were carried out as previously described (26). Briefly, 0.25 μg of low molecular weight (LMW) soluble Aβ (22 μm Aβ(1–42) without incubation), Aβ protofibril without apoE (22 μm Aβ(1–42) with incubation at 37 °C for 3 h), Aβ fibril (22 μm Aβ(1–42) after incubation at 37 °C for 24 h), Aβ protofibril with apoE3 or apoE4 (22 μm Aβ(1–42), and 220 nm apoE after incubation at 37 °C for 3 h) or PBS were injected into the hippocampus (anterior-posterior −2.5 mm, medial-lateral ±2.0 mm, dorsal-ventral −1.8 mm from Bregma) and the cortex (anterior-posterior −2.5 mm, medial-lateral ±2.0 mm, dorsal-ventral −1.0 mm from Bregma) in 8- or 12-month-old A7 and wt mice, respectively. At 4 months after injection, immunohistochemical staining for Aβ and the measurement of insoluble Aβ were carried out. For Aβ immunohistochemistry, paraffin-embedded sections of mouse brains were pretreated with microwave (550 W, 10 min) in citrate buffer (pH 6.0) followed by proteinase K treatment (100 μg/ml, 6 min) and then immunostained with an anti-Aβ mouse monoclonal antibody 82E1 (IBL) by avidin-biotin complex method using diaminobenzidine as chromogen. To biochemically quantitate the insoluble Aβ, the hippocampus of the injected side was extracted by stepwise homogenization by radioimmune precipitation assay buffer (50 mm of Tris-HCl, pH 7.4, 150 mm of NaCl, 1% of Nonidet P-40, 1% of sodium deoxycholate, 0.1% of SDS) buffer containing Complete protease inhibitor mixture (Roche), 2% SDS, and 70% formic acid. The levels of Aβ in formic acid fractions (insoluble Aβ) were measured by two-site ELISA. The ratio representing the increase in Aβ deposition was calculated as follows: {A7(Aβ) − A7(PBS)} − {wt(Aβ) − wt(PBS)}/A7(PBS), where A7(Aβ) is insoluble Aβ42 in the hippocampus of A7 mice injected with Aβ; A7(PBS) is insoluble Aβ42 in the hippocampus of A7 mice injected with PBS (i.e. contralateral side of Aβ injection); wt(Aβ) is insoluble Aβ42 in the hippocampus of wt mice injected with Aβ; and wt(PBS) is insoluble Aβ42 in the hippocampus of wt mice injected with PBS (i.e. contralateral side of Aβ injection).
In experiments comparing Aβ protofibril injection without apoE and with apoE3 or apoE4, the ratios of the levels of insoluble Aβ42 in the hippocampus injected with protofibril Aβ and apoE divided by those without apoE were calculated. In experiments comparing the effects of apoE3 and apoE4, the ratios of the levels of insoluble Aβ42 in the hippocampus injected with Aβ protofibrils with apoE4 divided by those with apoE3 were calculated.
In Vitro Assays for the Formation of Aβ Protofibrils and Fibrils
In vitro Aβ fibrillization assays were performed as previously described (21). Briefly, synthetic Aβ(1–42) was solubilized at a concentration of 22 μm without or in the presence of indicated proteins (apoE, α1-microglobulin, or α2M) at a concentration of 220 nm and incubated at 37 °C. Following incubation for the indicated times, 50-μl aliquots were put immediately on ice to prevent further fibril formation and were centrifuged at 17,000 × g for 5 min. After centrifugation, samples before and after centrifugation (total ThT and sup ThT, respectively) were mixed with 500 μl of 3 μm ThT (Tokyo Chemical) in 0.1 m glycine-NaOH (pH 8.5) to monitor fibril formation. Fluorescence levels were then assayed using a Hitachi F2500 fluorometer (lex = 443 nm and lem = 484 nm). The supernatants were fractionated by SEC on Superdex 75 column (GE Healthcare Bio-Sciences) attached to a series 1100 high performance liquid chromatograph (Hewlett-Packard) at a flow rate of 0.5 ml/min to monitor protofibril formation at room temperature. Aβ protofibrils and LMW Aβ were detected at elution times of ∼15 and ∼27 min, respectively, by UV absorbance at 214 nm. The start time of Aβ fibrillization was defined as the point when the difference of ThT binding between sample prior to and after centrifugation exceeds 1.0.
Negative Stain Electron Microscopy
Samples were spread on 400-mesh collodion-coated grids, negatively stained with 2% (w/v) phosphotungstic acid (pH 7.0; Wako Pure Chemical), and viewed in an electron microscope (JEOL 1200EXII), as described (21).
Immunoblot Analysis
SDS-PAGE was performed as previously described (21). Samples were loaded on 10–20% Tris-Tricine gradient gels (Cosmo Bio), and Aβ was detected by a mouse monoclonal antibody BAN50, as described (27). ApoE was detected by a mouse monoclonal antibody 3H1 (University of Ottawa Heart Institute Research Corporation) or a goat polyclonal antibody 11-S (Alpha Diagnostic Intl. Inc.). α1-Microglobulin was detected by a rabbit monoclonal anti-α1-microglobulin-specific antibody (Abcam). α2M was detected by a rabbit polyclonal anti-α2M-specific antibody (Dako).
Photoinduced Cross-linking of Unmodified Proteins (PICUP)
To examine the binding of Aβ and apoE, the PICUP method was applied, as previously described (16). Briefly, 22 μm Aβ(1–42) was incubated without or with apoE at 37 °C for the indicated times, and 1 μl of 1 mm Ru(Bpy) and 1 μl of 20 mm ammonium persulfate (Kanto Chemical) were added to 18 μl of Aβ samples. The mixture was irradiated exactly for 1 s, and the reaction was quenched immediately with 20 μl of 2× sample buffer containing 5% β-mercaptoethanol. Then the solution was subjected to immunoblot analysis.
Protofibril Stability Assay
Isolated Aβ protofibrils without or with apoE by SEC analysis was incubated at 37 °C for the indicated times. After incubation, the solution was centrifuged, followed by ThT binding assay of the total solution and the supernatants to evaluate the amount of Aβ protofibrils and fibrils.
Stability Assay for the SDS-stable Aβ-apoE Complex
To monitor the stability of SDS-stable Aβ-apoE complexes, Aβ(1–42) was incubated with apoE at 37 °C for 6 h, followed by the addition of urea (Nacalai Tesque) or HFIP (Kanto Chemical) at the indicated concentrations and incubated at 4 °C for 12 h. The solution was then subjected to immunoblot analysis.
Statistical Analysis
Statistical significance was determined by Student's t test for comparison of two groups or by one-way analysis of variance followed by a Tukey-Kramer post hoc test for multiple comparisons tests for comparison of more than two groups. The significance level was established at p < 0.05. The values are expressed as means ± S.D. or S.E.D.
Results
Aβ Protofibrils Exhibit a Seeding Effect on Aβ Deposition in the Brains of APP Transgenic Mice
In this study, we used APP transgenic mice (A7 line), which overexpress human APP with Swedish and Austrian double FAD-linked mutations (KM670/671NL + T714I) in neurons under the control of Thy1.2 promoter (25). A7 mice develop progressive Aβ deposition in the cerebral neocortices and hippocampi at the age of ∼11–12 months in an age-dependent manner.
