Abstract
Dacryocystorhinostomy (DCR) is the procedure of choice in patients with epiphora due to primary acquired nasolacrimal duct obstruction. The evolution of surgical tools, fiber-optic endoscopes, effective anesthesia techniques, and the adjunct use of antimetabolites intraoperatively; namely mitomycin-C (MMC) have significantly contributed to the advancement of DCR surgery. MMC is a systemic chemotherapeutic agent derived from Streptomyces caespitosus that inhibits the synthesis of DNA, cellular RNA, and protein by inhibiting the synthesis of collagen by fibroblasts. Even the cellular changes in the human nasal mucosal fibroblasts induced by MMC at an ultrastructural level have been documented. There, however, seems to be a lack of consensus regarding MMC: The dosage, the route of delivery/application, the time of exposure and subsequently what role each of these variables plays in the final outcome of the surgery. In this review, an attempt is made to objectively examine all the evidence regarding the role of MMC in DCR. MMC appears to improve the success rate of DCR.
Keywords: Dacryocystorhinostomy, intubation, lacrimal surgery, mitomycin-C, nasolacrimal duct obstruction, primary acquired nasolacrimal duct obstruction
Dacryocystorhinostomy (DCR) is the procedure of choice in patients with epiphora due to primary acquired nasolacrimal duct obstruction. Caldwell and Toti were the pioneers who first described endonasal and external DCR, respectively.[1,2] Subsequently, the evolution of surgical tools, the advent of fiber-optic endoscopes, better anesthesia techniques and the adjunct use of anti-metabolites intraoperatively and postoperatively by some; namely mitomycin-C (MMC) have significantly contributed to the advancement of DCR surgery. In experienced hands, DCR is a very successful procedure. The surgery may be performed either externally through a skin incision or endonasally with the help of fiber-optic endoscope. However, both surgical routes have reported failure rates ranging from 0% to 18%, due to blockage of osteotomy due to granulation tissue, scarring and formation of adhesions and synechiae in the nasal cavity.[3,4,5,6,7] During the postoperative healing process, scarring can further decrease the ostium size.[5] Therefore, the key to increasing the longevity of the success of a DCR, obviously lies in maintaining the patency of the ostium. The use of anti-metabolites, which inhibits circumosteal fibrous tissue growth and scarring is hence desirable.
Mitomycin-C is a systemic chemotherapeutic agent derived from Streptomyces caespitosus that inhibits the synthesis of DNA, cellular RNA, and protein by inhibiting the synthesis of collagen by fibroblasts.[8,9] Ugurbas et al. were one of the first to study the histopathology following the use MMC intraoperatively in endoscopic endonasal DCR. They found attenuated epithelium with intracytoplasmic vacuoles and loose subepithelial connective tissue that was hypocellular on histopathological examination.[10] Similarly, Kao et al. documented and compared outcomes of external DCR where intraoperative MMC was used versus DCRs without adjunctive MMC application.[11] They suggested that MMC favorably affects the ostium and may enhance the success of surgery. Kao et al. also found no adhesions in the MMC group. The late 1990s and early 2000s have subsequently seen multiple groups giving compelling evidence pointing toward a favorable opinion toward the use of MMC in DCR. On an ultrastructural level, MMC treated nasal mucosa showed significant changes involving the epithelial, glandular, vascular, and fibrocollagenous tissues when compared with a normal untreated tissue. However, these changes were restricted to treated areas only. These changes suggest that MMC may have a role in preventing the formation of cicatrix.[12]
The Evidence: Does Mitomycin-C Really Help?
