Abstract
A k-nearest neighbor (k-NN) classification model was constructed for 118 RDT NEDO (Repeated Dose Toxicity New Energy and industrial technology Development Organization; currently known as the Hazard Evaluation Support System (HESS)) database chemicals, employing two acute toxicity (LD50)-based classes as a response and using a series of eight PaDEL software-derived fingerprints as predictor variables. A model developed using Estate type fingerprints correctly predicted the LD50 classes for 70 of 94 training set chemicals and 19 of 24 test set chemicals. An individual category was formed for each of the chemicals by extracting its corresponding k-analogs that were identified by k-NN classification. These categories were used to perform the read-across study for prediction of the chronic toxicity, i.e., Lowest Observed Effect Levels (LOEL). We have successfully predicted the LOELs of 54 of 70 training set chemicals (77%) and 14 of 19 test set chemicals (74%) to within an order of magnitude from their experimental LOEL values. Given the success thus far, we conclude that if the k-NN model predicts LD50 classes correctly for a certain chemical, then the k-analogs of such a chemical can be successfully used for data gap filling for the LOEL. This model should support the in silico prediction of repeated dose toxicity.
Keywords: k-nearest neighbor, classification model, Estate fingerprint, LD50, LOEL, read-across, category formation
1. Introduction
The multiple target effect of toxicants is a significant hurdle for pharmaceutical research and for elucidating toxicity mechanisms [1]. Off-target toxicities are a particular challenge as they are commonly not readily predicted [2]. The extent to which such a target is influenced by a toxicant is also dependent upon its effective concentration [3].
Generally, an LD50 experiment uses a range of toxicant doses spanning from moderate to high when administered to a set of organisms (of the same species and strain). Several mechanisms take place in such an event including off-target and non-specific effects like inflammation, mitochondrial toxicity, liver toxicity, oxidative stress, competitive inhibition of transporters and drug metabolizing enzymes, etc. Together, all of these effects contribute to the result (LD50). On the other hand, in chronic toxicity experiments, the smallest dose administered every day for the time periods of 28 days, 91 days or two years that causes any detectable effect is known as the Lowest Observable Effect Level (LOEL). In order to derive an LOEL value, many effects (such as inflammation, hypothermia, locomotor activity, etc.) and levels of indicators (such as liver enzymes, choline esterase, albumin/globulin ratio, etc.) are recorded in the test animals. As LD50 and LOEL can both be influenced by multiple toxicity mechanisms, we have, in this study, attempted to utilize LD50 values for the prediction of LOEL values.
It is established that chemicals similar in molecular structure often have similar modes of action and thus exhibit similar properties [4]. This fundamental concept has been used to predict biological effects of chemicals by clustering them on the basis of their structural similarity; such a method of prediction is known as “read-across”. Thus, clustering a group of similar chemicals with a well categorized biological profile and then using them to predict biological effects of query chemicals is a powerful approach [5,6,7,8] and such a query chemical, along with its similar chemicals, could be considered as a category.
At present, there is no predefined basis for the acceptance or exclusion of a given chemical from a category [9]. Moreover, there are as yet no standard statistical tests for validation of such a category. To overcome these drawbacks, we used the “k-nearest neighbor (k-NN)” method to build a classification model that identified k neighbors for every chemical in dataset. The training and test set chemicals were considered as “queries” and their corresponding k-neighbors were considered as “analogs”; accordingly, a query together with its k-analogs was considered as a single category. The robustness of this classification model was tested using statistical validation tests. To perform the read-across study, we decided to use categories formed by k-NN classification models, for the following reasons: (1) the optimal validation parameters shall confirm that the categories formed through k-NN classification are robust enough to identify structurally similar k-analogs and (2) further validation of these categories shall be performed through the prediction of a class for each of the query (if such a prediction is correct).