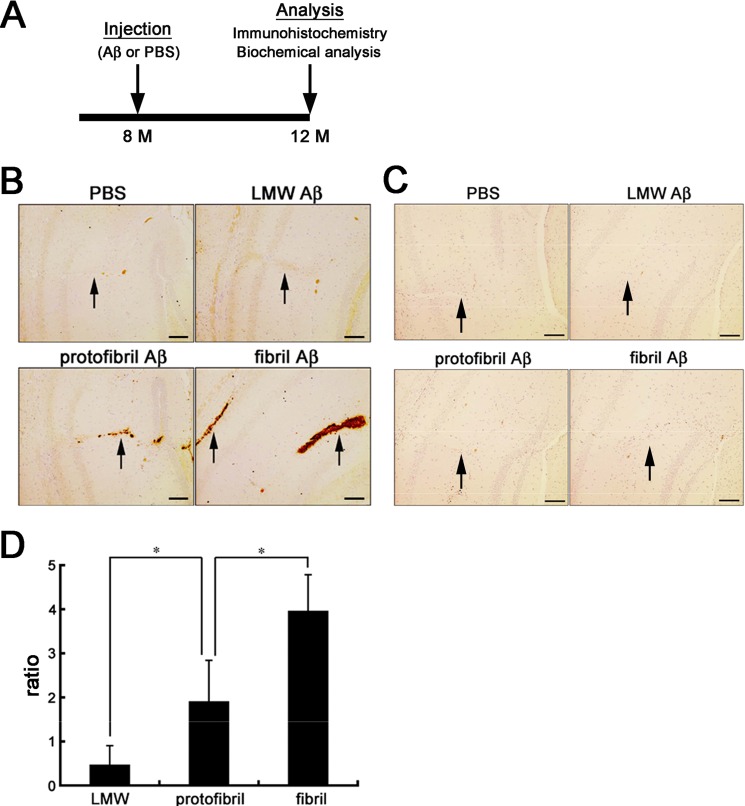
It has been reported that intracerebral inoculation of Aβ amyloid induces Aβ deposition in the brains of APP transgenic mice, acting as an aggregation seed for Aβ amyloid (26, 28). To elucidate whether Aβ protofibrils also work as an aggregation seed for Aβ amyloid in vivo, we prepared LMW Aβ, Aβ protofibrils, and Aβ fibrils using synthetic human Aβ(1–42) (21) and injected them individually into the brains of 8-month-old A7 or wt mice (Fig. 1A). We also injected PBS into the contralateral hemisphere as a control. After 4 months, immunohistochemical analyses of the brains of A7 mice showed that Aβ protofibrils and Aβ fibrils, but not LMW Aβ, induced Aβ deposition around the injection sites (Fig. 1B). In sharp contrast, no Aβ deposits were seen in the PBS-injected contralateral hemisphere (Fig. 1B) or in both hemispheres of wt mice (Fig. 1C). The levels of insoluble Aβ in the brains injected with Aβ protofibrils were significantly higher than those with LMW Aβ, and those in mice injected with Aβ fibrils were higher compared with those injected with Aβ protofibrils, as quantified by Aβ specific ELISA (Fig. 1D). These data suggested that not only Aβ fibrils but also Aβ protofibrils harbor a seeding potential on Aβ amyloidogenesis in vivo.
FIGURE 1.
In vivo seeding effects of Aβ protofibrils and fibrils in the brains of A7 mice. A, schematic representation of the timeline of experiments. A7 mice were injected with Aβ or PBS into the neocortex and hippocampus at 8 months. Then at 4 months after injection, the both hemispheres were immunohistochemically or biochemically analyzed. B and C, Aβ immunostaining of the hippocampi and neocortices of mouse brains injected with PBS, LMW, protofibril, or fibril Aβ. A7 mice (B) or wt (C) mice were injected with PBS, LMW, protofibril, or fibril Aβ, respectively, into the hippocampi and neocortices at 8 months old, and Aβ deposits around the trajectories of injection (arrows) were immunostained by 82E1 at 12 months. Representative images in each group (n = 4) are shown. Scale bar, 100 μm. D, relative levels of insoluble Aβ42 in the hippocampi of A7 mice injected with different Aβ preparations. Hippocampi of A7 mice injected with PBS, LMW, protofibril, or fibril Aβ, respectively, at 8 months old were dissected at 12 months, and the levels of insoluble Aβ42 were measured by two-site ELISA. The bars represent the mean ratios of the levels of insoluble Aβ42 after injection of LMW (n = 4), protofibril (n = 3), or fibril (n = 3) forms of Aβ divided by those injected with PBS. The mean values ± S.D. are shown. *, p < 0.05. One-way analysis of variance was used.
ApoE3, but Not ApoE4, Attenuates Aβ Deposition Induced by Aβ Protofibrils
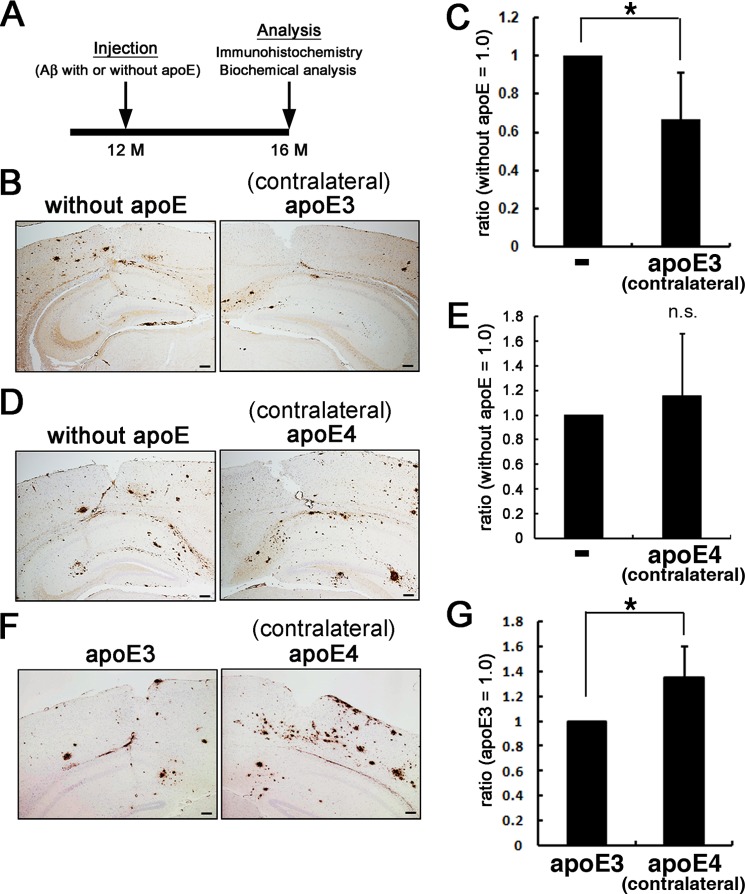
We hypothesized that apoE may affect the seeding effects of Aβ protofibrils in an isoform-dependent manner. To test this, we injected Aβ protofibrils alone into one side of the hippocampus of 12-month-old A7 mice and Aβ protofibrils with apoE3 or apoE4 into the other side (Fig. 2A). We found that Aβ protofibrils with apoE3 induced significantly less abundant Aβ deposition as revealed by immunohistochemistry (Fig. 2B) or as the amount of biochemically extractable insoluble Aβ compared with those injected with Aβ protofibrils alone (Fig. 2C). In contrast, Aβ protofibrils with apoE4 induced Aβ deposition at a similar extent to Aβ protofibrils alone (Fig. 2D). No significant difference in the amount of insoluble Aβ was seen between mice injected with Aβ protofibrils with apoE4 or Aβ protofibrils alone (Fig. 2E). We speculated that these contrasting results were due to the difference between the isoforms of injected apoE. To examine this idea, we injected Aβ protofibrils with apoE3 into one side, and Aβ protofibrils with apoE4 into the other side of the hippocampus of 12-month-old A7 mice and found that Aβ protofibrils with apoE4 induced higher levels of Aβ deposition as detected by immunohistochemistry and as insoluble Aβ by biochemistry compared with Aβ protofibrils with apoE3 (Fig. 2, F and G). These data indicated that apoE3, but not apoE4, attenuated Aβ deposition induced by Aβ protofibrils, suggesting that apoE may affect the seeding effect of Aβ protofibrils in an isoform-dependent manner.
FIGURE 2.