While the use of MMC has been increasingly popular in DCR, there is a lack of consensus regarding multiple variables; namely the dosage, the route of delivery/application, the time of exposure and subsequently what role each of these variables plays in the final outcome of the surgery. Ever since the intraoperative use of MMC has been a part of DCR, various studies have put forth their data with varying concentrations of MMC. The efficacy of different dosages of intraoperative MMC in external DCR in 50 eyes was studied by You and Fang.[9] In one group, 0.2 mg/ml of MMC was used for 5 min intraoperatively, and in the other group, 0.5 mg/ml of MMC was used for 5 min. These two groups were compared with a control group with no MMC. The patency rate and osteotomy size differences between the patients treated with MMC and the control group were statistically significant. However, there was no significant difference between the two groups in which MMC was used. Gonzalvo et al. studied the effects of intraoperative MMC on the clinical evolution and osteotomy size following an external DCR with helical computed tomography.[13] They concluded that intraoperative MMC may increase the success rates over the traditional DCR procedure and is effective in reducing the closure rate of the osteotomy after DCR.[13]
Rathore et al., in their study to find the role of topical MMC as a postoperative adjunct to endonasal DCR; placed a nasal pack soaked in 1 ml of 0.05% (2 mg in 5 ml of distilled water) MMC after DCR. This was in place for 48 h. In their study, they observed that postoperative retention of nasal packs for 48 h after endonasal DCR did not cause any major side effect. Improvement in clinical symptoms was noted in all patients who had nasal packing with MMC. Postoperatively, the nasal cavity which had been packed with MMC had healthy nasal mucosa during the entire follow-up, as compared to the control group where the saline nasal pack was used, where synechiae were seen in 65.2% of the patients.[14]
There have been further variations in the duration of application and concentration. Deka et al. used 3 groups: Control group 1, operated without MMC; experimental group 2, with MMC at a concentration of 0.05 mg/ml for 2 min; and experimental group 3, with MMC applied at a concentration of 0.4 mg/ml for 2 min. Furthermore, half of the cases in each group underwent single-flap DCR, and half underwent double-flap DCR surgery. They concluded that the ostium size in group 3 was found to be significantly bigger in comparison with group 1 and with group 2.[5] Nemet et al., reported their experience in treating distal and common canalicular obstruction by trephination followed by topical low-dose (0.03%) MMC and silicone intubation during endoscopic DCR 4 patients out of 5 remained asymptomatic with endoscopically seen open ostia for an average follow-up period of 15.4 months.[4]
While these reports have given a favorable verdict on the use of MMC in DCRs; there are few on the other end of the spectrum who have published evidence that is, equivocal. Zilelioglu et al. in one of the earlier studies to comment on the adjunctive use of MMC in endoscopic DCR; used topical 0.5 mg/ml solution of mitomycin intraoperatively and applied the drug for 2.5 min. They demonstrated histopathological evidence that 0.5 mg/ml of MMC for 2.5 min favorably affected wound healing in the osteotomy site. However, this limited series showed no benefit of using MMC intraoperatively as their surgical success rates with and without MMC had success rates of 77.8 and 77.3%, respectively, at a mean follow-up period of 18.2 months.[15] Yildirim et al. in a prospective randomized controlled study to study the adjunctive use of MMC in external DCRs noted that while the success rates of the MMC group were higher than those of the control group, the differences did not reach statistical significance.[16] Prasannaraj et al. in their results of endoscopic DCR where 38 patients were randomized into either an MMC group (0.2 mg/dL) or a control group, reported a success rate of 82.3% with MMC 85.7% without MMC. Granulations, adhesions, and obliterative sclerosis occurred in a similar number of patients in both groups, and the authors were of the opinion that granulations and adhesions did not have a bearing on the success rate in either group.[17]
While there are many reports which have shown the beneficial effect of MMC, it is equally important to note that there remains no study till date, which has reported a worse success rate for DCR with MMC when compared with DCRs without the use of MMC [Table 1].
Table 1.
Select studies (not exhaustive) which have used different concentrations of MMC intraoperatively during DCR and for varying durations
Most studies have found that intraoperative MMC application seems to be safe; furthermore no deleterious effects were noted with MMC application.[16] Mere eyeballing the data suggests that MMC plays a role in reducing the closure rate of the osteotomy site after a DCR.
