Abstract
Purpose:
To investigate the correlations between conjunctival inflammatory status and meibomian gland (MG) morphology in vernal keratoconjunctivitis (VKC) patients by using in vivo confocal microscopy (CM).
Materials and Methods:
Nineteen VKC patients (7 limbal, 7 tarsal, and 5 mixed forms) and 16 normal volunteers (controls) were enrolled. All subjects underwent CM scanning to obtain the images of upper palpebral conjunctiva and MGs. Inflammatory cell (IC) density in palpebral conjunctival epithelial and stromal layers, Langerhans cell (LC) density at lid margins and the stroma adjacent to the MG, and MG acinar unit density (MGAUD) were recorded. The longest and shortest diameters of MG acinar were measured. The Kruskal-Wallis test was used to compare the parameter differences whereas the Spearman's rank correlation analysis was applied to determine their correlations.
Results:
Among all groups, no significant statistical differences were found in epithelial and stromal IC densities, mean values of MG acinar unit densities, or longest and shortest diameters. Both LC parameters in the tarsal-mixed groups were significantly higher than those in the limbal and control groups. All LC densities of VKC patients showed a positive correlation with MGAUD and shortest diameter.
Conclusions:
In VKC patients, the conjunctival inflammatory status could be associated with the MG status. In vivo CM is a noninvasive, efficient tool in the assessment of MG status and ocular surface.
Keywords: Confocal microscopy, meibomian gland, meibomian gland microenvironment, papillary formation, vernal keratoconjunctivitis
Vernal keratoconjunctivitis (VKC) presents as a seasonally recurring, bilateral inflammation of cornea and conjunctiva and occurs predominantly in male children, who frequently have a personal or family history of atopy. Conjunctival hyperemia, intense itching, photophobia, tearing, and sticky mucus discharge are typical symptoms of VKC. Giant papillae, with a cobblestone-like appearance in the upper tarsal conjunctiva or at the limbus, are considered hallmarks of the disease.[1,2,3,4]
Based on the predominant involvement of either tarsal or limbal conjunctiva, VKC can be divided into three forms,[1,2] tarsal, limbal, and mixed. Tissue remodeling with the formation of large and sessile papillae is one of the most typical signs in the tarsal form, which may be less evident and limited at the peripheral cornea, in the limbal form. Because of tissue remodeling, the conjunctiva may become thickened along with subepithelial fibrosis and conjunctival scar formation. Because the tarsal conjunctival tissues lie adjacent to the meibomian gland (MG) layer, conjunctival changes could be related to anatomical alterations of MGs. However, MG changes and the impact of such changes on ocular surface disease have not been reported so far.
Laser scanning confocal microscopy (LSCM) is an alternative method of imaging MGs, and its fast and cellular level of observation enhances quantitative assessment of MG glandular structural changes and inflammatory status.[5,6,7,8,9,10]
The previous studies have been reported that LSCM-based parameters to be invaluable tools for assessment and evaluation of ocular surface and MG status in patients with MG dysfunction (MGD). Parameters such as MG acinar unit density (MGAUD), inflammatory cell (IC) density, MG acinar longest diameter, and MG acinar shortest diameter had an acceptable sensitivity and specificity in the diagnosis of MGD and correlated significantly with tear function test, MG dropout, and meibum expression grades.[7,8,9,10] Therefore, we used LSCM to identify the morphological changes in MGs and the conjunctival inflammatory status in VKC patients.
Materials and Methods
Subjects
This study was in compliance with the tenets of the declaration of Helsinki. Written informed consents were taken from all subjects or their guardians before the examination. The criteria for diagnosis of VKC patients included patients with symptoms of allergic conjunctivitis, such as ocular itching, redness, tearing, or ocular pain with seasonal aggravation, and presenting with a cobblestone-like appearance of the upper tarsal or limbal conjunctiva, associated with mucus discharge, corneal damage and intense itching. The recruited VKC subjects were first visit patients with no history of eye medication.