In vivo effects of apoE on the seeding effects of Aβ protofibrils. A, schematic representation of the timeline of experiments. A7 mice were injected with Aβ protofibrils preincubated with or without apoE into the neocortex and hippocampus at 12 months. At 4 months after injection, both hemispheres were immunohistochemically or biochemically analyzed. B and C, A7 mice were injected with Aβ protofibrils preincubated without apoE on one side of the neocortex and hippocampus and those with apoE3 on the contralateral side. The both hemispheres were immunohistochemically analyzed for Aβ using 82E1 antibody (B; n = 3 in each group) or subjected to biochemical quantification of insoluble Aβ (C; n = 5). Insoluble Aβ levels were quantitated by two-site ELISA, and the ratios of those in the contralateral side (i.e. injected with protofibrils preincubated with apoE3) divided by those in the side injected with Aβ protofibrils alone) were calculated (C). Similarly, those injected with protofibrils preincubated without apoE on one side and with apoE4 on the contralateral side (D; n = 3 in each group, E; n = 5) and those injected with protofibrils preincubated with apoE3 on one side and with apoE4 on the contralateral side (F; n = 3 in each group, G; n = 5) were immunohistochemically and biochemically analyzed. Scale bars, 100 μm. The mean values ± S.D. are shown. *, p < 0.05.
ApoE Suppresses the Protofibril to Fibril Conversion of Aβ in an Isoform-dependent Manner in Vitro
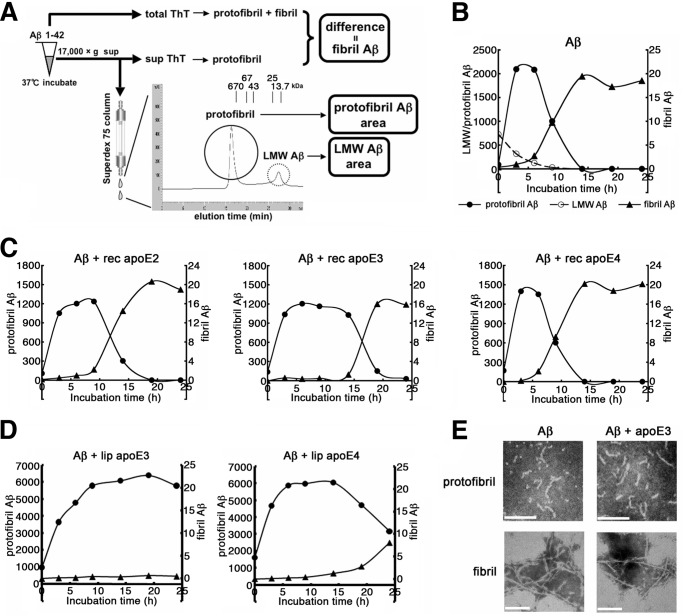
To elucidate the mechanism whereby apoE affects the seeding effects of Aβ protofibrils in an isoform-dependent manner, we investigated the role of apoE in the formation of Aβ protofibrils and fibrils in vitro by SEC analysis and ThT binding assay, respectively, as previously reported (21) (Fig. 3A). Briefly, the formation of Aβ protofibrils and LMW Aβ including monomer and lown oligomers, were quantitated as areas corresponding to the >100-kDa peak and the ∼10-kDa peak, respectively, detected by SEC analysis. Simultaneously, the formation of Aβ fibrils was evaluated as the difference of ThT binding between samples prior to and after centrifugation (total ThT and sup ThT, respectively). We defined the start time of Aβ fibril formation as the point when the difference of ThT binding between samples prior to and after centrifugation exceeds 1.0. When Aβ(1–42) was incubated, Aβ protofibrils were rapidly formed within 3 h, in parallel with the decrease in LMW Aβ, followed by a decrease in Aβ protofibrils; protofibrils disappeared by 14 h, whereas Aβ fibrils started to increase after 4.6 h of incubation, which leveled off at ∼14 h (Fig. 3B). Using these assays, we examined the effect of apoE on the formation of Aβ protofibrils and fibrils. In comparison with the incubation of Aβ alone (Fig. 3B), coincubation with apoE2 or apoE3 extended the lifetime of Aβ protofibrils and delayed the start of Aβ fibril formation by 7.6 and 13.9 h, respectively (Fig. 3C). In sharp contrast, coincubation with apoE4 did not extend the lifetime of Aβ protofibrils, and fibril formation started at 5.2 h (Fig. 3C). The time course of Aβ fibrillization in the presence of apoE4 was comparable to those in the absence of apoE (Fig. 3B). ApoE is known to be associated with high density lipoprotein particles in the brain and with very low density lipoprotein particles in the blood (29). To see whether lipidation of apoE is critical to the effects on the formation of Aβ protofibrils and fibrils, we used lipidated apoE particles purified from culture media of human immortalized astrocytes overexpressing apoE3 or apoE4 as described (24, 30). We incubated Aβ with or without lipidated apoE particles, monitored the formation of Aβ protofibrils and fibrils, and found that the presence of lipidated apoE particles extended the lifetime of Aβ protofibrils (Fig. 3D); notably, the capacity of lipidated apoE4 particles to prolong the lifetime of Aβ protofibrils was smaller than that of lipidated apoE3 (>24 h in apoE3 versus 14.3 h in apoE4; Fig. 3D). To determine whether apoE altered the structure of protofibrils or fibrils of Aβ, we observed the ultrastructure of Aβ protofibrils or fibrils by negative stain electron microscopy. Aβ protofibrils presented with a short and curved fibril-like structure and Aβ fibrils exhibited a long, straight, and unbranching structure as described (15), which were almost identical between samples of Aβ protofibrils and fibrils in the presence or absence of apoE3 (Fig. 3E). These data suggested that apoE2 and apoE3 potentially suppress the conversion of Aβ protofibrils to fibrils, resulting in the prolongation of the lifetime of Aβ protofibrils and delay in the start of Aβ fibril formation. In contrast, apoE4 has a lesser effect on the conversion of Aβ protofibrils to fibrils compared with apoE2 or apoE3, thereby allowing Aβ protofibrils with apoE4 to rapidly form fibrils compared with those with apoE2 or apoE3.
FIGURE 3.
Isoform-dependent effects of apoE on the formation of Aβ protofibrils and fibrils in vitro. A, schematic depiction of the method for the measurement of LMW, protofibril, and fibril forms of Aβ. The levels of LMW Aβ and protofibril Aβ are quantitated as areas corresponding to the ∼10-kDa peak and >100-kDa peak, respectively. The levels of Aβ fibrils are evaluated as the differences of ThT binding between samples prior to (total ThT) and after centrifugation (sup ThT). B, time course of the formation of LMW, protofibril, and fibril Aβ as monitored by SEC and ThT binding. 22 μm of Aβ(1–42) was incubated for 0, 3, 6, 9, 14, 19, or 24 h, and the levels of LMW Aβ (open circles) and protofibril Aβ (filled circles) were quantitatively evaluated by SEC and those of Aβ fibrils (triangles) by ThT binding, respectively. Representative data out of four independent experiments (that showed similar profiles) are shown. C, the effects of recombinant (rec) apoE on the formation of protofibril and fibril Aβ. 22 μm of Aβ(1–42) was incubated for 0, 3, 6, 9, 14, 19, or 24 h with 220 nm of rec apoE2 (left panel, n = 4), rec apoE3 (middle panel, n = 5), or rec apoE4 (right panel, n = 4), and the time course of the formation of Aβ protofibrils (circles) and fibrils (triangles) was monitored as in B. The starting time points of fibril formation were estimated to be 7.6, 13.9, and 5.2 h, respectively, in preparations incubated with rec apoE2, apoE3, and apoE4. The mean values are shown. D, the effects of the lipidated (lip) apoE particles on the formation of protofibril and fibril Aβ. 22 μm of Aβ(1–42) was incubated for 0, 3, 6, 9, 14, 19, or 24 h with 220 nm of lipid apoE3 particles (left panel, n = 4) or lipid apoE4 particles (right panel, n = 4), and the time course of the formation of protofibrils (circles) and fibrils (triangles) were monitored as in C. Fibril formation was observed starting at >24 h and 24 h of incubation, respectively, in preparations incubated with lipidated (lip) apoE3 and apoE4. The mean values are shown. E, negative stain electron microgram of protofibril and fibril forms of Aβ(1–42) incubated alone (left column) or with apoE3 (right column) for 6 h (protofibrils, upper row) and 24 h (fibrils, lower row), respectively. Scale bar, 100 nm.