The 3R principle, i.e., to reduce, refine and replace, has been widely accepted as an ethical framework for conducting animal experiments for the purpose of research [10]. In response to this, many in vitro and in silico methods have been adopted to reduce the use of animal experiments in the last few decades. In the case of repeated dose toxicity studies, the use of in vitro methods has been not validated to date [11]. In repeated dose toxicity studies, an endpoint can represent a multitude of biological effects that take place through different mechanisms, occur in different organ tissues, and progress with different time frames. Accordingly, this poses a challenge for quantitative structure-activity relationship (QSAR) modelers and can explain why very few attempts have been made so far to model this endpoint [12]. In this study, we have made an attempt to construct a better model for the prediction of repeated dose toxicity.
The LOEL and LD50 values are typically measured in milligrams per kilograms per day, i.e., milligrams of chemical per kilogram of body weight administered per day. We assumed that if lethal doses (LD50) are in the same range (e.g., LD50 of query and corresponding k-analogs in the range of 1 to 2000 mg/kg/day or in other similar range) or within an order of magnitude in a certain chemical category, it can be possible to predict LOEL of query using LOELs of its k-analogs within that category. To test this hypothesis, we decided to construct k-NN classification models using two classes that are based on the magnitudes of LD50 values and use them as response variables. We then derived k-analogs for the chemicals (queries) within the Repeated Dose Toxicity New Energy and industrial technology Development Organization i.e., RDT NEDO database. When this classification model predicts the correct class of a query, its category can be considered qualified for the further task of read-across study. In the read-across study, LOEL values of queries from the qualified categories will be calculated by taking arithmetic means of LOELs of their respective k-analogs.
2. Results and Discussion
The RDT NEDO database documented 235 effects (refer to Table S1), 41 organ tissues were examined and 11 examination items were recorded on six strains of rats of both genders. The database was then screened for “Examination item-LOEL”, “Effect-Total effect”, “Organ tissue-Whole body”, “Organism-Rat”, “Gender-Male”, “Strain-Crj:CD(SD)” and “Route-Oral (gavage)”.
2.1. k-NN Classification
A classification model is a mathematical relationship between a set of fingerprints and response variables. The k-nearest neighbor (k-NN) method is a standard and sensitive classification technique [13,14,15,16,17,18]. The k-NN algorithm is based on the k-nearest neighbors classification rule described by Hart et al. [19]. In this algorithm, a class of each query is predicted based on the majority class of its closest k-neighbors (e.g., for the category where k = 3, if two of the three analogs are from class 2, then predicted class for the query is class 2). The closest neighbors are identified on the basis of distance matrix. Several methods of distance calculations between queries on the basis of binary data (here, fingerprints) exist to date [20]. We have selected the “Jaccard-Tanimoto” distance method for the calculation of distance matrices [21].
Using the k-NN method, we constructed eight classification models for the respective fingerprint types. Statistical parameters of those models are given in Table 1. After examination of the Non-Error Rate (NER), sensitivity, specificity and class error, we observed that the optimal k-NN classification model was built from Estate fingerprints. The model consisted of 79 Estate fingerprints, was associated with NERcv of 0.74 for the internal set and of 0.81 for the external test set. Selection by fivefold cross validation identified an optimal value of k as equal to 3.
Table 1.
Parameters of eight k-NN classification models.