Feng et al. in their meta-analysis of primary external DCR with and without MMC included nine randomized controlled trials reporting on a total of 562 DCRs including patients in the age range 30–57 years. They mentioned that there was a significantly higher success rate in the MMC group in comparison with the control group (odds ratio, 2.11; 95% confidence interval, 1.19–3.74, P = 0. 01).[41] The meta-analysis also found that intraoperative MMC application seems to be a safe adjuvant and helps in maintaining the patency of the ostium. Cheng et al. in their recently published meta-analysis of endoscopic DCRs to compare the clinical results with and without MMC concluded that in addition to being safe, MMC helps reduce the closure rate of the osteotomy and enhance the success rate after both primary and revision endonasal DCR.[8]
Ali et al. evaluated the ultrastructural effects of topical and circumostial injection of mitomycin-C (COS-MMC) on nasal mucosa and compared them with the controls (untreated naïve nasal mucosa). This was the first study to document detailed subcellular effects and classify the transmission electron microscopy findings as those of epithelial, glandular, vascular, and fibro collagenous. They reported that both, topical and COS-MMC showed profound changes in nasal mucosa, but more marked changes were observed in the COS-MMC group. The nasal fibroblasts showed a dramatic structural response to MMC: Intracellular edema, pleomorphic and vesicular mitochondria, dilated smooth and rough endoplasmic reticulum, and chromatin condensation.[12]
The Dosage and Duration
It is evident from the varying concentrations of MMC that different studies have used that there is no agreement on the issue. It is further unclear as to how researchers arrived at each of these arbitrary concentrations before applying it to practice. Ali et al. studied the effect of varying concentrations of MMC and treatment durations on cellular proliferation and viability of the fibroblasts. Nasal mucosa harvested from patients undergoing a DCR was used to establish primary cultures by explant culture method. The cells were then treated with different concentrations of MMC (0.1–0.5 mg/ml) for different time periods (3, 5, and 10 min). Cell viability, cellular proliferation, and the actin cytoskeletons of fibroblasts were studied. The significant findings of this study were that the doubling time of cultured nasal mucosal fibroblasts was found to be approximately 24 h. MMC at 0.4 mg/ml beyond 5 min and 0.5 mg/ml concentration at all time points were lethal and caused extensive cell death when compared with controls. The minimum effective concentration appeared to be 0.2 mg/ml for 3 min as it prevented cell proliferation of the fibroblasts by inducing cell cycle arrest, without causing extensive apoptosis.[42]
As mentioned in the discussion above, there have been different routes of application of MMC to the nasal and lacrimal sac mucosal surfaces. Most studies have used a cotton-tip applicator soaked in MMC applied under the nasal and lacrimal flaps for the desired duration followed by copious irrigation with normal saline.[41] Nemet et al. used 0.03% solution of MMC in a 2 ml syringe with a 26 gauge lacrimal cannula to irrigate the newly trephined canaliculus via the punctum into the nose, with the stents in place.[4] A neurosurgical patty was preplaced in the nose to soak up any excess MMC and finally, the conjunctival sac was immediately irrigated with normal saline. Rathore et al. in their series of endonasal DCRs, packed the nasal cavity with 0.05% MMC nasal pack for 48 h.[14]
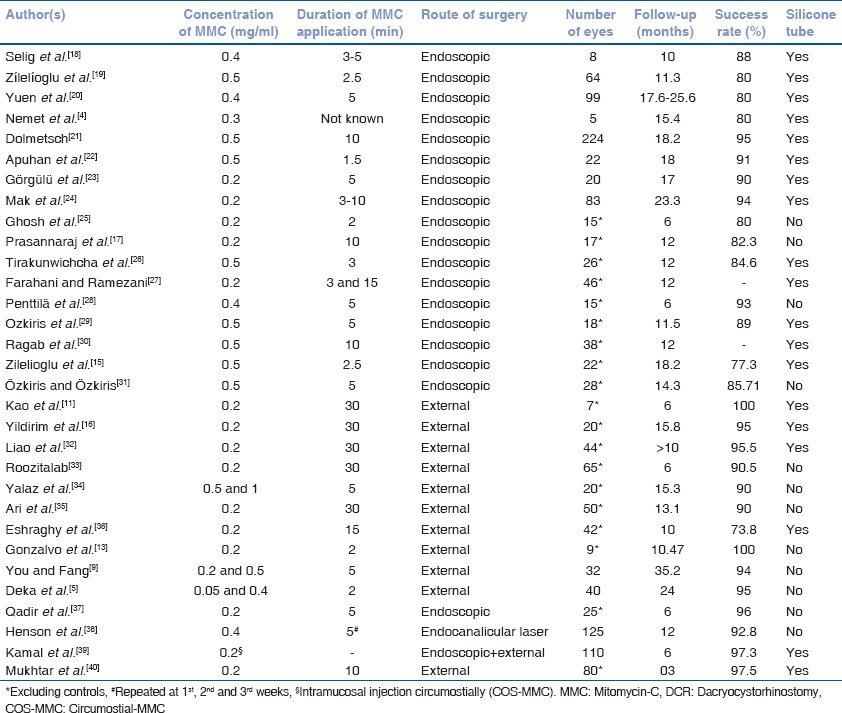