ApoE Forms SDS-stable Complex with Aβ Protofibrils
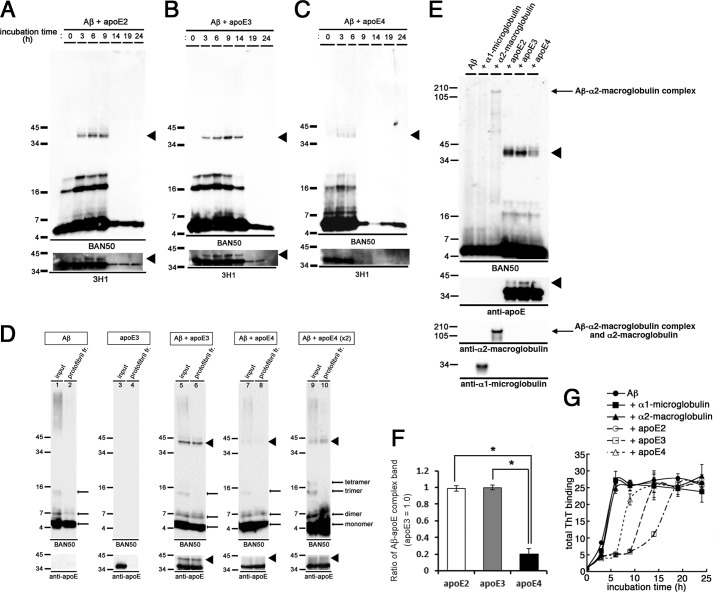
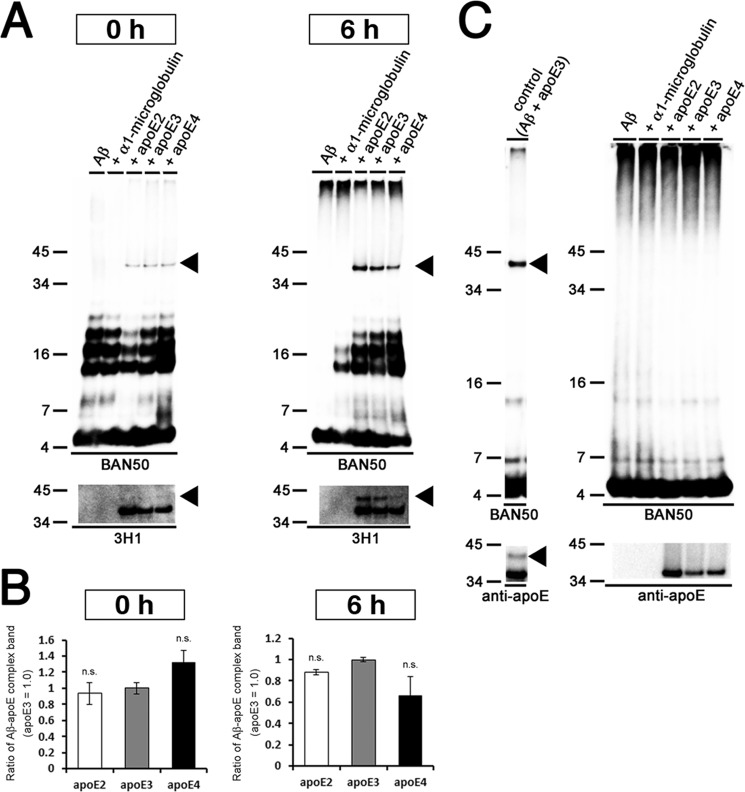
Because apoE affected the protofibril to fibril conversion of Aβ in an isoform-dependent manner, we next examined the interaction of apoE with Aβ protofibrils, as well as its isoform dependence. To this end, we coincubated Aβ with different isoforms of apoE in vitro and evaluated their interaction by immunoblotting. It has been reported that apoE forms an SDS-stable complex with Aβ (31–33). Upon coincubation of Aβ with apoE2 or apoE3, an anti-Aβ antibody (BAN50) revealed ∼40-kDa bands in addition to the Aβ monomer and oligomer bands (Fig. 4, A and B, upper panels). The ∼40-kDa bands were also labeled with an anti-apoE antibody (3H1) (Fig. 4, A and B, lower panels), indicating that the ∼40-kDa bands represented the SDS-stable complex of Aβ and apoE2 or apoE3. Intriguingly, the ∼40-kDa bands appeared in a time-dependent manner: the ∼40-kDa band did not exist at 0 h, which emerged during 3–9 h upon coincubation with apoE2 or 3–14 h upon coincubation with apoE3, concomitantly with the Aβ protofibril formation (Fig. 3C). After 9 h of coincubation with apoE2 or 14 h of coincubation with apoE3, the ∼40-kDa bands disappeared, which coincided with the emergence of Aβ fibrils (Fig. 3C). These findings are consistent with the idea that apoE was precipitated with Aβ fibrils. In contrast, the ∼40-kDa bands were present at 3–6 h of incubation when Aβ was coincubated with apoE4 (Fig. 4C). The time course of the emergence of the ∼40-kDa bands was coincident with that of Aβ protofibril formation in the presence of apoE4 (Fig. 3C).
FIGURE 4.
Formation of SDS-stable Aβ-apoE complex in vitro. A–C, time course of formation of the SDS-stable Aβ-apoE complex. 22 μm of Aβ(1–42) was incubated for 0, 3, 6, 9, 14, 19, or 24 h with rec apoE2 (A), rec apoE3 (B), or rec apoE4 (C). After centrifugation, SDS-stable Aβ-apoE complex was monitored by immunoblot analyses with an anti-Aβ antibody (BAN50, upper panel) and an anti-apoE antibody (3H1, bottom panel). Arrowheads indicate the SDS-stable Aβ-apoE complex bands (n = 3). D, formation of SDS-stable Aβ-apoE complex in the Aβ protofibril fraction. Immunoblot analyses of isolated protofibrils without apoE (lanes 1 and 2), with rec apoE3 (lanes 5 and 6), with rec apoE4 (lanes 7–10), and of rec apoE3 alone (lanes 3 and 4) with an anti-Aβ antibody (BAN50, upper panels) and an anti-apoE antibody (bottom panels). Lanes 9 and 10 were loaded with double the amount of samples. Arrowheads indicate the bands corresponding to SDS-stable Aβ-apoE complex migrating at ∼40 kDa (n = 3). E, 22 μm of Aβ(1–42) was incubated alone (Aβ) or with rec α1-microglobulin, rec α2M, rec apoE2, rec apoE3, or rec apoE4 for 6 h. After centrifugation at 17,000 × g for 5 min, SDS-stable complex was monitored by immunoblotting analyses using an anti-Aβ antibody (BAN50, upper panel), an anti-apoE antibody (3H1, the second panel from the top), an anti-α2M antibody (the third panel from the top), and an anti-α1-microglobulin antibody (bottom panel). Arrowheads indicate ∼40-kDa bands corresponding to the SDS-stable Aβ-apoE complex, and the arrow indicates the SDS-stable Aβ-α2M complex migrating at ∼190 kDa. F, quantitative measurement of the band intensities in E. The mean values ± S.D. in three independent experiments are shown as ratios relative to the values of apoE3 as 1.0. *, p < 0.05. One-way analysis of variance was used. G, in vitro Aβ fibrillization assay in the presence of binding proteins. 22 μm of Aβ(1–42) was incubated in the absence (filled circles) or presence of rec α1-microglobulin (the molar ratio of Aβ/α1-microglobulin at 100:1, filled squares), rec α2M (the molar ratio of Aβ/α2M at 100:1, filled triangles), rec apoE2 (the molar ratio of Aβ/apoE2 at 100:1, open circles), rec apoE3 (the molar ratio of Aβ/apoE3 at 100:1, open squares), or rec apoE4 (the molar ratio of Aβ/apoE4 at 100:1, open triangles) for 0, 3, 6, 9, 14, 19, and 24 h, and then ThT fluorescence was measured. The mean values in three independent experiments are shown.