Patients with blepharitis, contact lens wearers, atopic dermatitis, continuous eye drops use, history of eye surgery, systemic diseases or being treated with systemic medication were excluded. The exclusion criteria for controls included blepharitis, obvious eyelid or ocular surface disorders, contact lens wearers, atopic dermatitis, continuous eye drops use, history of eye surgery, and systemic or ocular disease that would interfere with lid anatomical structure, tear film production or function. The right eye was preferentially included in the study. If the right eye met the exclusive criteria, data from the left eye were used.
Clinical evaluations
Slit lamp microscopic (SLM) examinations were performed to classify the VKC patients into three forms. During the ocular examination, particular attention was paid to eyelid margins, tarsal and bulbar conjunctiva, and cornea. The tarsal and mixed forms were combined into one group for convenient analysis of tarsal papillae. Papillary diameters were measured by ImageJ software (http://www.rsbweb.nih.gov/ij/)[7] based on the SLM photos or confocal images. The clinical grading of patients was based on the criteria developed by Bonini et al.[2]
In vivo laser scanning confocal microscopy
Laser scanning confocal microscopy was performed on all subjects along with Heidelberg retinal tomography (HRT II/Rostock cornea module; Heidelberg Engineering GmbH, Dosenheim, Germany), as described previously.[5,6,7,8,9,10] After the eyelid was everted, the center of the Tome-Cap was aplanated onto the upper palpebral conjunctiva, and then scanning was begun at the most superficial tissues and progressed down to the deepest ones visualized with satisfactory resolution. The two-dimensional image sizes were 384 × 384 pixels with a 400 × 400 μm field of view.
We analyzed three randomly chosen, nonoverlapping, high-quality digital images of conjunctival epithelial and stromal layers, as well as lid margins and MGs. The following variables were quantified: (1) IC Densities in the upper palpebral conjunctival epithelial and stromal layers, and Langerhans cell (LC) densities in the lid margin and the stroma adjacent to MGs. These were calculated automatically within the manually identified largest available region of interest (Cell Count software; Heidelberg Engineering GmbH, Dosenheim,Germany), (2) the density of manually identified MGs inside each 400 × 400 μm frame was calculated automatically with the Cell Count software, and (3) in the confocal image containing MGs, six best-focused acinar units were selected for measuring the longest and shortest diameters of MG acinar with ImageJ software.
Statistical analysis
Data were processed using the Statistical Package for the Social Sciences software (SPSS 13.0 for Windows; IBM, Chicago, IL, USA). All data are shown as the mean ± standard deviation. The Kruskal-Wallis test was used to compare the parameters among control, limbal, and tarsal-mixed groups. The Spearman's rank correlation analysis was applied to determine the correlation among IC, LC, and MG parameters. A P < 0.05 was considered as statistically significant.
Results
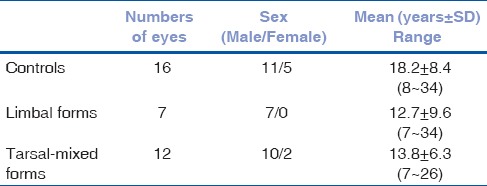
Nineteen VKC patients, including 7 limbal forms (7 were male; mean age, 12.7 ± 9.6 years; range: 7–34 years), 7 tarsal, and 5 mixed forms (10 were male and 2 were female; mean age, 13.8 ± 6.3 years; range: 7–26 years) were enrolled in the study. Sixteen age-matched healthy volunteer controls (11 were male and 5 were female; mean age, 18.2 ± 8.4 years; range: 8–34 years) were consecutively enrolled [Table 1].
Table 1.
Details of all participants
The clinical grading of tarsal-mixed cases were Grade 4 in 1 case (8.3%), Grade 2A in 2 cases (16.7%), and Grade B in 9 cases (75%). The limbal cases were graded as Grade 2B in 3 cases (50%) and Grade 3 in 3 cases (50%). None of the patients had meibomian orifice obstruction or metaplasia.
