Abstract
Improvement in quality of life (QoL) is a primary treatment goal for patients with peripheral arterial disease (PAD). The current study aimed to quantify improvement in the health status of PAD patients following peripheral revascularization using the peripheral artery questionnaire (PAQ) and ankle-brachial index (ABI), and to evaluate possible correlation between the two methods. The PAQ and ABI were assessed in 149 symptomatic PAD patients before, and three months after peripheral revascularization. Mean PAQ summary scores improved significantly three months after revascularization (+49.3 ± 15 points, p < 0.001). PAQ scores relating to patient symptoms showed the largest improvement following revascularization. The smallest increases were seen in reported treatment satisfaction (all p’s < 0.001). As expected the ABI of treated limbs showed significant improvement post-revascularization (p < 0.001). ABI after revascularization correlated with patient-reported changes in the physical function and QoL domains of the PAQ. Twenty-two percent of PAD patients were identified as having a poor response to revascularization (increase in ABI < 0.15). Interestingly, poor responders reported improvement in symptoms on the PAQ, although this was less marked than in patients with an increase in ABI > 0.15 following revascularization. In conclusion, data from the current study suggest a significant correlation between improvement in patient-reported outcomes assessed by PAQ and ABI in symptomatic PAD patients undergoing peripheral revascularization.
Keywords: peripheral artery disease, revascularization, questionnaire, ankle-brachial index
1. Introduction
Patients with peripheral arterial disease (PAD) have greater functional impairment and faster rates of functional decline than the healthy population [1]. PAD prevalence dramatically increases with age, and most patients with PAD have one or more cardiovascular disease risk factors that should be targeted for therapy [2]. The selection of optimal medical therapy and ultimately revascularization procedure depends on quantifying PAD patients’ health status, since these interventions are primarily intended to improve symptoms, functional status, and quality of life (QoL) [3]. The ankle-brachial index (ABI) has been used as a sensitive, valuable, and cost-effective outcome measure and screening tool for PAD. However, recent publications have suggested that ABI measured by pulse palpation or automatic blood pressure devices is unreliable and correlates poorly with changes in health status scores [4]. Currently, the rates of lower extremity revascularization and related medical costs are increasing and the application of direct, patient-centered outcome assessments is necessary to quantify treatment success. The Peripheral Artery Questionnaire (PAQ) was developed to measure the health status of PAD patients based on their symptoms, functional status, and QoL [5]. We have previously translated and validated a Korean version of the PAQ in Korean PAD patients and demonstrated that PAQ scores strongly correlate with ABI [6,7]. Thus, screening programs using the PAQ in conjunction with ABI may improve the ability to detect PAD patients when assessing individuals with suspicious lower-limb symptoms. However, few studies have investigated the correlation between patient-reported symptoms and ABI following peripheral revascularization as a means to assess treatment outcomes. The aim of this study was to quantify improvement in the health status of symptomatic PAD patients undergoing peripheral revascularization via ABI and PAQ, and to investigate whether patient-reported symptom outcomes correlated with medically measured outcomes.
2. Results
One hundred and forty-nine PAD patients undergoing peripheral revascularization were recruited to this study. The mean age of the population was 70 ± 10 years, and 84% (n = 125) were men. Almost half (46%) were active or past smokers, 64% were hypertensive, 62% had dyslipidemia, and 39% were diabetic. One hundred patients underwent endovascular revascularlization. This included percutaneous transluminal angioplasty (PTA) alone of the superficial femoral and/or popliteal artery (n = 25), stent insertion in the iliac artery (n = 26)/superficial femoral artery (n = 6)/popliteal artery (n = 5), stent insertion with PTA of the superficial femoral and/or popliteal artery (n = 25), and PTA of the superficial femoral and/or popliteal artery (n = 13). Forty-nine patients underwent bypass surgery including aorto-iliac bypass (n = 3), ilio-femoral bypass (n = 3), femoro-femoral bypass (n = 18), and above knee femoro-popliteal bypass (n = 20), and below knee femoro-popliteal bypass (n = 5). No complications such as procedure-related death, all-cause death, major target limb amputation, and target vessel thrombosis were observed within 30 days after lower limb revascularization. Three patients developed an access-site hematoma. The baseline characteristics of enrolled patients and comparisons between percutaneous and surgical revascularization are shown in Table 1.
Table 1.