Because the formation of SDS-stable Aβ-apoE complex coincided with the protofibril formation of Aβ, we further examined whether apoE was directly bound to Aβ protofibrils by incubating Aβ with or without apoE for 6 h and isolating the Aβ protofibril fraction using SEC. When Aβ was incubated without apoE, Aβ-positive bands corresponding to Aβ monomer and oligomers were detected in the protofibril fraction (Fig. 4D, lane 2), suggesting the dissociation of Aβ protofibrils during SDS-PAGE into monomer or oligomers. When apoE3 was incubated alone, no apoE-positive band was detected in the Aβ protofibril fraction (Fig. 4D, lane 4). Importantly, when Aβ was coincubated with apoE3, Aβ monomer, oligomers, apoE3, and the ∼40-kDa SDS-stable Aβ-apoE3 complex were detected in the protofibril fraction (Fig. 4D, lane 6), indicating that apoE3 directly interacted with Aβ protofibrils and formed the ∼40-kDa SDS-stable complex with Aβ protofibrils. In addition, the ∼40-kDa band corresponding to the SDS-stable Aβ-apoE4 complex was also detected in the Aβ protofibril fraction by coincubation of Aβ with apoE4 (Fig. 4D, lanes 8 and 10). It is notable that the amount of SDS-stable Aβ-apoE4 complex in the Aβ protofibril fraction was markedly smaller than that of SDS-stable Aβ-apoE2 or Aβ-apoE3 complex (Fig. 4, D–F), whereas the levels of Aβ in these fractions were similar. These data suggest that apoE formed a SDS-stable complex with Aβ protofibrils in an isoform-dependent manner. To further ascertain the specificity of apoE in the formation of SDS-stable complex with Aβ protofibrils, we coincubated Aβ with α1-microglobulin, α2M, recombinant apoE2, apoE3, or apoE4 and examined the level of SDS-stable complex by immunoblotting (Fig. 4E). We confirmed that apoE2 formed the ∼40-kDa SDS-stable Aβ-apoE complex at a similar extent to apoE3, whereas apoE4 formed smaller amount of SDS-stable Aβ-apoE complex compared with apoE3 (Fig. 4, E and F). α1-Microglobulin did not form the SDS-stable complex with Aβ, whereas α2M, which is capable of binding with Aβ, formed Aβ-α2M SDS-stable complex as a ∼190-kDa band. Because α2M did not affect the Aβ fibrillization in vitro (Fig. 4G), we presumed that the interaction of α2M with Aβ did not affect the conversion of Aβ from protofibrils to fibrils.
Aβ Interacts with apoE Prior to Protofibril Formation
Since apoE formed the SDS-stable complex with Aβ protofibrils in an isoform-dependent manner, we examined whether the binding affinity of apoE to Aβ differs among apoE isoforms. To examine the interaction between apoE and Aβ in solution, we performed chemical cross-linking assay using the PICUP method (16). We found that all apoE isoforms bound to Aβ at 0 or 6 h with no difference in the levels of the Aβ-apoE complex of ∼40 kDa (Fig. 5, A and B). To determine whether apoE binds to the preformed Aβ protofibrils, we coincubated preformed Aβ protofibrils with apoE. No bands corresponding to SDS-stable Aβ-apoE complex were detected (Fig. 5C), suggesting that apoE initially binds to soluble Aβ and that apoE may form SDS-stable complex with Aβ protofibrils in an isoform-dependent manner during the course of Aβ fibrillization.
FIGURE 5.
Chemical cross-linking analyses of the interaction between Aβ protofibrils and apoE. A, 22 μm of Aβ(1–42) was incubated alone (Aβ) or with rec α1-microglobulin, rec apoE2, rec apoE3, or rec apoE4 for 0 or 6 h and cross-linked by PICUP. The binding between Aβ and apoE was assayed by immunoblot analyses using an anti-Aβ antibody (BAN50, upper panel) and an anti-apoE antibody (3H1, bottom panel). Arrowheads indicate bands corresponding to the SDS-stable Aβ-apoE complex. B, quantitative measurement of the band intensities in A. The mean values ± S.D. in three independent experiments are shown as ratios relative to the values of apoE3 as 1.0. n.s. means no significant difference. One-way analysis of variance was used. C, formation of SDS-stable Aβ-apoE complex from the preformed protofibrils. Post lanes, 22 μm of Aβ(1–42) was incubated for 6 h to generate protofibrils, and thus formed protofibrils were again incubated alone or with rec α1-microglobulin, rec apoE2, rec apoE3, or rec apoE4 for 8 h at 37 °C. Control lane, 22 μm of Aβ(1–42) was incubated for 6 h with rec apoE3. SDS-stable Aβ-apoE complex was detected by immunoblot analyses with an anti-Aβ antibody (BAN50, upper panels) or an anti-apoE antibody (bottom panels). Arrowheads indicate the bands corresponding to SDS-stable Aβ-apoE complex.
ApoE4 Forms a Less Stable Complex with Aβ Protofibrils Compared with ApoE2 or ApoE3
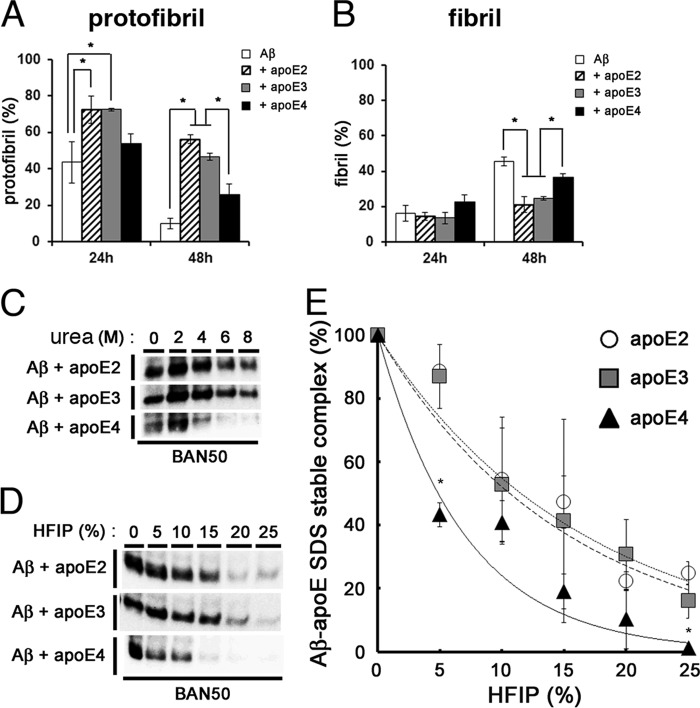
It remained unknown why apoE2 or apoE3 suppressed the conversion of Aβ protofibrils to fibrils more efficiently than apoE4 and formed a more SDS-stable complex with Aβ protofibrils than apoE4. We hypothesized that apoE may have a role in stabilizing Aβ protofibrils in an isoform-dependent manner. To test this, we first evaluated the stability of Aβ protofibrils isolated by SEC. We incubated the SEC-isolated Aβ protofibrils at 37 °C for 24 and 48 h, respectively, centrifuged, and monitored the remaining Aβ protofibrils in the supernatants by ThT binding (Fig. 6A). We found that ∼56.2% and ∼46.7% of Aβ protofibrils formed in the presence of apoE2 and apoE3, respectively, remained after 48 h of incubation. In contrast, ∼9.9% and ∼26.0% of Aβ protofibrils formed without apoE or in the presence of apoE4, respectively, persisted after incubation. Simultaneously, we measured the levels of Aβ fibrils, which were formed during the incubation period, by ThT binding assay. The ratios of Aβ fibrils formed from Aβ protofibrils incubated with apoE2 and apoE3 were ∼21.1% and ∼24.7%, respectively, after 48 h of incubation (Fig. 6B), whereas those with apoE4 or without apoE were ∼46.0% and ∼36.8%, respectively (Fig. 6B). These data strongly suggested that apoE3 had the capacity to stabilize Aβ protofibrils by suppressing the conformational change into fibrils, whereas apoE4 had a weaker effect on the stabilization of Aβ protofibrils.
