Abstract
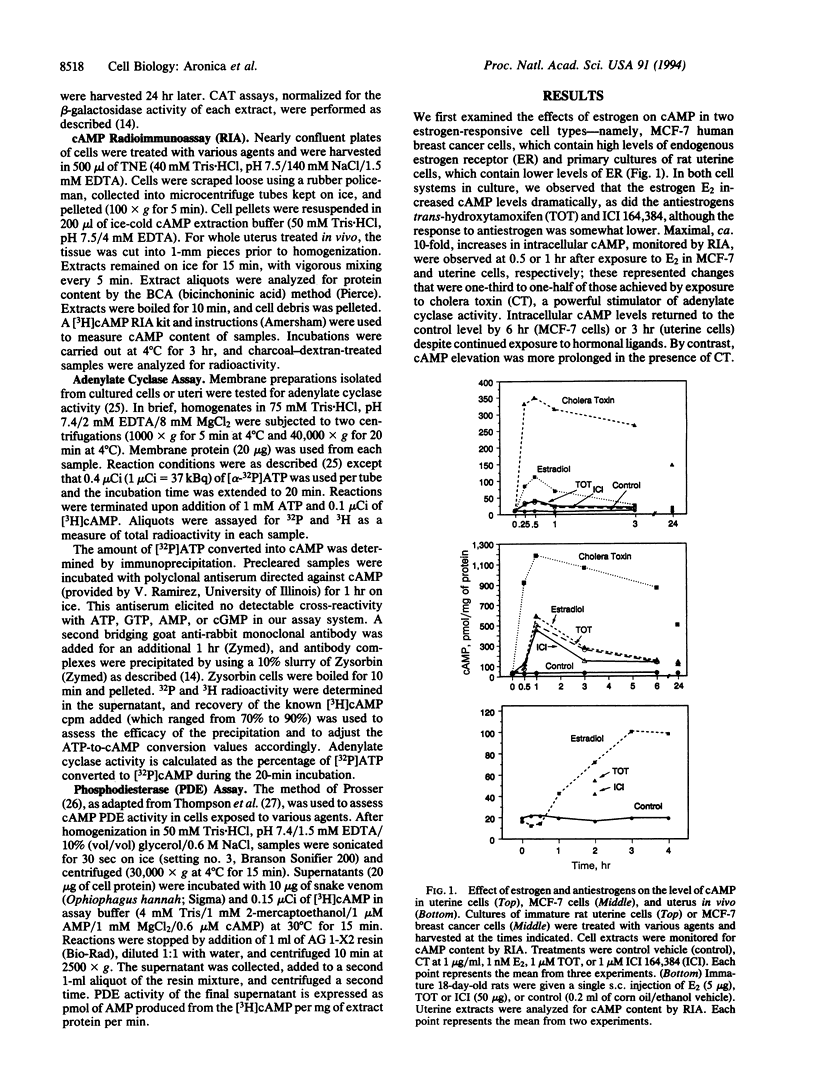
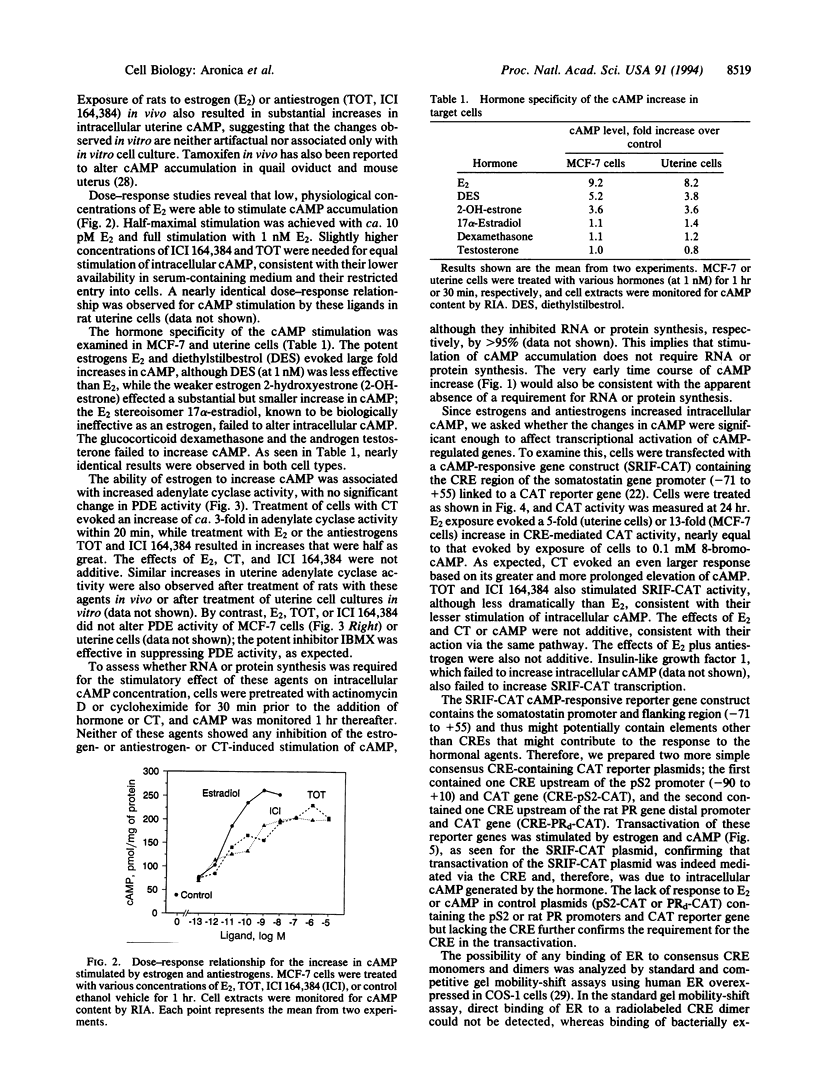
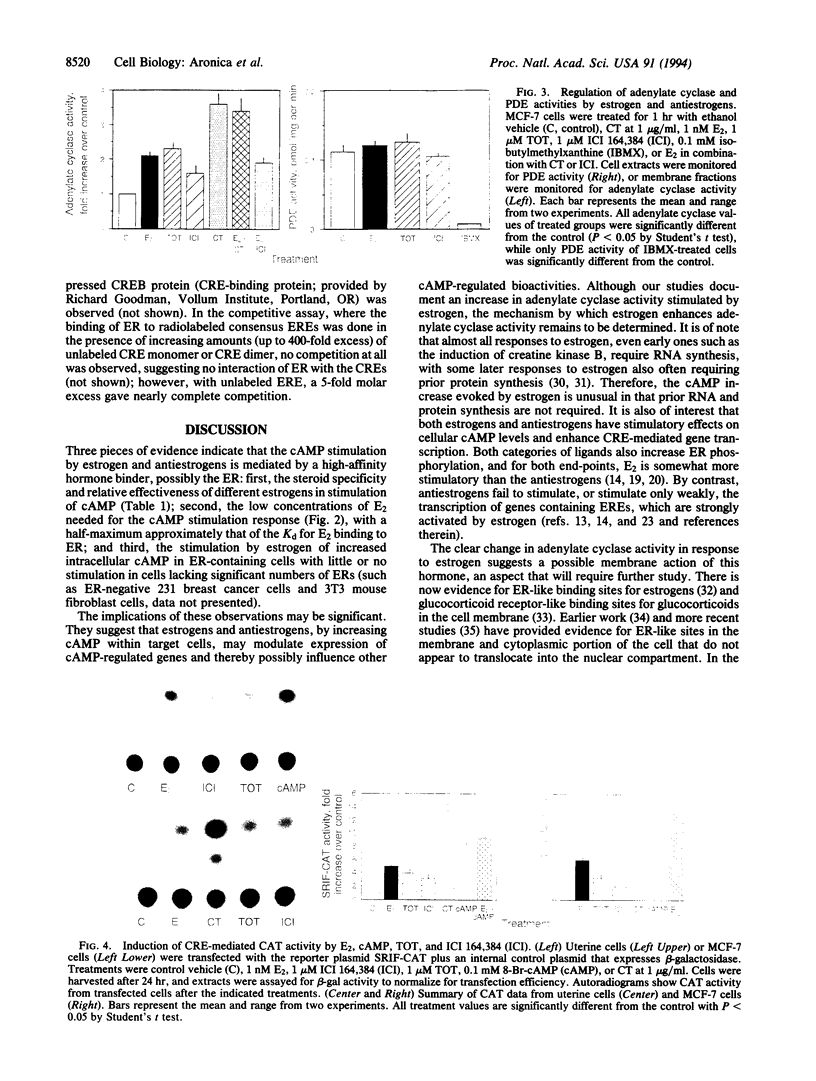
Estrogenic hormones, believed to exert most of their effects via the direct interaction of their receptors with chromatin, are found to increase cAMP in target breast cancer and uterine cells in culture and in the intact uterus in vivo. Increases in intracellular cAMP are evoked by very low concentrations of estradiol (half maximal at 10 pM) and by other physiologically active estrogens and antiestrogens, but not by an inactive estrogen stereoisomer. These increases in cAMP result from enhanced membrane adenylate cyclase activity by a mechanism that does not involve genomic actions of the hormones (are not blocked by inhibitors of RNA and protein synthesis). The estrogen-stimulated levels of cAMP are sufficient to activate transcription from cAMP response element-containing genes and reporter plasmid constructs. Our findings document a nongenomic action of estrogenic hormones that involves the activation of an important second-messenger signaling system and suggest that estrogen regulation of cAMP may provide an additional mechanism by which this steroid hormone can alter the expression of genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Metzger D., Bornert J. M., Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993 Mar;12(3):1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica S. M., Katzenellenbogen B. S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993 Jun;7(6):743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- Barsony J., Marx S. J. Receptor-mediated rapid action of 1 alpha,25-dihydroxycholecalciferol: increase of intracellular cGMP in human skin fibroblasts. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1223–1226. doi: 10.1073/pnas.85.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Moyer M. L., Nordeen S. K., Edwards D. P. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4441–4445. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew C. S., Rinard G. A. Estrogenic regulation of uterine cyclic AMP metabolism. Biochim Biophys Acta. 1974 Oct 8;362(3):493–500. doi: 10.1016/0304-4165(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Cho H., Katzenellenbogen B. S. Synergistic activation of estrogen receptor-mediated transcription by estradiol and protein kinase activators. Mol Endocrinol. 1993 Mar;7(3):441–452. doi: 10.1210/mend.7.3.7683375. [DOI] [PubMed] [Google Scholar]
- Dupont-Mairesse N., Van Sande J., Rooryck J., Fastrez-Boute A., Galand P. Mechanism of estrogen action--independence of several responses of the rat uterus from the early increase in adenosine 3',5'-cyclic monophosphate. J Steroid Biochem. 1974 Apr;5(2):173–177. doi: 10.1016/0022-4731(74)90125-3. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Ahnadi C., Fayard J. M., Pageaux J. F., Laugier C. Opposite regulation of cAMP concentration in the quail oviduct and the mouse uterus by tamoxifen. Correlation with estrogen-antagonist and estrogen-agonist activity. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):571–577. doi: 10.1016/0960-0760(92)90386-w. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Katzenellenbogen B. S. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: antiestrogen selectivity and promoter dependence. Mol Endocrinol. 1994 Mar;8(3):296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Watson C. S., Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J. 1993 Oct;7(13):1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- Gorski J., Furlow J. D., Murdoch F. E., Fritsch M., Kaneko K., Ying C., Malayer J. R. Perturbations in the model of estrogen receptor regulation of gene expression. Biol Reprod. 1993 Jan;48(1):8–14. doi: 10.1095/biolreprod48.1.8. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Gruol D. J., Altschmied J. Synergistic induction of apoptosis with glucocorticoids and 3',5'-cyclic adenosine monophosphate reveals agonist activity by RU 486. Mol Endocrinol. 1993 Jan;7(1):104–113. doi: 10.1210/mend.7.1.8383286. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Teng C. T., Ross K. A., Parker M. G., Korach K. S., McLachlan J. A. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol. 1993 Aug;7(8):992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S. Dynamics of steroid hormone receptor action. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Montano M. M., Katzenellenbogen B. S. Cloning of the rat progesterone receptor gene 5'-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993 Dec;7(12):1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Ham E. A., Zanetti M. E., Sanford C. H., Nicol S. E., Goldberg N. D. Estrogen-related increases in uterine guanosine 3':5'-cyclic momophosphate levels. Proc Natl Acad Sci U S A. 1974 May;71(5):1866–1870. doi: 10.1073/pnas.71.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff P., Montano M. M., Schodin D. J., Katzenellenbogen B. S. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994 Feb 11;269(6):4458–4466. [PubMed] [Google Scholar]
- Lieberherr M., Grosse B., Kachkache M., Balsan S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Miner Res. 1993 Nov;8(11):1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Migliaccio A., Pagano M., Auricchio F. Immediate and transient stimulation of protein tyrosine phosphorylation by estradiol in MCF-7 cells. Oncogene. 1993 Aug;8(8):2183–2191. [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979 Oct;11(4):1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Rangarajan P. N., Umesono K., Evans R. M. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992 Sep;6(9):1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Differential DNA-binding abilities of estrogen receptor occupied with two classes of antiestrogens: studies using human estrogen receptor overexpressed in mammalian cells. Nucleic Acids Res. 1991 Dec 11;19(23):6595–6602. doi: 10.1093/nar/19.23.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., O'Malley B. W. Steroid hormones: effects on adenyl cyclase activity and adenosine 3',5'-momophosphate in target tissues. Science. 1970 Apr 10;168(3928):253–255. doi: 10.1126/science.168.3928.253. [DOI] [PubMed] [Google Scholar]