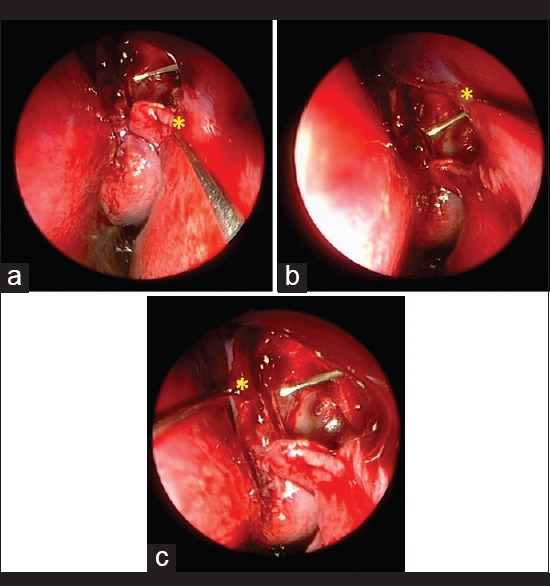
Another promising variation in the delivery technique of MMC is COS-MMC introduced by Kamal et al., where after fashioning the mucosal flaps, intramucosal injection of 0.02% MMC was injected at four points (0.1 ml at each point) along the edges of the freshly created ostium [Figs. 1 and 2]. Their high clinical success rates along with basic science evidence indicate that COS-MMC may be an effective adjunctive modality in high-risk cases like revision and posttraumatic DCR's.[39]
Figure 1.
(a-c) The sites of injection of intramucosal (nasal mucosa) circumostial mitomycin-C seen endoscopically (sites marked with yellow asterisks). A lacrimal probe has been introduced transcanalicular route and is seen at the ostium
Figure 2.
Endoscopic view, the ostium of a 2-year-old child who underwent dacryocystorhinostomy with circumostial mitomycin-C. Note the large ostium, which is unusual given the exuberant healing response commonly seen in the pediatric population
Postoperative MMC application has also been studied by Henson et al.[38] An 8 mm cotton ball was soaked with 0.5 ml of MMC (0.4 mg/dl) and placed on the tip of a curved mosquito forceps. This was endoscopically guided intranasally and placed on the osteotomy site for 5 min. This 5 min application of MMC (0.4 mg/ml), without irrigation, was done on the 1st, 2nd, and 3rd week. The success rate at 12 months postoperatively was 92.8%. All failures were due to cicatricial closure of the ostium. More importantly, no significant intra-operative and postoperative nasal complications from the MMC were recorded.[38]
Other Confounders
The major disadvantage of all the clinical studies is the heterogeneity with regards to multiple factors, and this prevents head to head comparisons and subsequent drawing of useful conclusions. Some studies have included revision cases and others have not; some surgeons prefer to wash off the MMC after application while others do not; variable techniques, dosages, and follow-ups are some of the many confounders while comparing the outcomes DCRs with and without MMC. The expertise of the surgeon often plays a role in the outcome of a surgery: In a DCR, a beginner is more likely to traumatize the nasal mucosa and, in particular, the nasal septum which can promote the formation of septal adhesions. Other factors such as racial variations, age and sex of the patient can also affect the eventual outcome of the surgery.
The Way Forward
While the core surgical principle of DCR remains constant; newer techniques are constantly evolving toward making it a lesser invasive, safer procedure with long lasting success. The role that MMC has played in improving the success rate of DCR appears to be useful in the wake of recent evidence; regardless of the route chosen: External or endoscopic endonasal.[43] Having more or less established the safety and efficacy of MMC, the focus perhaps could shift on newer delivery techniques of the drug: Intra mucosal depot injection of MMC intra-operatively or even an MMC-coated intubation tube which could provide sustained drug delivery and that may affect the outcomes of the surgery. The short-term action of MMC still remains a point of contention. The search for the ideal dosage, the most efficacious mode of drug delivery and minimum effective duration of application of the drug is still on. The journey of MMC in DCR from experimentation to implementation has yielded promising results; the next step, naturally is standardization. The answer lies in larger, multi-centric, uniform protocol guided, double-masked randomized control trials.