| Entry | Fingerprint | NER | k | Sensitivity | Specificity | |||
|---|---|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 1 | Class 2 | |||||
| 1 | CDK | Fitting | 0.69 | 9 | 0.77 | 0.61 | 0.61 | 0.77 |
| CV | 0.76 | 9 | 0.84 | 0.68 | 0.68 | 0.84 | ||
| External | 0.74 | 9 | 0.79 | 0.70 | 0.70 | 0.79 | ||
| 2 | Estate | Fitting | 0.75 | 3 | 0.73 | 0.76 | 0.76 | 0.73 |
| CV | 0.74 | 3 | 0.77 | 0.71 | 0.71 | 0.77 | ||
| External | 0.81 | 3 | 0.71 | 0.90 | 0.90 | 0.71 | ||
| 3 | Extended CDK | Fitting | 0.70 | 9 | 0.80 | 0.61 | 0.61 | 0.80 |
| CV | 0.74 | 9 | 0.80 | 0.68 | 0.68 | 0.80 | ||
| External | 0.79 | 9 | 0.79 | 0.80 | 0.80 | 0.79 | ||
| 4 | Graph | Fitting | 0.68 | 3 | 0.75 | 0.61 | 0.61 | 0.75 |
| CV | 0.70 | 3 | 0.79 | 0.61 | 0.61 | 0.79 | ||
| External | 0.76 | 3 | 0.71 | 0.80 | 0.80 | 0.71 | ||
| 5 | Klekoth-Roth | Fitting | 0.68 | 10 | 0.70 | 0.66 | 0.66 | 0.70 |
| CV | 0.77 | 10 | 0.77 | 0.76 | 0.76 | 0.77 | ||
| External | 0.72 | 10 | 0.64 | 0.80 | 0.80 | 0.64 | ||
| 6 | MACCS | Fitting | 0.76 | 7 | 0.79 | 0.74 | 0.74 | 0.79 |
| CV | 0.73 | 7 | 0.77 | 0.68 | 0.68 | 0.77 | ||
| External | 0.72 | 7 | 0.64 | 0.80 | 0.80 | 0.64 | ||
| 7 | Pubchem | Fitting | 0.77 | 1 | 0.80 | 0.74 | 0.74 | 0.80 |
| CV | 0.73 | 1 | 0.77 | 0.68 | 0.68 | 0.77 | ||
| External | 0.77 | 1 | 0.64 | 0.90 | 0.90 | 0.64 | ||
| 8 | Substructure | Fitting | 0.68 | 5 | 0.73 | 0.63 | 0.63 | 0.73 |
| CV | 0.66 | 5 | 0.80 | 0.53 | 0.53 | 0.80 | ||
| External | 0.71 | 5 | 0.71 | 0.70 | 0.70 | 0.71 | ||
The sensitivity of a model represents its ability to correctly recognize a class for a given chemical (query) while specificity characterizes an ability of a particular class to decline chemicals (queries) of all other classes. The Estate fingerprint-based k-NN model has demonstrated a 77% success rate in predicting toxic queries (class 1) and 71% for non-harmful queries (class 2) for the training set. Similarly, this model could predict the toxic queries (class 1) and the non-harmful queries (class 2) with specificity rates of 0.71 and 0.77, respectively. A class error of 0.26 was associated with the training set queries of both the classes (i.e., class 1 and 2). For the test set, the Estate fingerprint-based k-NN model has demonstrated a sensitivity rate of 0.71 and 0.90, and a specificity rate of 0.90 and 0.71 for class 1 and 2 queries, respectively. The class errors associated with this model were 0.19 for the queries of both the classes (i.e., class 1 and 2). This model was able to correctly classify 70 of 94 training set queries and 19 of 24 test set queries. More details are provided in Tables S3 and S4.
Thus, we have confirmed that the Estate fingerprint-based model is the most statistically robust, justifying its use in read-across studies.
2.2. Read-Across for LOEL Prediction
The LOEL predictions of all training set and test set queries are shown in Table 2 and Table 3, respectively, along with the LOELs of their corresponding k-nearest neighbors. The ratio of actual and predicted LOELs has been calculated and is referred to as fold difference (Fold_diff).
Table 2.