Baseline characteristics of enrolled PAD patients.
| Variables | Total (n = 149) | Endovascular Revascularization (n = 100) | Bypass Surgery (n = 49) | p Value |
|---|---|---|---|---|
| Age, years | 70.3 ± 9.7 | 70.1 ± 9.9 | 70.7 ± 9.3 | 0.704 |
| Male, % | 125 (84%) | 82 (82%) | 43 (88%) | 0.258 |
| Systolic BP, mmHg | 125.1 ± 18.2 | 124.4 ± 19.8 | 126.7 ± 14.6 | 0.424 |
| Diastolic BP, mmHg | 77.8 ± 10.6 | 77.6 ± 10.6 | 78.2 ± 10.6 | 0.749 |
| Heart rate, bpm | 80.4 ± 13.0 | 80.6 ± 13.6 | 80.2 ± 11.9 | 0.870 |
| Total cholesterol | 163.8 ± 48.2 | 162.4 ± 53.7 | 166.6 ± 34.5 | 0.576 |
| LDL, mg/dL | 103.4 ± 47.5 | 105.6 ± 47.1 | 98.9 ± 48.9 | 0.568 |
| HDL, mg/dL | 40.6 ± 28.8 | 41.6 ± 33.9 | 38.5 ± 14.1 | 0.572 |
| C-reactive protein, mg/L | 4.2 ± 16.1 | 3.85 ± 15.4 | 0.96 ± 2.65 | 0.350 |
| Ankle brachial index | 0.75 ± 0.24 | 0.77 ± 0.24 | 0.70 ± 0.24 | 0.181 |
| Hypertension, % | 95 (64%) | 63 (63%) | 32 (65%) | 0.465 |
| Diabetes, % | 58 (39%) | 38 (38%) | 20 (41%) | 0.438 |
| Dyslipidemia, % | 92 (62%) | 62 (62%) | 30 (61%) | 0.533 |
| Current smoker, % | 48 (32%) | 33 (33%) | 15 (31%) | 0.461 |
| Ex-smoker, % | 21 (14%) | 11 (11%) | 10 (20%) | 0.099 |
| Aspirin, % | 87 (58%) | 60 (60%) | 27 (55%) | 0.329 |
| Clopidogrel, % | 29 (20%) | 20 (20%) | 9 (18%) | 0.500 |
| Cilostazol, % | 10 (7%) | 8 (8%) | 2 (4%) | 0.302 |
| BP medication, % | 88 (59%) | 59 (59%) | 29 (59%) | 0.782 |
| Statin, % | 62 (42%) | 48 (48%) | 14 (29%) | 0.024 |
| Location | ||||
| Aorto-iliac | 48 (32.2%) | 46 (46%) | 2 (4%) | <0.001 |
| Femoropopliteal | 101 (67.8%) | 54 (54%) | 47 (96%) | <0.001 |
Values are expressed as means ± SD. PAD: peripheral artery disease; BP: blood pressure; LDL: low density lipoprotein; HDL: high density lipoprotein.
2.1. Post-Revascularization Outcome Assessments
Post-revascularization ABI of the treated limb was significantly improved compared to baseline (Table 2). Mean PAQ summary scores also improved significantly after revascularization (p < 0.001). All six PAQ domains improved (Table 2). The greatest post-revascularization improvement was observed in the symptoms category, whereas the smallest increase was seen in the treatment satisfaction domain.
Table 2.
PAQ scores and ABI prior to and three months after revascularization.
| Assessments | Pre-Revascularization | Post-Revascularization | Changes (Δ) | p Value |
|---|---|---|---|---|
| ABI in non-treated limb | 0.94 ± 0.22 | 0.97 ± 0.16 | 0.02 ± 0.17 | 0.360 |
| ABI in treated limb | 0.65 ± 0.17 | 0.94 ± 0.20 | 0.31 ± 0.23 | <0.001 |
| Physical function | 22.4 ± 12.9 | 81.6 ± 19.9 | 59.2 ± 19.8 | <0.001 |
| Symptom | 22.7 ± 19.6 | 83.2 ± 16.7 | 60.5 ± 25.2 | <0.001 |
| Stability | 41.6 ± 14.8 | 87.6 ± 19.4 | 46.0 ± 24.3 | <0.001 |
| Treatment satisfaction | 36.8 ± 10.6 | 69.4 ± 16.1 | 32.6 ± 17.5 | <0.001 |
| Quality of life | 22.1 ± 9.5 | 73.0 ± 18.5 | 50.9 ± 20.4 | <0.001 |
| Social limitation | 41.8 ± 19.1 | 87.8 ± 15.5 | 46.0 ± 20.7 | <0.001 |
| Summary | 32.1 ± 11.3 | 81.4 ± 16.4 | 49.3 ± 15.8 | <0.001 |
Values are expressed as means ± SD. ABI: ankle brachial index; PAQ: peripheral artery questionnaire.
Baseline ABI of the affected limb was higher in the patients undergoing endovascular treatment compared to those having open surgery; however, post-revascularization increases in ABI were comparable between the groups. Patients receiving endovascular treatment reported a more significant increase within the social limitation domain of the PAQ than those undergoing open surgery (p = 0.037). No post-surgical differences were observed between the groups in the other PAQ domains, or PAQ summary score (Table 3).
Table 3.