Financial support and sponsorship
MJA receives royalties from Springer for his textbook “Principles and Practice of Lacrimal Surgery.”
Conflict of interest
There are no conflict of interest.
References
- 1.Toti A. Nuovometodoconservatore di cura radicle delle sup-purazonicroniche del saccolacrimale (Dacriocistorinostomia) Clin Mod Fir. 1904;10:385–7. [Google Scholar]
- 2.Caldwell GW. Two new operations for obstruction of the nasal duct with preservation of the canaliculi and an incidental description of a new lachrymal probe. NY Med J. 1893;57:581. [Google Scholar]
- 3.Allen K, Berlin AJ. Dacryocystorhinostomy failure: Association with nasolacrimal silicone intubation. Ophthalmic Surg. 1989;20:486–9. [PubMed] [Google Scholar]
- 4.Nemet AY, Wilcsek G, Francis IC. Endoscopic dacryocystorhinostomy with adjunctive mitomycin C for canalicular obstruction. Orbit. 2007;26:97–100. doi: 10.1080/01676830601174627. [DOI] [PubMed] [Google Scholar]
- 5.Deka A, Bhattacharjee K, Bhuyan SK, Barua CK, Bhattacharjee H, Khaund G. Effect of mitomycin C on ostium in dacryocystorhinostomy. Clin Experiment Ophthalmol. 2006;34:557–61. doi: 10.1111/j.1442-9071.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 6.Hallum AV. The Dupuy-Dutemps dacryocystorhinostomy. Am J Ophthalmol. 1949;32:1197–206. doi: 10.1016/s0002-9394(49)90973-3. [DOI] [PubMed] [Google Scholar]
- 7.Mcpherson TR, Egleston DB. Dacryocystorhinostomy. Am J Ophthalmol. 1959;47:328–31. [PubMed] [Google Scholar]
- 8.Cheng SM, Feng YF, Xu L, Li Y, Huang JH. Efficacy of mitomycin C in endoscopic dacryocystorhinostomy: A systematic review and meta-analysis. PLoS One. 2013;8:e62737. doi: 10.1371/journal.pone.0062737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You YA, Fang CT. Intraoperative mitomycin C in dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2001;17:115–9. doi: 10.1097/00002341-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ugurbas SH, Zilelioglu G, Sargon MF, Anadolu Y, Akiner M, Aktürk T. Histopathologic effects of mitomycin-C on endoscopic transnasal dacryocystorhinostomy. Ophthalmic Surg Lasers. 1997;28:300–4. [PubMed] [Google Scholar]
- 11.Kao SC, Liao CL, Tseng JH, Chen MS, Hou PK. Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology. 1997;104:86–91. doi: 10.1016/s0161-6420(97)30357-1. [DOI] [PubMed] [Google Scholar]
- 12.Ali MJ, Baig F, Lakshman M, Naik MN. Electron microscopic features of nasal mucosa treated with topical and circumostial injection of mitomycin C: Implications in dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2015;31:103–7. doi: 10.1097/IOP.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalvo Ibáñez FJ, Fuertes Fernández I, Fernández Tirado FJ, Hernández Delgado G, Rabinal Arbués F, Honrubia López FM. External dacryocystorhinostomy with mitomycin C. Clinical and anatomical evaluation with helical computed tomography. Arch Soc Esp Oftalmol. 2000;75:611–7. [PubMed] [Google Scholar]
- 14.Rathore PK, Kumari Sodhi P, Pandey RM. Topical mitomycin C as a postoperative adjunct to endonasal dacryocystorhinostomy in patients with anatomical endonasal variants. Orbit. 2009;28:297–302. doi: 10.3109/01676830902856328. [DOI] [PubMed] [Google Scholar]