Summary of predicted lowest observed effect levels (LOELs) of all training set queries obtained by arithmetic means of LOELs of corresponding k-nearest analogs (3 analogs) from Estate fingerprint based k-NN model.
| Entry | LOEL | Fold_diff | ||||
|---|---|---|---|---|---|---|
| Query | Analog 1 | Analog 2 | Analog 3 | Predicted | ||
| 1 | 30 | 50 | 625 | 1.2 | 225.40 | 7.51 |
| 2 | 50 | 30 | 625 | 1.2 | 218.73 | 4.37 |
| 3 | 200 | 100 | 750 | 150 | 333.33 | 1.67 |
| 4 | 10 | 10 | 250 | 30 | 96.67 | 9.67 |
| 5 | 30 | 20 | 30 | 30 | 26.67 | 1.12 |
| 6 | 70 | 300 | 20 | 20 | 113.33 | 1.62 |
| 7 | 5 | 150 | 200 | 6 | 118.67 | 23.73 |
| 8 | 100 | 200 | 750 | 200 | 383.33 | 3.83 |
| 9 | 150 | 200 | 100 | 30 | 110.00 | 1.36 |
| 10 | 1000 | 1000 | 11 | 100 | 370.33 | 2.70 |
| 11 | 30 | 1000 | 100 | 150 | 416.67 | 13.89 |
| 12 | 0.75 | 5 | 6 | 50 | 20.33 | 27.11 |
| 13 | 30 | 20 | 200 | 10 | 76.67 | 2.56 |
| 14 | 3130 | 1000 | 100 | 600 | 566.67 | 1.84 |
| 15 | 100 | 300 | 750 | 40 | 363.33 | 3.63 |
| 16 | 10 | 10 | 250 | 30 | 96.67 | 9.67 |
| 17 | 30 | 60 | 60 | 50 | 56.67 | 1.89 |
| 18 | 1000 | 30 | 1000 | 1000 | 676.67 | 1.47 |
| 19 | 60 | 30 | 60 | 50 | 46.67 | 1.29 |
| 20 | 600 | 3130 | 160 | 62.5 | 1117.50 | 1.86 |
| 21 | 20 | 30 | 200 | 10 | 80.00 | 4.00 |
| 22 | 750 | 1000 | 100 | 200 | 433.33 | 1.73 |
| 23 | 25 | 30 | 250 | 10 | 96.67 | 3.87 |
| 24 | 200 | 300 | 100 | 750 | 383.33 | 1.92 |
| 25 | 1000 | 3130 | 100 | 100 | 1110.00 | 1.11 |
| 26 | 250 | 200 | 3130 | 70 | 1133.33 | 4.53 |
| 27 | 200 | 30 | 20 | 200 | 83.33 | 2.40 |
| 28 | 300 | 200 | 200 | 100 | 166.67 | 1.80 |
| 29 | 160 | 600 | 1000 | 1000 | 866.67 | 5.42 |
| 30 | 350 | 1000 | 625 | 625 | 750.00 | 2.14 |
| 31 | 60 | 200 | 750 | 40 | 330.00 | 5.50 |
| 32 | 100 | 240 | 250 | 10 | 166.67 | 1.67 |
| 33 | 100 | 200 | 100 | 1000 | 433.33 | 4.33 |
| 34 | 30 | 30 | 30 | 100 | 53.33 | 1.78 |
| 35 | 3130 | 2500 | 10 | 350 | 953.33 | 1.09 |
| 36 | 1000 | 1000 | 11 | 30 | 347.00 | 2.88 |
| 37 | 40 | 30 | 60 | 60 | 50.00 | 1.25 |
| 38 | 100 | 40 | 20 | 30 | 30.00 | 1.11 |
| 39 | 1000 | 750 | 100 | 100 | 316.67 | 1.05 |
| 40 | 300 | 70 | 20 | 250 | 113.33 | 2.65 |
| 41 | 200 | 150 | 100 | 30 | 93.33 | 2.14 |
| 42 | 30 | 100 | 300 | 200 | 200.00 | 6.67 |
| 43 | 30 | 30 | 20 | 30 | 26.67 | 1.12 |