Changes in PAQ scores and ABI following lower limb revascularization via endovascular or open surgery.
| Assessments | Endovascular Revascularization (n = 100) | Open Surgery (n = 49) | p Value |
|---|---|---|---|
| Baseline ABI in treated limb | 0.68 ± 0.18 | 0.59 ± 0.12 | 0.007 |
| Postoperative ABI in treated limb | 0.96 ± 0.20 | 0.93 ± 0.20 | 0.519 |
| ΔABI in treated limb | 0.29 ± 0. 24 | 0.34 ± 0.19 | 0.391 |
| Baseline physical function | 21.3 ± 11.9 | 24.7 ± 14.7 | 0.164 |
| Postoperative physical function | 80.0 ± 21.9 | 84.9 ± 14.6 | 0.105 |
| ΔPhysical function | 58.7 ± 18.8 | 60.3 ± 19.2 | 0.641 |
| Baseline symptom | 19.8 ± 17.9 | 28.6 ± 21.7 | 0.016 |
| Postoperative symptom | 82.6 ± 17.8 | 84.2 ± 14.4 | 0.584 |
| Δsymptom | 62.9 ± 23.3 | 55.7 ± 28.5 | 0.127 |
| Baseline stability | 41.7 ± 13.9 | 41.3 ± 16.8 | 0.890 |
| Postoperative stability | 86.0 ± 20.8 | 90.8 ± 15.9 | 0.120 |
| Δstability | 44.3 ± 24.2 | 49.5 ± 24.4 | 0.224 |
| Baseline treatment satisfaction | 37.4 ± 9.7 | 35.6 ± 12.3 | 0.311 |
| Postoperative treatment satisfaction | 70.8 ± 16.0 | 66.7 ± 16.2 | 0.147 |
| ΔTreatment satisfaction | 33.3 ± 16.1 | 31.1 ± 20.1 | 0.505 |
| Baseline quality of life | 20.0 ± 7.7 | 26.5 ± 11.3 | 0.001 |
| Postoperative quality of life | 72.8 ± 16.0 | 73.5 ± 18.3 | 0.845 |
| ΔQuality of life | 52.8 ± 18.8 | 47.0 ± 23.0 | 0.125 |
| Baseline social limitation | 38.4 ± 17.2 | 48.6 ± 21.0 | 0.002 |
| Postoperative social limitation | 86.9 ± 16.5 | 89.6 ± 13.2 | 0.283 |
| ΔSocial limitation | 48.5 ± 17.8 | 41.0 ± 25.0 | 0.037 |
| Baseline summary | 30.6 ± 10.0 | 35.4 ± 13.1 | 0.025 |
| Postoperative summary | 80.6 ± 17.4 | 83.1 ± 14.0 | 0.353 |
| Δsummary | 50.0 ± 14.0 | 47.7 ± 19.0 | 0.438 |
Values are expressed as means ± SD. ABI: ankle brachial index; PAQ: peripheral artery questionnaire.
2.2. Correlation between PAQ Score and ABI
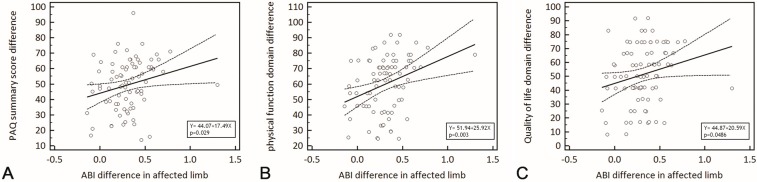
Post-surgical scores for all PAQ domains, except for symptom stability, were significantly correlated with the post-revascularization ABI of the treated limb, as shown in Table 4. In contrast, the changes in scores from before and after revascularization were not as strongly associated with post-procedure ABIs. The change in summary score (r = 0.243, p = 0.029, Figure 1A), change in physical function (r = 0.326, p = 0.003, Figure 1B) and change in QoL (r = 0.220, p = 0.048, Figure 1C) domains showed modest correlation to the change in ABI. No significant correlation between change in symptom (r = 0.185, p = 0.098), change in symptom stability (r = −0.102, p = 0.363), change in treatment satisfaction (r = 0.208, p = 0.063) and change in social limitation (r = 0.046, p = 0.683) with change in ABI were observed.
Table 4.
Correlation between scores of each PAQ domain and ABI after revascularization.
| PAQ Domain | Correlation Coefficient | p Value |
|---|---|---|
| Physical function | 0.337 | 0.001 |
| Stability | −0.122 | 0.231 |
| Symptom | 0.376 | <0.001 |
| Treatment satisfaction | 0.427 | <0.001 |
| Quality of life | 0.371 | 0.001 |
| Social limitation | 0.333 | 0.001 |
| Summary score | 0.374 | <0.001 |
Figure 1.
Correlation analysis assessing the association between changes in PAQ scores and change in ABI in the affected limb after revascularization. The change in summary scores (A); physical function (B); and Quality of Life domain (C) scores were significantly correlated with the changes in ABI.