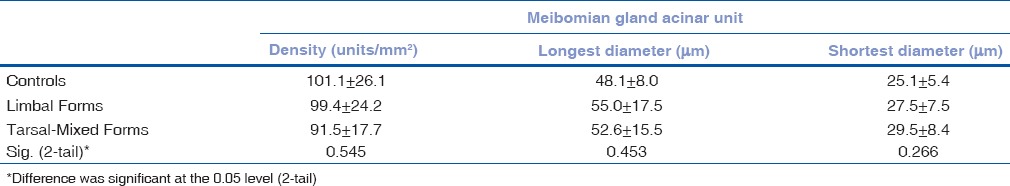
The in vivo LSCM images disclosed morphologic alternations of MG in patients with VKC, including extensive periglandular LC infiltration, blurred MG lumen contours [Fig. 1a] and hyper reflective solid matter in the lumen [Fig. 1b] compared with control subjects. No obvious differences were found in the densities of epithelial and stromal ICs among limbal, tarsal-mixed, and normal groups, while the values of LC densities were significantly higher in the tarsal-mixed group than in the limbal and control groups [Table 2]. The mean values of all MG variables had no significant differences among the three groups [Table 3].
Figure 1.
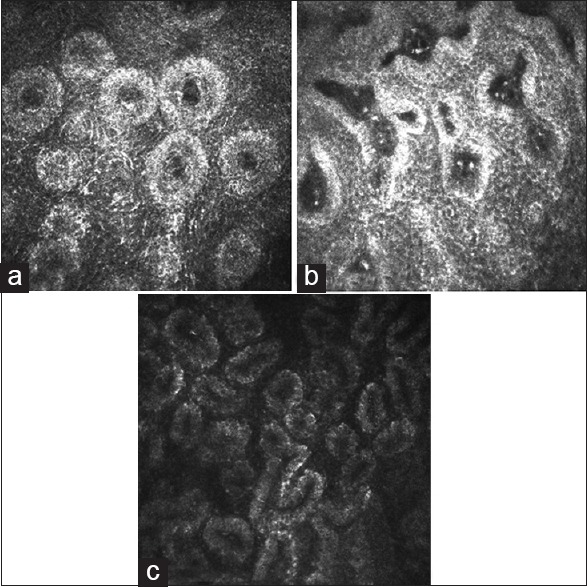
(a) A confocal image from a mixed form patient. Hyper reflective edematous acinars with lumen contour became blurred were notable. (b) A confocal image from a representative limbal form patient. Notable hyperreflective solid matters in the meibomian gland lumen were showed. (c) A confocal image from a representative control. Numerous and compact acinar units were notable
Table 2.
Inflammatory status of three group
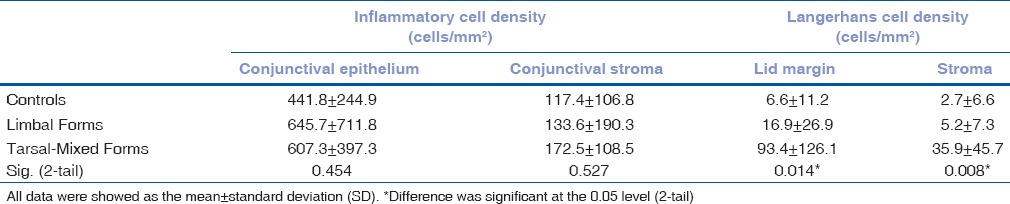
Table 3.
MG acinar unit density, longest and shortest diameter
Spearman's rank correlation analysis showed that LC density values of lid margins positively correlated with MGAUD and shortest diameters. Similar correlations were found between LC density in the stromal layer and MGAUD. Negative correlations were observed between IC densities and MG parameters.