- 15.Zilelioglu G, Ugurbas SH, Anadolu Y, Akiner M, Aktürk T. Adjunctive use of mitomycin C on endoscopic lacrimal surgery. Br J Ophthalmol. 1998;82:63–6. doi: 10.1136/bjo.82.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildirim C, Yaylali V, Esme A, Ozden S. Long-term results of adjunctive use of mitomycin C in external dacryocystorhinostomy. Int Ophthalmol. 2007;27:31–5. doi: 10.1007/s10792-007-9057-6. [DOI] [PubMed] [Google Scholar]
- 17.Prasannaraj T, Kumar BY, Narasimhan I, Shivaprakash KV. Significance of adjunctive mitomycin C in endoscopic dacryocystorhinostomy. Am J Otolaryngol. 2012;33:47–50. doi: 10.1016/j.amjoto.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Selig YK, Biesman BS, Rebeiz EE. Topical application of mitomycin-C in endoscopic dacryocystorhinostomy. Am J Rhinol. 2000;14:205–7. doi: 10.2500/105065800782102672. [DOI] [PubMed] [Google Scholar]
- 19.Zílelíoglu G, Tekeli O, Ugurba SH, Akiner M, Aktürk T, Anadolu Y. Results of endoscopic endonasal non-laser dacryocystorhinostomy. Doc Ophthalmol. 2002;105:57–62. doi: 10.1023/a:1015702902769. [DOI] [PubMed] [Google Scholar]
- 20.Yuen KS, Lam LY, Tse MW, Chan DD, Wong BW, Chan WM. Modified endoscopic dacryocystorhinostomy with posterior lacrimal sac flap for nasolacrimal duct obstruction. Hong Kong Med J. 2004;10:394–400. [PubMed] [Google Scholar]
- 21.Dolmetsch AM. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010;117:1037–40. doi: 10.1016/j.ophtha.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Apuhan T, Yildirim YS, Eroglu F, Sipahier A. Effect of mitomycin C on endoscopic dacryocystorhinostomy. J Craniofac Surg. 2011;22:2057–9. doi: 10.1097/SCS.0b013e3182319863. [DOI] [PubMed] [Google Scholar]
- 23.Görgülü O, Ozdemir S, Görgülü FF, Altin A, Selçuk T, Akbas Y. Adjunctive use of mitomycin C in endoscopic revision dacryocystorhinostomy. B-ENT. 2012;8:123–6. [PubMed] [Google Scholar]
- 24.Mak ST, Io IY, Wong AC. Prognostic factors for outcome of endoscopic dacryocystorhinostomy in patients with primary acquired nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2013;251:1361–7. doi: 10.1007/s00417-012-2228-9. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Roychoudhury A, Roychaudhuri BK. Use of mitomycin C in endo-DCR. Indian J Otolaryngol Head Neck Surg. 2006;58:368–9. doi: 10.1007/BF03049597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tirakunwichcha S, Aeumjaturapat S, Sinprajakphon S. Efficacy of mitomycin C in endonasal endoscopic dacryocystorhinostomy. Laryngoscope. 2011;121:433–6. doi: 10.1002/lary.21292. [DOI] [PubMed] [Google Scholar]
- 27.Farahani F, Ramezani A. Effect of intraoperative mitomycin C application on recurrence of endoscopic dacryocystorhinostomy. Saudi Med J. 2008;29:1354–6. [PubMed] [Google Scholar]
- 28.Penttilä E, Smirnov G, Seppä J, Kaarniranta K, Tuomilehto H. Mitomycin C in revision endoscopic dacryocystorhinostomy: A prospective randomized study. Am J Rhinol Allergy. 2011;25:425–8. doi: 10.2500/ajra.2011.25.3676. [DOI] [PubMed] [Google Scholar]
- 29.Ozkiris M, Ozkiris A, Göktas S. Effect of mitomycin C on revision endoscopic dacryocystorhinostomy. J Craniofac Surg. 2012;23:e608–10. doi: 10.1097/SCS.0b013e31826c7cf7. [DOI] [PubMed] [Google Scholar]
- 30.Ragab SM, Elsherif HS, Shehata EM, Younes A, Gamea AM. Mitomycin C-enhanced revision endoscopic dacryocystorhinostomy: A prospective randomized controlled trial. Otolaryngol Head Neck Surg. 2012;147:937–42. doi: 10.1177/0194599812450280. [DOI] [PubMed] [Google Scholar]