2.3. Differences in PAQ Scores in Patients Who Did or Did not Respond to Revascularization
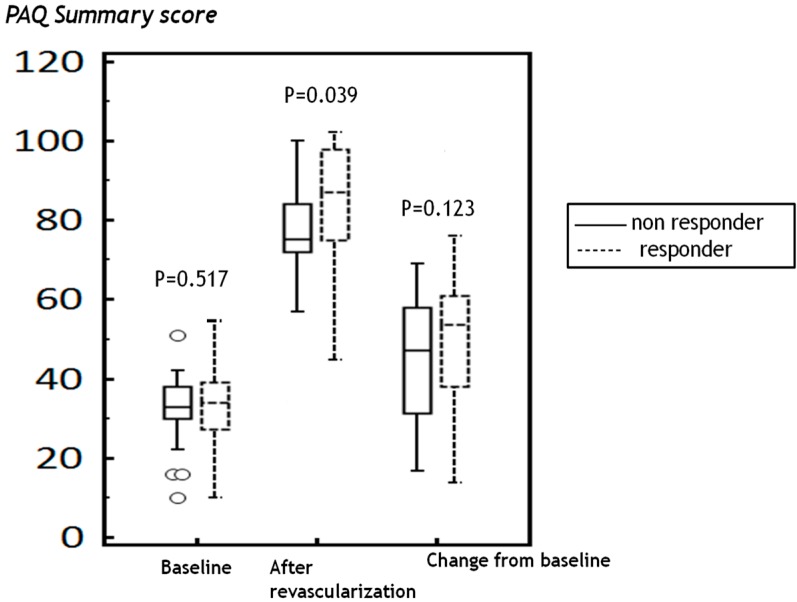
Patients were then reclassified as those who responded poorly to revascularization (post-surgical increase in ABI of the treated limb <0.15), and those who responded well (increase in ABI > 0.15). Using these criteria, 33 (22%) of patients responded poorly to revascularization. Demographic characteristics of good- and poor-responders are shown in Supplementary Table S1. Post-revascularization PAQ summary score was higher in the good-responder group (Table 5, Figure 2). Although the post-revascularization changes in PAQ scores were lower in non-responders, they were only significantly different for the treatment satisfaction domain (Table 5).
Table 5.
Comparison of PAQ scores and ABI between responders and non-responders.
| Assessments | Responders (n = 116) | Non-Responders (n = 33) | p Value |
|---|---|---|---|
| ΔABI, ischemic side | 0.39 ± 0.18 | 0.02 ± 0.10 | <0.001 |
| Post-physical function | 85.4 ± 16.7 | 77.1 ± 16.3 | 0.066 |
| Post-symptom score | 86.3 ± 15.2 | 77.3 ± 16.5 | 0.033 |
| Post-stability | 88.1 ± 21.5 | 88.9 ± 17.6 | 0.886 |
| Post-treatment satisfaction | 73.0 ± 14.5 | 63.0 ± 19.8 | 0.020 |
| Post-quality of life | 75.7 ± 17.9 | 67.1 ± 18.2 | 0.080 |
| Post-social limitation | 90.2 ± 14.0 | 81.5 ± 17.7 | 0.031 |
| Post-summary score | 84.4 ± 15.0 | 75.8 ± 16.4 | 0.039 |
| ΔPhysical function | 61.9 ± 18.5 | 53.3 ± 15.2 | 0.051 |
| ΔSymptom | 61.3 ± 25.3 | 53.7 ± 22.8 | 0.234 |
| ΔStability | 44.9 ± 26.0 | 47.9 ± 21.7 | 0.620 |
| ΔTreatment satisfaction | 35.5 ± 17.7 | 25.9 ± 18.7 | 0.047 |
| ΔQuality of life | 53.1 ± 20.8 | 44.7 ± 22.3 | 0.165 |
| ΔSocial limitation | 46.5 ± 20.5 | 43.5 ± 20.5 | 0.585 |
| ΔSummary | 51.0 ± 16.3 | 44.2 ± 16.0 | 0.123 |
Values are expressed as means ± SD. ABI: ankle brachial index; PAQ: peripheral artery questionnaire.
Figure 2.
After revascularization, peripheral artery questionnaire summary scores were higher in the responder group; however, the scores from the baseline were not significantly different between the groups.
3. Discussion
In this descriptive study, we found that both ABI and PAQ showed significant improvement following revascularization therapy regardless of the surgical method. The average post-revascularization changes were 0.31 in ABI of the symptomatic limb and 49.3 points in the PAQ summary score. Comparisons between different methods of revascularization showed similar changes in ABI and PAQ summary scores, but social limitation was less compromised after endovascular revascularization. The PAQ summary score was well-correlated with ABI, and the physical function domain showed the strongest correlation.
PAD is a chronic arterial occlusive disease of the lower extremities caused by atherosclerosis. Previous studies reported a prevalence of PAD-related symptoms (mainly intermittent claudication) ranging from 5.3% to 18.9% among the elderly population [8]. This low prevalence was attributed to elderly people not walking enough to experience symptoms, either because of impaired vascularization in the extremities or other disorders such as osteoarthritis. There is little correlation between clinical parameters of vascular disease, such as ABI, and the patient’s self-reported level of disability [9]. In fact, ABI is considered most beneficial in identifying asymptomatic individuals, rather than those with symptomatic disease being considered for intervention [3]. Another study found that most atherosclerotic events occurred in symptomatic patients with ABI < 0.9 [10,11].