- Sanborn B. M., Bhalla R. C., Korenman S. G. Use of a modified radioligand assay to measure the effect of estradiol on uterine adenosine 3',5'-cyclic monophosphate. Endocrinology. 1973 Feb;92(2):494–499. doi: 10.1210/endo-92-2-494. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Tung L., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993 May 5;268(13):9262–9266. [PubMed] [Google Scholar]
- Sheffield L. G., Welsch C. W. Cholera-toxin-enhanced growth of human breast cancer cell lines in vitro and in vivo: interaction with estrogen. Int J Cancer. 1985 Oct 15;36(4):479–483. doi: 10.1002/ijc.2910360411. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Strickland P., Trumpbour V., Coleman S., Daniel C. W. In vivo, cAMP stimulates growth and morphogenesis of mouse mammary ducts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4950–4954. doi: 10.1073/pnas.81.15.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. P., DeFranco D. B. Effects of okadaic acid, a protein phosphatase inhibitor, on glucocorticoid receptor-mediated enhancement. Mol Endocrinol. 1992 Jan;6(1):26–34. doi: 10.1210/mend.6.1.1310797. [DOI] [PubMed] [Google Scholar]
- Stewart P. J., Webster R. A. Intrauterine injection of cholera toxin induces estrogen-like uterine growth. Biol Reprod. 1983 Oct;29(3):671–679. doi: 10.1095/biolreprod29.3.671. [DOI] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Inhibition of estrogen-induced elevation of cyclic 3',5'-adenosine monophosphate in rat uterus. I. By beta-adrenergic receptor-blocking drugs. Mol Pharmacol. 1969 Sep;5(5):470–480. [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Tischkau S. A., Ramirez V. D. A specific membrane binding protein for progesterone in rat brain: sex differences and induction by estrogen. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1285–1289. doi: 10.1073/pnas.90.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Grady L. H., Judy B. M., Jordan V. C., Preziosi D. E. Subcellular compartmentalization of MCF-7 estrogen receptor synthesis and degradation. Mol Cell Endocrinol. 1993 Aug;94(2):183–194. doi: 10.1016/0303-7207(93)90167-i. [DOI] [PubMed] [Google Scholar]