Discussion
The MGs secrete lipids onto the ocular surface. These lipids constitute outer layer of tear film, act as a barrier to cutaneous sebum to prevent contamination, prevent rapid evaporation of the tear film, facilitate lubrication to reduce friction from blinks on the ocular surface, and provide a smooth optical surface.[11,12,13] Although previous study showed that VKC patients had a lower tear break-up time value and a normal Schirmer's test value when compared with normal subjects,[14] there has been no report investigating the MG alternation and their impact on the ocular surface disease so far. VKC patients with papillae formation show conjunctival tissue remodeling, and based on a previous report, MGs likely undergo anatomical structural changes, which show MG duct distortion as in patients with perennial allergic conjunctivitis.[15] Therefore, it is important to determine whether VKC patients with papillae formation have MGDs.
As a new technology, in vivo LSCM not only makes a quantitative assessment of conjunctival inflammation as well as identifies either neutrophils or LCs, but can also detect phenotypic alterations in MGs. By introducing new diagnostic parameters, such as MGAUD and longest and shortest diameters, LSCM can reflect histopathological changes such as glandular atrophy and acinar/ductal dilatation.[7,8,9,10] To the best of our knowledge, LSCM has not been used previously to determine in vivo alterations of MGs in VKC patients with tarsal papillae.
In VKC patients with papillary formation, the infiltration of ICs with diameters in the range of 12–15 μm were mainly found in conjunctival epithelia (depth of approximately 11 μm) and stroma (depth of approximately 100 μm), with the appearance resembling multinucleated granulocytes. LCs, which showed as hyperreflective corpuscular particles with dendritic processes, infiltrated into the lid margin and the stroma, peripheral to the MGs [Fig. 2a and b]. In contrast, only a few numbers of ICs dispersed into the conjunctival epithelium, and even fewer into the deeper stromal layer, in the normal control group. The papillary formation was not observed in the controls. Apart from inflammatory infiltration, fibrosis, and neovascularization in the stromal layers, some MG lumen contours became blurred [Fig. 1a] in the VKC patients compared with normal controls [Fig. 1c]. This was accompanied by hyperreflective solid matter [Fig. 1b] in the lumen.
Figure 2.
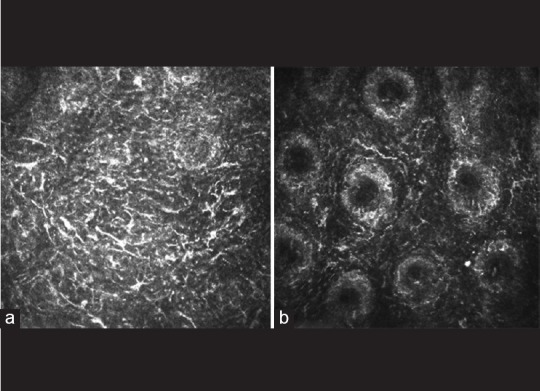
(a) A confocal image from a mixed form patient. Extensive Langerhans cells (LCs) dispersed in lid margin. (b) A confocal image from a mixed form patient. LCs which showed as hyper reflective corpuscular particles with dendritic processes infiltrated in the stroma peripheral to meibomian glands
At the present study, all enrolled patients had papillary formations in the upper lid when observed under SLM or LSCM. Although the presence of meibomian orifice obstruction or metaplasia did not show in the patients, blurred MG lumen contours and intralumenal hyper reflective solid matter were found, using LSCM. Additionally, it is notable that moderate to extensive LCs were observed in surrounding MGs of all patients, especially in the tarsal-mixed group patients (P < 0.05). Despite no significant differences found among the values of all MG parameters, in the three groups, Spearman's rank correlation results showed LCs had closely related MGAUD, which suggested that immunological inflammation initiated by LCs may eliminate MGs, influence MG structure, and could consequently cause MG abnormality. Therefore, in vivo LSCM examination of MGs in patients with VKC should be helpful in evaluating the MG microenvironment inflammatory status, and guide the selection of a treatment protocol for VKC patients.