Outcome assessments of revascularization therapy that focus on anatomic end points, such as degree of lumen narrowing and restenosis, are surrogate end points that effectively assess the physiologic impact of lower limb atherothrombosis and its treatment. These measures do not adequately describe the impact of the disease from the patients’ perspective or the benefits or adverse effects of treatment on QoL. In studies of PAD patients not receiving revascularization, ABI, a measure integrating the impact of all stenoses in a limb, is poorly associated with function [12,13]. So far, the relationship of restenosis or occlusion to symptoms and function after revascularization is poorly studied and likely to vary between individuals. Traditionally, patient-reported symptom measures and objective functional performance have been included as clinical trial end points [14,15,16,17,18,19]. Among the instruments for the assessment of patients’ symptoms, previous studies suggest that the PAQ is more sensitive to changes in patients’ health status than generic instruments, such as the EuroQol 5 Dimensions (EQ5D) [14], the health associated questionnaire (HAQ) [6], and EuroQol-Visual Analogue Scale (EQ VAS) [15]. According to our previous study, the reliability of the Korean version of PAQ was adequate with an intra-class correlation coefficient of 0.71, and Cronbach’s alpha for the summary score was 0.94, indicating good internal consistency and congruence with the original English version [6]. Recent trials have also assessed intermittent claudication-related symptoms and functional impairment using the Walking Impairment Questionnaire (WIQ) and PAQ [16]. Compared to the WIQ, which mainly focuses on the patient’s walking distance and ability, the PAQ assesses a broader range of domains, including PAD-related physical limitation, symptoms, quality of life, social function and treatment satisfaction. The clinical validity of the PAQ proved to be good in the discrimination of patients with or without symptomatic PAD and its severity as defined by walking distance [15]. Moreover, PAQ subscales were correlated with the risk factors relevant for PAD [15], and we have previously shown that the PAQ might increase the pre-test probability of ABI screening among patients with suspicious symptoms [7]. This study extends this prior work by demonstrating that the improvement in disease-specific PAQ is significantly associated with changes in ABI after revascularization.
Since the patients’ main concerns are symptom relief and improvement in daily functioning, revascularization treatment should be assessed for its success in improving patient health status [20,21,22,23,24,25]. Although ABI is known to correlate poorly with changes in health status scores [3], ABI is currently one of the most widely used tools for evaluation of peripheral revascularization. However, in patients with intermittent claudication, there is no evidence that endovascular therapy alone provides improved outcome over supervised exercise alone based on the improvement of ABI and treadmill walking [24]. Recently, six-month disease-specific QoL results in the Claudication: Exercise versus Endoluminal Revascularization (CLEVER) study showed that maximal treadmill walking time showed good correlation between self-reported symptom improvement in patients treated with stent revascularization, but not in patients with supervised exercise [25]. This finding indicates that traditional objective treadmill test outcomes may not correlate well with symptom relief in patients with claudication.
Considering the limited use of quality of life tools to assess outcomes in clinical practice, and the need for more patient-centered assessments of the effects of treatment, we believe the disease-specific PAQ to be the preferred choice or studying outcomes in PAD patients. However, comparison between ABI and PAQ was needed to prove clinical efficacy of PAQ-based outcome assessments after revascularization in patients with intermittent claudication. The current study firstly demonstrated that revascularization treatment resulted in significant improvements in both ABI and subjective QoL outcomes assessed by PAQ. Secondly, data show that the PAQ summary score was well-correlated with ABI in PAD patients. Changes in ABI and summary score of PAQs were similar between endovascular treatment and bypass surgery, but the improvement of social limitation was significantly higher in patients with endovascular treatment, which, if replicated, could be a benefit of endovascular treatment as compared with open surgery.
The benefits of revascularization in patients with PAD may be closely tied to improvements in the ABI after revascularization [26]. In the current study, ~22% of patients showed a poor response to revascularization evidenced by an increase <0.15 units in ABI within the treated limb three months after surgery. Nevertheless, these “non-responders” experienced significant improvements in their health status, although not as much as the responder group. This suggests that the technical success of the operation measured by ABI failed to capture the benefits experienced by patients. Thus, the ABI change has a complementary, but not a superior, role to the PAQ in patient outcome assessment after revascularization. Conversely, a previous study showed that despite a technically successful procedure in 98% of patients, 21% of patients did not achieve the minimal clinically important improvement of an eight-point change in PAQ summary score after revascularization [14]. The reasons for the discrepancies between improvement in ABI and PAQ in the current study are unclear.