- 31.Özkiris M, Özkiris A. Endoscopic dacryocystorhinostomy not using canalicular silicone intubation tube with and without mitomycin C: A comparative study. Eur J Ophthalmol. 2012;22:320–5. doi: 10.5301/ejo.5000048. [DOI] [PubMed] [Google Scholar]
- 32.Liao SL, Kao SC, Tseng JH, Chen MS, Hou PK. Results of intraoperative mitomycin C application in dacryocystorhinostomy. Br J Ophthalmol. 2000;84:903–6. doi: 10.1136/bjo.84.8.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roozitalab MH, Amirahmadi M, Namazi MR. Results of the application of intraoperative mitomycin C in dacryocystorhinostomy. Eur J Ophthalmol. 2004;14:461–3. doi: 10.1177/112067210401400602. [DOI] [PubMed] [Google Scholar]
- 34.Yalaz M, Firinciogullari E, Zeren H. Use of mitomycin C and 5-fluorouracil in external dacryocystorhinostomy. Orbit. 1999;18:239–45. doi: 10.1076/orbi.18.4.239.2686. [DOI] [PubMed] [Google Scholar]
- 35.Ari S, Gun R, Surmeli S, Atay AE, Caca I. Use of adjunctive mitomycin C in external dacryocystorhinostomy surgery compared with surgery alone in patients with nasolacrimal duct obstruction: A prospective, double-masked, randomized, controlled trial. Curr Ther Res Clin Exp. 2009;70:267–73. doi: 10.1016/j.curtheres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshraghy B, Raygan F, Tabatabaie SZ, Tari AS, Kasaee A, Rajabi MT. Effect of mitomycin C on success rate in dacryocystorhinostomy with silicone tube intubation and improper flaps. Eur J Ophthalmol. 2012;22:326–9. doi: 10.5301/ejo.5000007. [DOI] [PubMed] [Google Scholar]
- 37.Qadir M, Ahangar A, Dar MA, Hamid S, Keng MQ. Comparative study of dacryocystorhinostomy with and without intraoperative application of Mitomycin C. Saudi J Ophthalmol. 2014;28:44–8. doi: 10.1016/j.sjopt.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henson RD, Cruz HL, Henson RG, Jr, Ali MJ, Kakizaki H. Postoperative application of mitomycin-C in endocanalicular laser dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2012;28:192–5. doi: 10.1097/IOP.0b013e31824a48f3. [DOI] [PubMed] [Google Scholar]
- 39.Kamal S, Ali MJ, Naik MN. Circumostial injection of mitomycin C (COS-MMC) in external and endoscopic dacryocystorhinostomy: Efficacy, safety profile, and outcomes. Ophthal Plast Reconstr Surg. 2014;30:187–90. doi: 10.1097/IOP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 40.Mukhtar SA, Jamil AZ, Ali Z. Efficacy of external dacryocystorhinostomy (DCR) with and without mitomycin-C in chronic dacryocystitis. J Coll Physicians Surg Pak. 2014;24:732–5. doi: 10.2014/JCPSP.732735. [DOI] [PubMed] [Google Scholar]
- 41.Feng YF, Yu JG, Shi JL, Huang JH, Sun YL, Zhao YE. A meta-analysis of primary external dacryocystorhinostomy with and without mitomycin C. Ophthalmic Epidemiol. 2012;19:364–70. doi: 10.3109/09286586.2012.733792. [DOI] [PubMed] [Google Scholar]
- 42.Ali MJ, Mariappan I, Maddileti S, Ali MH, Naik MN. Mitomycin C in dacryocystorhinostomy: The search for the right concentration and duration - A fundamental study on human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2013;29:469–74. doi: 10.1097/IOP.0b013e3182a23086. [DOI] [PubMed] [Google Scholar]
- 43.Marcet MM, Kuk AK, Phelps PO. Evidence-based review of surgical practices in endoscopic endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction and other new indications. Curr Opin Ophthalmol. 2014;25:443–8. doi: 10.1097/ICU.0000000000000084. [DOI] [PubMed] [Google Scholar]