FIGURE 6.
Isoform-dependent effects of apoE on the stability of Aβ protofibrils. A and B, the effects of apoE on the stability of Aβ protofibrils (A) and formation of fibrils (B). Aβ protofibrils isolated by SEC were incubated alone (white column), with rec apoE2 (hatched column), with rec apoE3 (gray column), or rec apoE4 (black column) for 24 and 48 h. After centrifugation at 17,000 × g for 5 min, the levels of protofibrils were monitored by the ThT fluorescence of supernatants, and those of fibrils were determined by the difference in ThT fluorescence between samples before and after centrifugation. The percentage of levels of protofibrils (A) and fibrils (B) at 24 or 48 h that comprise those prior to incubation as 100% (mean values ± S.D. in three independent experiments) are shown. *, p < 0.05. One-way analysis of variance was used. C, stability of SDS-stable Aβ-apoE complex against urea. 22 μm of Aβ(1–42) was incubated for 6 h with rec apoE2, rec apoE3, or rec apoE4, followed by addition of urea at 0, 2, 4, 6, or 8 m of final concentrations and additionally incubated for 12 h. SDS-stable Aβ-apoE complex was detected by immunoblot analyses with an anti-Aβ antibody (BAN50). D, stability of SDS-stable Aβ-apoE complex against HFIP. 22 μm of Aβ(1–42) was incubated for 6 h with rec apoE2, rec apoE3, or rec apoE4, followed by addition of HFIP at 0, 5, 10, 15, 20, or 25% of final concentrations and additionally incubated for 12 h. SDS-stable Aβ-apoE complex was detected by immunoblot analyses with an anti-Aβ antibody (BAN50). E, quantitation of data in D. The levels of Aβ-apoE2 (circles), Aβ-apoE3 (squares), and Aβ-apoE4 (triangles) complexes after addition of indicated concentrations of HFIP were evaluated by densitometry. The mean values ± S.D. in three independent experiments are shown. *, p < 0.05. One-way analysis of variance was used.
To further examine the effect of apoE2 or apoE3 on the stability of Aβ protofibrils, we tested the stability of the protofibril Aβ-apoE complex by chemical-induced denaturation. We incubated the SDS-stable Aβ-apoE complex in different concentrations of urea solution (0, 2, 4, 6, or 8 m) and found that complexes of Aβ-apoE2 or Aβ-apoE3 were stable, even in the presence of 8 m urea, whereas SDS-stable Aβ-apoE4 complex was dissociated in 4, 6, or 8 m of urea solution (Fig. 6C). We also incubated the SDS-stable Aβ-apoE complex in 0, 5, 10, 15, 20, or 25% of HFIP and found that the Aβ-apoE2 or Aβ-apoE3 complex was more stable than the Aβ-apoE4 complex (Fig. 6, D and E). These data also suggested that apoE4 rendered the Aβ protofibrils less stable compared with apoE2 or apoE3. Taken together, we concluded that apoE2 or apoE3 forms a more stable complex with Aβ protofibrils, resulting in the suppression of structural conversion of Aβ protofibrils to fibrils, whereas apoE4 does not have this effect.
Discussion
In this study, we showed that both Aβ protofibrils and fibrils are capable of inducing Aβ deposition in the brain as an amyloid seed, similarly to Aβ amyloid extracted from the brains of AD patients or APP transgenic mice (26, 28), and that apoE3, but not apoE4, had a suppressive effect on the Aβ deposition induced by Aβ protofibrils. We further showed in in vitro Aβ protofibril/fibril formation assays that apoE2 and apoE3 prolonged the lifetime of Aβ protofibrils and suppressed the conversion of Aβ protofibrils to fibrils, whereas apoE4 had a lesser effect on the retention of Aβ protofibrils and failed to inhibit the conversion of Aβ protofibrils to fibrils compared with apoE2 or apoE3. Furthermore, we found that Aβ protofibrils interacted with apoE and formed a SDS-stable complex. Finally we showed that SDS-stable Aβ-apoE4 complex was less stable than those containing apoE2 or apoE3, resulting in the lower level of SDS-stable Aβ-apoE4 complex compared with those with apoE2 or apoE3. These data suggest that apoE2 or apoE3 plays a role in the retention of Aβ protofibrils, thereby suppressing the conversion of Aβ protofibrils to fibrils, whereas apoE4 is incapable of sustaining the lifetime of Aβ protofibrils in a loss of function manner. This view is consistent with the results that apoE3 suppressed Aβ fibril formation in vitro and attenuated the protofibril-induced Aβ deposition in the brains of APP transgenic mice, whereas apoE4 did not have these effects. It has been reported that the Aβ burden in the brains of patients with AD is increased with ϵ4 allele in a dose-dependent manner (10, 34). Overexpression of human apoE4 in the absence of endogenous murine apoE increased the deposition of fibrillar Aβ in the hippocampus of mice, whereas that of apoE3 did not have this effect (35, 36). Taken together, these data support the notion that apoE4 promotes the amyloid fibril formation through the lack of suppression of conversion of Aβ protofibrils to fibrils.
We coincubated Aβ and apoE in vitro, separated the protofibril fraction by SEC (∼600-kDa ranges; Fig. 3A), and detected a ∼40-kDa SDS-stable band by SDS-PAGE that is positive both for Aβ and apoE on immunoblots (Fig. 4, A–D). We speculated that the ∼40-kDa band was derived from a subfraction of Aβ protofibrils-apoE complex and examined their amount or stability in detail, although it remains to be determined whether the characteristics of the SDS-stable Aβ-apoE complex represents those of Aβ protofibrils potentially interacting with apoE, because the SDS-stable Aβ-apoE complex comprised a relatively minor fraction of apoE or Aβ (Fig. 4D). Nonetheless, we have shown that the stabilizing activity of apoE4 on Aβ protofibrils was lower than those of apoE2 or apoE3, underscoring the differential effects of apoE isoforms on the stability of protofibrils (Fig. 6A).
It has been reported that the level of Aβ monomer-apoE4 complex was smaller than that of Aβ monomer-apoE3 complex in vitro by immunoblotting (32). Recently, it has also been reported that soluble Aβ-apoE4 complex is less stable than Aβ-apoE2 or Aβ-apoE3 in vitro as measured by Aβ-apoE complex-specific ELISA and that the levels of soluble Aβ-apoE complex are lower in 5xFAD-apoE4TR mice compared with those in 5xFAD-apoE3TR or 5xFAD-apoE2TR mice in vivo (37). In the present study, we show that the level of SDS-stable Aβ-apoE4 protofibril complex in the Aβ protofibril fraction was lower than that of SDS-stable Aβ-apoE3 protofibril complex. These data suggest an isoform-specific effect of apoE on the stability of the Aβ-apoE complex, i.e. Aβ-apoE3 complex being more stable than Aβ-apoE4 complex (38). Furthermore, we show that the stability of Aβ protofibril-apoE4 complex was lower than that of Aβ protofibril-apoE2 or Aβ protofibril-apoE3 complex in vitro, suggesting that apoE isoforms differentially stabilize both protofibrils and monomers of Aβ. However, the mechanism of the isoform-specific stabilization of Aβ protofibrils by apoE remains unclear. The Aβ protofibril-apoE4 complex may have a thermodynamically higher energy state compared with Aβ protofibril-apoE2 or apoE3 complexes; this could enable the faster transition of the conformation of Aβ protofibrils with apoE4 into fibrils compared with apoE2 or apoE3, because of the lower activation barrier. It has been documented that apoE4 forms a compact structure by the salt bridge between Arg-61 and Glu-255, whereas apoE2 and apoE3 have an open structure without the salt bridge (39, 40). Moreover, substitution of Thr for Arg-61 in apoE4, which mimics the open structure of apoE3, formed more SDS-stable Aβ-apoE complex than apoE4 (41). These observations support the notion that the difference in the tertiary structure of apoE affects the isoform-dependent differences in the stability of Aβ protofibril-apoE complexes, which may result in affecting the rate of the conformational changes of Aβ protofibrils to fibrils.