There are several limitations of the present study. First, this is an observational study with relatively few subjects. Compared to clinical trials, however, our study comprises a rather heterogeneous population and is more representative of daily clinical practice. Second, the interval between revascularization therapy and the post-revascularization assessment of treatment was relatively short. To understand whether restenosis or disease progression affect PAQ scores will require further study. Because over 80% of participants in our study were male, the current results may not be generalizable to the correlation of ABI and PAQ in women. Finally, results of treadmill tests or other objective measures of patient symptoms like the WIQ, intermittent claudication questionnaire (ICQ), EQ-5D, PAD Quality of Life Questionnaire (PADQOL) and Vascular Quality of Life Questionnaire (VascuQoL) were not examined in the current study. No Korean versions of these questionnaires are currently available. Further large, prospective studies should be conducted to ascertain the correlation of PAQ and other objective measures of patient symptoms in Korean patients.
4. Experimental Section
4.1. Participants and Selection Criteria
Between January 2009 and August 2012, 218 consecutive patients underwent peripheral revascularization for symptomatic PAD. To be eligible for this study, PAD participants must have been diagnosed with PAD in one of the noninvasive vascular laboratories and have an ABI ≤ 0.90 at their baseline study visit. Patients were excluded if they were below 18 years of age, refused revascularization therapy, had a history of thoracic or brain surgery, or had comorbidities such as cancer or a life-threatening illness. Among the 218 patients who underwent revascularization, 25 were excluded due to these criteria, 30 patients did not complete pre- or post-revascularization PAQ, and 14 refused to provide written informed consent. Thus, a total of 149 patients were enrolled and completed a pre- and post-revascularization PAQ, as well as post-revascularization ABI. The study protocol was approved by the Institutional Review Boards of Pusan National University Yangsan Hospital, (IRB no; 04-2011-034) and all patients provided written informed consent. Characteristics of study subjects including demographics, established cardiovascular risk factors (smoking, type 2 diabetes mellitus (DM), hypertension, or dyslipidemia, as defined by the TASC II report), family history, past medical history, history of atherothrombotic events, medications in the last six months (including anti-hypertensives, anti-diabetics, lipid-lowering drugs, and antithrombotic agents), and smoking status were obtained using a standard questionnaire. Blood pressure, heart rate, body weight, height, body mass index (BMI), waist circumference, and physical examination findings specific to PAD (absence of pedal pulses, arterial bruits, and trophic changes of the foot) were also recorded. The revascularization strategy was decided at the surgeon’s discretion. Surgical revascularization was performed using standard techniques, such as aorto-iliac bypass, ilio-femoral bypass, femoro-femoral bypass, and femoro-popliteal bypass. Femoro-popliteal bypass was performed using autologous greater saphenous vein grafts, and the extent of revascularization was left to the discretion of the surgeon. Peripheral endovascular revascularization was performed using various interventional devices, including stents. The extent of the intervention and choice of equipment were also at the discretion of the operating surgeon. Either open or endovascular revascularization was selected by consensus between the vascular surgeon and interventionist using the TASC II classification [27].
4.2. Questionnaires Administered
Patient-reported symptom outcomes were measured by the translated Korean version of the PAQ [6], a disease-specific instrument for assessing health status in patients with PAD [5,6]. The PAQ is a self-administered, 20-item health status measure for patients with PAD. Scores are separated into six domains, including physical limitation (the degree to which PAD limits the patient’s routine activities), symptoms (frequency and discomfort of intermittent claudication and the frequency of rest pain), symptom stability (recent improvement or deterioration in patient symptoms), treatment satisfaction (patient satisfaction with current treatment), social functioning (limitations in patients’ ability to interact with others), and QoL (personal evaluation that reflects the patient’s current symptoms and limitations as compared with their desired level of functioning). A summary score is calculated as the average of the physical limitation, symptoms, QoL, and social functioning scores. Scores range from 0 to 100, where higher scores indicate less functional limitation, fewer symptoms, better treatment satisfaction, higher social functioning, and better QoL. A symptom stability score of 50 represents no change over the preceding four weeks, whereas scores above or below 50 represent recent improvement or worsening of symptoms, respectively. The pre-revascularization PAQ inquired about symptoms attributable to PAD over the four weeks before revascularization, and the post-revascularization PAQ was administered three months after revascularization therapy. The changes in PAQ scores were calculated by subtracting pre-revascularization PAQ scores from the post-revascularization PAQ scores.
4.3. Measurement of Ankle Brachial Index
ABI was measured in all patients using an automatic waveform analyzer (VP-1000; Colin Co., Komaki, Japan) in a quiet room, with the patient supine for at least five minutes before measurement. The VP-1000 simultaneously recorded pulse waves, blood pressure (BP) in both arms and ankle, and electrocardiography. The ABI was then calculated as the ratio of the ankle systolic BP divided by the ipsilateral arm systolic BP, using the lower value of the ankle systolic BP for the calculation. The treated leg ABI was used for analysis. An increase in ABI of ≥0.15 in the revascularized leg at three months after surgery was considered a clinically meaningful ABI improvement as previously described [26]. If a participant underwent bilateral revascularization, the leg with the lowest ABI before revascularization was considered in the analysis as the lowest leg ABI most closely associated with degree of functional impairment [28]. The change in ABI was calculated as subtracting baseline ABI from the ABI after revascularization.