In accordance with previous studies,[16] higher numbers of eosinophils were observed in the epithelium and stroma of VKC patients’ conjunctiva, even though no significant difference was found in IC densities among normal, limbal, and tarsal-mixed groups. Several reasons could account for this result. The confocal images of tarsal epithelia were very difficult to obtain from the exact same tissue depth because of the difficulty in controlling the movement of the head. The limited number of patients in the current study should also be taken into consideration. The current study found that tarsal conjunctival inflammation had no effect on the MG and its microenvironment. Thus, we hypothesize that it is not useful to assess MG inflammation status from the tarsal conjunctival presentation.
Our study suggests that MG status in VKC patients requires careful attention from clinicians to avoid the disturbances in tear film, which can increase the inflammatory status through vicious circles. Although five MG parameters enabled us to understand comprehensively the MG glandular structure and microenvironmental changes, further research will be taken to investigate the tear evaporation rates, tear function and ocular surface tests in VKC patients.
Conclusion
We have reported the first study to elucidate the alterations of MG morphology in VKC disease by in vivo LSCM. Our study suggests that conjunctival inflammatory status, rather than upper tarsal conjunctival tissue remodeling, may affect the MG status in VKC patients. In vivo LSCM is helpful to elucidate the alterations of MGs in VKC patients with the papillary formation, and may increase our understanding of the pathogenesis of VKC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kumar S. Vernal keratoconjunctivitis: A major review. Acta Ophthalmol. 2009;87:133–47. doi: 10.1111/j.1755-3768.2008.01347.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonini S, Sacchetti M, Mantelli F, Lambiase A. Clinical grading of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7:436–41. doi: 10.1097/ACI.0b013e3282efb726. [DOI] [PubMed] [Google Scholar]
- 3.Bielory L. Allergic and immunologic disorders of the eye. Part II: Ocular allergy. J Allergy Clin Immunol. 2000;106:1019–32. doi: 10.1067/mai.2000.111238. [DOI] [PubMed] [Google Scholar]
- 4.Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond) 2004;18:345–51. doi: 10.1038/sj.eye.6700675. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Adan ES, Matsumoto Y, Dogru M, Fukagawa K, Takano Y, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Mol Vis. 2007;13:1379–89. [PubMed] [Google Scholar]
- 6.Wakamatsu TH, Okada N, Kojima T, Matsumoto Y, Ibrahim OM, Dogru M, et al. Evaluation of conjunctival inflammatory status by confocal scanning laser microscopy and conjunctival brush cytology in patients with atopic keratoconjunctivitis (AKC) Mol Vis. 2009;15:1611–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–72. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Shimazaki J, et al. In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology. 2012;119:1961–8. doi: 10.1016/j.ophtha.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Shigeno Y, Sato EA, Ibrahim OM, Saiki M, Negishi K, et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247:821–9. doi: 10.1007/s00417-008-1017-y. [DOI] [PubMed] [Google Scholar]
- 11.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–51. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- 12.Goto E, Endo K, Suzuki A, Fujikura Y, Matsumoto Y, Tsubota K. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–9. doi: 10.1167/iovs.02-0170. [DOI] [PubMed] [Google Scholar]
- 13.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–70. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Matsumoto Y, Dogru M, Okada N, Igarashi A, Fukagawa K, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62:917–25. doi: 10.1111/j.1398-9995.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 15.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Tomidokoro A, et al. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea. 2010;29:858–60. doi: 10.1097/ICO.0b013e3181ca3668. [DOI] [PubMed] [Google Scholar]
- 16.Le Q, Hong J, Zhu W, Sun X, Xu J. In vivo laser scanning confocal microscopy of vernal keratoconjunctivitis. Clin Experiment Ophthalmol. 2011;39:53–60. doi: 10.1111/j.1442-9071.2010.02379.x. [DOI] [PubMed] [Google Scholar]