Our in vivo experiments showed that the injection of Aβ protofibrils into the brain of APP transgenic mice induced Aβ deposition. Our results indicate that Aβ protofibrils are capable of acting as an aggregation seed in vivo. This also suggests that the Aβ protofibrils act as “on pathway” intermediates that promote Aβ amyloid formation. Aβ protofibrils have been identified as a toxic, soluble, and fibril-like intermediate in an in vitro Aβ fibrillization assay (22, 23). It has been reported that Aβ protofibrils were detected in the brain and cerebrospinal fluid of APP transgenic mice using an antibody specific for Aβ protofibrils (42) and that passive immunization with the Aβ protofibril-specific antibody significantly reduced the amyloid burden in the brains of APP transgenic mice (43). In addition, it has been reported that E22G (Arctic) FAD mutation that alters an amino acid residue within Aβ region accelerates protofibril formation in vitro (20), as well as amyloid deposition in the brains of APP transgenic mice (44). These data suggest that Aβ protofibrils play critical roles in Aβ fibrillization and in Aβ amyloid deposition in the brains of AD patients as an aggregation seed. Furthermore, we showed that apoE3, but not apoE4, attenuated the Aβ deposition induced by inoculation of Aβ protofibrils into the brains of APP transgenic mice. These results suggest that apoE affects the seeding effect of Aβ protofibrils in an isoform-dependent manner.
In the in vitro protofibril/fibril formation study, the lifetime of Aβ protofibrils coincubated with apoE4 was shorter than that of protofibrils with apoE2 or apoE3. This apparently contradicts with the finding that the FAD-linked E22G (Arctic) mutation increases the level of Aβ protofibrils (20). The discrepancy may partly be explained by the limited amount of Aβ peptides within the reaction tube in the in vitro experimental setting, resulting in the monophasic emergence of Aβ protofibrils and subsequent conversion to fibrils. In human brains in vivo, Aβ is continuously produced and supplied to the brain parenchyma; once Aβ starts to aggregate in the brain, we postulate that the level of protofibrils is maintained at a plateau, at an equilibrium between formation of Aβ protofibrils and structural conversion to fibrils, resulting in an increase in the level of Aβ deposition in the brain, without reducing the level of pathogenic Aβ protofibrils. We have previously reported that H6R (English) and D7N (Tottori) FAD-linked mutations accelerated Aβ fibril formation without an increase in the protofibril formation in vitro (21), suggesting that the acceleration of structural conversion of Aβ protofibrils to fibrils may be a mechanism causative for AD. Recently, it has been reported that apoE4 increases the formation of soluble Aβ oligomers more markedly than apoE2 or apoE3 in the brains of AD patients (30, 37). Taken together, it is conceivable that the entire process of Aβ fibrillization is suppressed by apoE2 or apoE3, thereby causing the attenuation of amyloid deposition in the brain, whereas apoE4 does not have these effects, thereby causing the enhanced amyloid deposition phenotype observed in APOE4 carriers in AD. It also remains possible that Aβ protofibrils complexed with apoE4 have a higher neuronal toxicity compared with those with apoE2 or apoE3.
In present study, we specifically investigated the effects of apoE on the process of Aβ fibrillization and deposition. Recent studies also highlight the roles of apoE in the metabolism and clearance of Aβ in the brain (45). Using an in vivo microdialysis technique, it is reported that apoE-null mice, in which reduced Aβ deposition has been documented (46), had a significantly shorter half-life of soluble Aβ in the brain interstitial fluids (47). These data suggest that the effects of apoE on the metabolism and clearance of Aβ should also be taken into account in the understanding of Aβ economy in brains.
In summary, we show that apoE is involved in the process of Aβ fibrillization and Aβ deposition in an isoform-dependent manner: apoE2 or apoE3 suppresses the conversion of Aβ protofibrils to fibrils and attenuates Aβ deposition, whereas apoE4 is incapable of suppressing the conversion, presumably by the lack of a stabilizing activity. These findings add to our understanding as to why APOE ϵ4 allele is a major risk factor for AD and support the idea that regulating the interaction between Aβ and apoE may be a promising therapeutic target for the inhibition of fibrillization and deposition of Aβ in the brain.
Acknowledgments
We thank Dr. T. Wakabayashi and other lab members for helpful discussions.
This work was supported by Core Research for Evolutional Science and Technology of Japan Science and Technology Agency, the Strategic Research Program for Brain Science from MEXT, and the Health Labor Sciences Research Grant on Amyloidosis.
- AD
- Alzheimer disease
- Aβ
- amyloid β peptide
- FAD
- familial AD
- APP
- Aβ precursor protein
- apoE
- apolipoprotein E
- ThT
- thioflavin T
- HFIP
- 1,1,1,3,3,3-hexafluoro-2-propanol
- α2M
- α2-macroglobulin
- PICUP
- photoinduced cross-linking of unmodified protein
- SEC
- size exclusion chromatography
- LMW
- low molecular weight
- rec
- recombinant.
References
- 1. Selkoe D. J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 [DOI] [PubMed] [Google Scholar]
- 2. Dickson D. W. (1997) The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 56, 321–339 [DOI] [PubMed] [Google Scholar]
- 3. Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. (1985) Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J. Clin. Invest. 76, 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. (1987) Astrocyte synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 917, 148–161 [DOI] [PubMed] [Google Scholar]
- 5. Nakai M., Kawamata T., Taniguchi T., Maeda K., Tanaka C. (1996) Expression of apolipoprotein E mRNA in rat microglia. Neurosci. Lett. 211, 41–44 [DOI] [PubMed] [Google Scholar]
- 6. Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 8. Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166 [DOI] [PubMed] [Google Scholar]
- 9. Näslund J., Thyberg J., Tjernberg L. O., Wernstedt C., Karlström A. R., Bogdanovic N., Gandy S. E., Lannfelt L., Terenius L., Nordstedt C. (1995) Characterization of stable complexes involving apolipoprotein E and the amyloid β peptide in Alzheimer's disease brain. Neuron 15, 219–228 [DOI] [PubMed] [Google Scholar]
- 10. Rebeck G. W., Reiter J. S., Strickland D. K., Hyman B. T. (1993) Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron 11, 575–580 [DOI] [PubMed] [Google Scholar]
- 11. Jarrett J. T., Berger E. P., Lansbury P. T., Jr. (1993) The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 [DOI] [PubMed] [Google Scholar]
- 12. Lomakin A., Teplow D. B., Kirschner D. A., Benedek G. B. (1997) Kinetic theory of fibillogenesis of amyloid β-protein. Proc. Natl. Acad. Sci. U.S.A. 94, 7942–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans K. C., Berger E. P., Cho C. G., Weisgraber K. H., Lansbury P. T., Jr. (1995) Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 92, 763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naiki H., Gejyo F., Nakakuki K. (1997) Concentration-dependent inhibitory effects of apolipoprotein E on Alzheimer's β-amyloid fibril formation in vitro. Biochemistry 36, 6243–6250 [DOI] [PubMed] [Google Scholar]
- 15. Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., Teplow D. B. (1997) Amyloid β-protein fibrillogenesis: detection of protofibrillar intermediate. J. Biol. Chem. 272, 22364–22372 [DOI] [PubMed] [Google Scholar]