4.4. Statistical Analyses
The Kolmogorov-Smirnov test was used for the evaluation of variable distribution. Normally distributed data are presented as mean ± standard deviation, and not normally distributed data are expressed as median (inter quartile range). Categorical variables are presented as percentages or numbers. Comparisons of all measurements were made with paired t-tests and Wilcoxon signed-rank test for continuous variables and chi-squared and Fischer’s exact tests for categorical variables. The independent Student’s t-test was used to determine the difference in normally distributed data, and the Mann-Whitney U test was used for comparison of medians for not normally distributed variables. Comparison of patient-reported symptoms between responders and non-responders based on changes in ABI was analyzed with the use of independent t-tests. Correlations were made with the Pearson correlation test. All p-values were two-sided, and a probability value of p < 0.05 was considered a significant difference. Statistical analysis was performed using SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
5. Conclusions
Our data suggest that patient-reported improvement in PAD symptoms following lower extremity revascularization is associated with, but distinct from, post-revascularization improvements in the ABI. Although patients with a post-surgical increase in ABI >0.15 showed a greater improvement in symptoms than those with ABI increases <0.15, patients who had a poor hemodynamic response to revascularization also reported important improvements in their health status. Thus revascularization can offer symptomatic relief independent of significant increases in ABI. Use of quality of life measurement tools such as the PAQ is likely to be beneficial in assessing the outcomes in PAD patients after revascularization.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/16/05/11355/s1.
Author Contributions
Kyoung Im Cho designed the study, Hyung Gon Je and Bo Hyun Kim directed its implementation, including the study’s analytic strategy. Hyung Gon Je and Yong Hyun Park collected the data, and John Spertus and Jae Sik Jang helped conduct the literature review. Kyoung Im Cho prepared the Methods and the Discussion sections of the text and had the overall responsibility. Hyung Gon Je and Bo Hyun Kim contributed equally to this article and share first authorship.
Conflicts of Interest
John Spertus discloses that he owns the copyright to the PAQ. The other authors declare no conflict of interest.
References
- 1.McDermott M.M., Liu K., Greenland P., Guralnik J.M., Criqui M.H., Chan C., Pearce W.H., Schneider J.R., Ferrucci L., Celic L., et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E., Erlinger T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Mehta T., Venkata S.A., Chetter I., McCollum P. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur. J. Vasc. Endovasc. Surg. 2006;31:46–52. doi: 10.1016/j.ejvs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V., Lacroix P., Doucet S., Preux P.M., Criqui M.H., Laskar M. Diagnosis of peripheral arterial disease in general practice: can the ankle-brachial index be measured either by pulse palpation or an automatic blood pressure device? Int. J. Clin. Pract. 2008;62:1001–1007. doi: 10.1111/j.1742-1241.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 5.Spertus J., Jones P., Poler S., Rocha-Singh K. The peripheral artery questionnaire: A new disease-specific health status measure for patients with peripheral arterial disease. Am. Heart J. 2004;147:301–308. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.H., Cho K.I., Spertus J., Kim S.M. Cross-cultural adaptation and validation of the Peripheral Artery Questionnaire: Korean version for patients with peripheral vascular diseases. Vasc. Med. 2012;17:215–222. doi: 10.1177/1358863X12445104. [DOI] [PubMed] [Google Scholar]
- 7.Kim B.H., Cho K.I., Spertus J., Park Y.H., Je H.G., Shin M.S., Lee J.H., Jang J.S. Peripheral Artery Questionnaire improves ankle brachial index screening in symptomatic patients with peripheral artery disease. Int. J. Clin. Pract. 2014;68:1488–1495. doi: 10.1111/ijcp.12494. [DOI] [PubMed] [Google Scholar]
- 8.Meijer W.T., Hoes A.W., Rutgers D., Bots M.L., Hofman A., Grobbee D.E. Peripheral arterial disease in the elderly: The rotterdam study. Arterioscler. Thromb. Vasc. Biol. 1998;18:185–192. doi: 10.1161/01.ATV.18.2.185. [DOI] [PubMed] [Google Scholar]
- 9.Feinglass J., McCarthy W.J., Slavensky R., Manheim L.M., Martin G.J. Effect of lower extremity blood pressure on physical functioning in patients with intermittent claudication. J. Vasc. Surg. 1996;24:503–511. doi: 10.1016/S0741-5214(96)70066-6. [DOI] [PubMed] [Google Scholar]
- 10.Newman A.B., Shemanski L., Manolio T.A., Cushman M., Mittelmark M., Polak J.F., Powe N.R., Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 1999;19:538–545. doi: 10.1161/01.ATV.19.3.538. [DOI] [PubMed] [Google Scholar]