- 16. Bitan G., Lomakin A., Teplow D. B. (2001) Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J. Biol. Chem. 276, 35176–35184 [DOI] [PubMed] [Google Scholar]
- 17. Harper J. D., Wong S. S., Lieber C. M., Lansbury P. T. (1997) Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 4, 119–125 [DOI] [PubMed] [Google Scholar]
- 18. Bitan G., Kirkitadze M. D., Lomakin A., Vollers S. S., Benedek G. B., Teplow D. B. (2003) Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. U.S.A. 100, 330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilsberth C., Westlind-Danielsson A., Eckman C. B., Condron M. M., Axelman K., Forsell C., Stenh C., Luthman J., Teplow D. B., Younkin S. G., Näslund J., Lannfelt L. (2001) The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Aβ protofibril formation. Nat. Neurosci. 4, 887–893 [DOI] [PubMed] [Google Scholar]
- 21. Hori Y., Hashimoto T., Wakutani Y., Urakami K., Nakashima K., Condron M. M., Tsubuki S., Saido T. C., Teplow D. B., Iwatsubo T. (2007) The Tottori (D7N) and English (H6R) familial Alzheimer disease mutations accelerate Aβ fibril formation without increasing protofibril formation. J. Biol. Chem. 282, 4916–4923 [DOI] [PubMed] [Google Scholar]
- 22. Hartley D. M., Walsh D. M., Ye C. P., Diehl T., Vasquez S., Vassilev P. M., Teplow D. B., Selkoe D. J. (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh D. M., Hartley D. M., Kusumoto Y., Fezoui Y., Condron M. M., Lomakin A., Benedek G. B., Selkoe D. J., Teplow D. B. (1999) Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952 [DOI] [PubMed] [Google Scholar]
- 24. Morikawa M., Fryer J. D., Sullivan P. M., Christopher E. A., Wahrle S. E., DeMattos R. B., O'Dell M. A., Fagan A. M., Lashuel H. A., Walz T., Asai K., Holtzman D. M. (2005) Production of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-β. Neurobiol. Dis. 19, 66–76 [DOI] [PubMed] [Google Scholar]
- 25. Yamada K., Yabuki C., Seubert P., Schenk D., Hori Y., Ohtsuki S., Terasaki T., Hashimoto T., Iwatsubo T. (2009) Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high-affinity to soluble Aβ. J. Neurosci. 29, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A. L., Vigouret J. M., Paganetti P., Walsh D. M., Mathews P. M., Ghiso J., Staufenbiel M., Walker L. C., Jucker M. (2006) Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto T., Wakabayashi T., Watanabe A., Kowa H., Hosoda R., Nakamura A., Kanazawa I., Arai T., Takio K., Mann D. M., Iwatsubo T. (2002) CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 21, 1524–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kane M. D., Lipinski W. J., Callahan M. J., Bian F., Durham R. A., Schwarz R. D., Roher A. E., Walker L. C. (2000) Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 20, 3606–3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribalta J., Vallvé J. C., Girona J., Masana L. (2003) Apolipoprotein and apolipoprotein receptor genes, blood lipids and disease. Curr. Opin. Clin. Nutr. Metab. Care 6, 177–187 [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto T., Serrano-Pozo A., Hori Y., Adams K. W., Takeda S., Banerji A. O., Mitani A., Joyner D., Thyssen D. H., Bacskai B. J., Frosch M. P., Spires-Jones T. L., Finn M. B., Holtzman D. M., Hyman B. T. (2012) Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J. Neurosci. 32, 15181–15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aleshkov S., Abraham C. R., Zannis V. I. (1997) Interaction of nascent ApoE2, ApoE3, and ApoE4 isoforms expressed in mammalian cells with amyloid peptide β (1–40): relevance to Alzheimer's disease. Biochemistry 36, 10571–10580 [DOI] [PubMed] [Google Scholar]
- 32. LaDu M. J., Falduto M. T., Manelli A. M., Reardon C. A., Getz G. S., Frail D. E. (1994) Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406 [PubMed] [Google Scholar]
- 33. Yang D. S., Smith J. D., Zhou Z., Gandy S. E., Martins R. N. (1997) Characterization of the binding of amyloid-β peptide to cell culture-derived apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J. Neurochem. 68, 721–725 [DOI] [PubMed] [Google Scholar]
- 34. Gomez-Isla T., West H. L., Rebeck G. W., Harr S. D., Growdon J. H., Locascio J. J., Perls T. T., Lipsitz L. A., Hyman B. T. (1996) Clinical and pathological correlates of apolipoprotein E ϵ4 in Alzheimer's disease. Ann. Neurol. 39, 62–70 [DOI] [PubMed] [Google Scholar]
- 35. Holtzman D. M., Bales K. R., Tenkova T., Fagan A. M., Parsadanian M., Sartorius L. J., Mackey B., Olney J., McKeel D., Wozniak D., Paul S. M. (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 97, 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fagan A. M., Watson M., Parsadanian M., Bales K. R., Paul S. M., Holtzman D. M. (2002) Human and murine ApoE markedly alters Aβ metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol. Dis. 9, 305–318 [DOI] [PubMed] [Google Scholar]
- 37. Tai L. M., Bilousova T., Jungbauer L., Roeske S. K., Youmans K. L., Yu C., Poon W. W., Cornwell L. B., Miller C. A., Vinters H. V., Van Eldik L. J., Fardo D. W., Estus S., Bu G., Gylys K. H., Ladu M. J. (2013) Levels of soluble apolipoprotein E/amyloid-β (Aβ) complex are reduced and oligomeric Aβ increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J. Biol. Chem. 288, 5914–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tai L. M., Mehra S., Shete V., Estus S., Rebeck G. W., Bu G., LaDu M. J. (2014) Soluble apoE/Aβ complex: mechanism and therapeutic target for APOE4-induced AD risk. Mol. Neurodegener. 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong L. M., Wilson C., Wardell M. R., Simmons T., Mahley R. W., Weisgraber K. H., Agard D. A. (1994) Human apolipoprotein E: role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J. Biol. Chem. 269, 22358–22365 [PubMed] [Google Scholar]
- 40. Dong L. M., Weisgraber K. H. (1996) Human apolipoprotein E4 domain interaction: arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem. 271, 19053–19057 [DOI] [PubMed] [Google Scholar]
- 41. Bentley N. M., Ladu M. J., Rajan C., Getz G. S., Reardon C. A. (2002) Apolipoprotein E structural requirements for the formation of SDS-stable complexes with β-amyloid-(1–40): the role of salt bridge. Biochem. J. 366, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Englund H., Sehlin D., Johansson A. S., Nilsson L. N., Gellerfors P., Paulie S., Lannfelt L., Pettersson F. E. (2007) Sensitive ELISA detection of amyloid-β protofibrils in biological samples. J. Neurochem. 103, 334–345 [DOI] [PubMed] [Google Scholar]
- 43. Lord A., Englund H., Söderberg L., Tucker S., Clausen F., Hillered L., Gordon M., Morgan D., Lannfelt L., Pettersson F. E., Nilsson L. N. (2009) Amyloid-β protofibril levels correlate with spatial learning in Arctic Alzheimer's disease transgenic mice. FEBS J. 276, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng I. H., Palop J. J., Esposito L. A., Bien-Ly N., Yan F., Mucke L. (2004) Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat. Med. 10, 1190–1192 [DOI] [PubMed] [Google Scholar]
- 45. Bu G. (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogensis and therapy. Nat. Rev. Neurosci. 10, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bales K. R., Verina T., Dodel R. C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E. M., Little S. P., Cummins D. J., Piccardo P., Ghetti B., Paul S. M. (1997) Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 17, 263–264 [DOI] [PubMed] [Google Scholar]
- 47. DeMattos R. B., Cirrito J. R., Parsadanian M., May P. C., O'Dell M. A., Taylor J. W., Harmony J. A., Aronow B. J., Bales K. R., Paul S. M., Holtzman D. M. (2004) ApoE and clusterin cooperatively suppress Aβ level and deposition: evidence that apoE regulates extracellular Aβ metabolism in vivo. Neuron 41, 193–202 [DOI] [PubMed] [Google Scholar]