- 11.Doobay A.V., Anand S.S. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: A systematic review. Arterioscler. Thromb. Vasc. Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 12.Atkins L.M., Gardner A.W. The relationship between lower extremity functional strength and severity of peripheral arterial disease. Angiology. 2004;55:347–355. doi: 10.1177/000331970405500401. [DOI] [PubMed] [Google Scholar]
- 13.Szuba A., Oka R.K., Harada R., Cooke J.P. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc. Med. 2006;11:155–163. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 14.Safley D.M., House J.A., Laster S.B., Daniel W.C., Spertus J.A., Marso S.P. Quantifying improvement in symptoms, functioning, and quality of life after peripheral endovascular revascularization. Circulation. 2007;115:569–575. doi: 10.1161/CIRCULATIONAHA.106.643346. [DOI] [PubMed] [Google Scholar]
- 15.Hoeks S.E., Smolderen K.G., Scholte O.R.W.J., Verhagen H.J., Spertus J.A., Poldermans D.J. Clinical validity of a disease-specific health status questionnaire: the peripheral artery questionnaire. Vasc. Surg. 2009;49:371–377. doi: 10.1016/j.jvs.2008.08.089. [DOI] [PubMed] [Google Scholar]
- 16.Murphy T.P., Cutlip D.E., Regensteiner J.G., Mohler E.R., Cohen D.J., Reynolds M.R., Massaro J.M., Lewis B.A., Cerezo J., Oldenburg N.C., et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the claudication: Exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiatt W.R., Hirsch A.T., Regensteiner J.G., Brass E.P. Clinical trials for claudication: Assessment of exercise performance, functional status, and clinical end points. Circulation. 1995;92:614–621. doi: 10.1161/01.CIR.92.3.614. [DOI] [PubMed] [Google Scholar]
- 18.Murphy T.P., Soares G.M., Kim H.M., Ahn S.H., Haas R.A. Quality of life and exercise performance after aortoiliac stent placement for claudication. J. Vasc. Interv. Radiol. 2005;16:947–953. doi: 10.1097/01.RVI.0000161140.33944.ED. [DOI] [PubMed] [Google Scholar]
- 19.Bosch J.L., Hunink M.G. Comparison of the Health Utilities Index Mark 3 (HUI3) and the EuroQol EQ-5D in patients treated for intermittent claudication. Qual. Life Res. 2000;9:591–601. doi: 10.1023/A:1008929129537. [DOI] [PubMed] [Google Scholar]
- 20.Taft C., Karlsson J., Gelin J., Jivegard L., Sandstrom R., Arfvidsson B., Dahllöf A.G., Lundholm K., Sullivan M. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients II: One-year results of health-related quality of life. Eur. J. Vasc. Endovasc. Surg. 2001;22:114–123. doi: 10.1053/ejvs.2001.1406. [DOI] [PubMed] [Google Scholar]
- 21.Chetter I.C., Spark J.I., Scott D.J., Kester R.C. Does angioplasty improve the quality of life for claudicants?: A prospective study. Ann. Vasc. Surg. 1999;13:93–103. doi: 10.1007/s100169900226. [DOI] [PubMed] [Google Scholar]
- 22.Patterson R.B., Pinto B., Marcus B., Colucci A., Braun T., Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J. Vasc. Surg. 1997;25:312–318. doi: 10.1016/S0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 23.Spronk S., Bosch J.L., Veen H.F., den Hoed P.T., Hunink M.G. Intermittent claudication: functional capacity and quality of life after exercise training or percutaneous transluminal angioplasty—Systematic review. Radiology. 2005;235:833–842. doi: 10.1148/radiol.2353040457. [DOI] [PubMed] [Google Scholar]
- 24.Ahimastos A.A., Pappas E.P., Buttner P.G., Walker P.J., Kingwell B.A., Golledge J. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J. Vasc. Surg. 2011;54:1511–1521. doi: 10.1016/j.jvs.2011.06.106. [DOI] [PubMed] [Google Scholar]
- 25.Murphy T.P., Reynolds M.R., Cohen D.J., Regensteiner J.G., Massaro J.M., Cutlip D.E., Mohler E.R., Cerezo J., Oldenburg N.C., Thum C.C., et al. Correlation of patient-reported symptom outcomes and treadmill test outcomes after treatment for aortoiliac claudication. J. Vasc. Interv. Radiol. 2013;24:1427–1435. doi: 10.1016/j.jvir.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott M.M., Kibbe M., Guralnik J.M., Pearce W.H., Tian L., Liao Y., Zhao L., Criqui M.H. Comparative effectiveness study of self-directed walking exercise, lower extremity revascularization, and functional decline in peripheral artery disease. J. Vasc. Surg. 2013;57:990–996. doi: 10.1016/j.jvs.2012.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setacci C., de Donato G., Teraa M., Moll F.L., Ricco J.B., Becker F., Robert-Ebadi H., Cao P., Eckstein H.H., de Rango P., et al. Chapter IV: Treatment of critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2011;42:S43–S59. doi: 10.1016/S1078-5884(11)60014-2. [DOI] [PubMed] [Google Scholar]
- 28.McDermott M.M., Criqui M.H., Liu K., Guralnik J.M., Greenland P., Martin G.J., Pearce W. Lower ankle brachial index calculated by averaging the dorsalis pedis and posterior tibial arterial pressures is most closely associated with leg functioning in peripheral arterial disease. J. Vasc. Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.