| 44 | 5 | 10 | 3130 | 2500 | 1880.00 | 376.00 |
| 45 | 40 | 100 | 300 | 1000 | 466.67 | 11.67 |
| 46 | 2 | 20 | 30 | 30 | 26.67 | 13.33 |
| 47 | 1.2 | 30 | 625 | 500 | 385.00 | 320.83 |
| 48 | 240 | 100 | 250 | 10 | 120.00 | 2 |
| 49 | 6 | 11 | 100 | 150 | 87.00 | 14.50 |
| 50 | 250 | 100 | 240 | 40 | 126.67 | 1.97 |
| 51 | 11 | 1000 | 6 | 1000 | 668.67 | 60.79 |
| 52 | 2 | 30 | 1000 | 30 | 353.33 | 176.67 |
| 53 | 62.5 | 6 | 600 | 3130 | 1245.33 | 19.93 |
| 54 | 100 | 1000 | 1000 | 300 | 766.67 | 7.67 |
| 55 | 40 | 200 | 100 | 150 | 150.00 | 3.75 |
| 56 | 10 | 200 | 100 | 200 | 166.67 | 16.67 |
| 57 | 20 | 70 | 300 | 1000 | 456.67 | 22.83 |
| 58 | 200 | 300 | 100 | 100 | 166.67 | 1.20 |
| 59 | 300 | 100 | 1000 | 40 | 380.00 | 1.27 |
| 60 | 100 | 1000 | 30 | 1000 | 676.67 | 6.77 |
| 61 | 30 | 30 | 1000 | 0.78 | 343.59 | 11.45 |
| 62 | 20 | 70 | 20 | 300 | 130.00 | 6.50 |
| 63 | 20 | 30 | 30 | 100 | 53.33 | 2.67 |
| 64 | 30 | 100 | 150 | 30 | 93.33 | 3.11 |
| 65 | 500 | 250 | 1.2 | 10 | 87.07 | 1.91 |
| 66 | 200 | 781 | 40 | 60 | 293.67 | 1.47 |
| 67 | 1000 | 1000 | 100 | 240 | 446.67 | 2.23 |
| 68 | 625 | 1.2 | 30 | 60 | 30.40 | 6.85 |
| 69 | 10 | 2500 | 3130 | 1000 | 2210.00 | 221.00 |
| 70 | 2500 | 10 | 3130 | 1000 | 1380.00 | 1.81 |
| 71 | 100 | 100 | 150 | 30 | 93.33 | 1.07 |
| 72 | 60 | 30 | 60 | 50 | 46.67 | 1.29 |
| 73 | 50 | 30 | 60 | 60 | 50.00 | 1.00 |
| 74 | 1000 | 350 | 1000 | 30 | 460.00 | 2.17 |
| 75 | 625 | 625 | 625 | 350 | 533.33 | 1.17 |
| 76 | 0.78 | 350 | 625 | 625 | 533.33 | 683.76 |
| 77 | 40 | 100 | 240 | 250 | 196.67 | 4.92 |
| 78 | 5 | 5 | 10 | 3130 | 1048.33 | 209.67 |
| 79 | 30 | 1.2 | 625 | 500 | 375.40 | 12.51 |
| 80 | 250 | 20 | 100 | 500 | 206.67 | 1.21 |
| 81 | 2 | 30 | 60 | 60 | 50.00 | 25.00 |
| 82 | 250 | 10 | 100 | 240 | 116.67 | 2.14 |
| 83 | 30 | 10 | 10 | 250 | 90.00 | 3.00 |
| 84 | 20 | 100 | 100 | 750 | 316.67 | 15.83 |
| 85 | 6 | 62.5 | 3130 | 70 | 1087.50 | 181.25 |
| 86 | 60 | 781 | 200 | 350 | 443.67 | 7.39 |
| 87 | 6 | 625 | 625 | 15 | 421.67 | 70.28 |
| 88 | 30 | 30 | 60 | 60 | 50.00 | 1.67 |
| 89 | 100 | 625 | 625 | 15 | 421.67 | 4.22 |
| 90 | 100 | 150 | 30 | 200 | 126.67 | 1.27 |
| 91 | 781 | 60 | 200 | 30 | 96.67 | 2.69 |
| 92 | 625 | 625 | 625 | 350 | 533.33 | 1.17 |
| 93 | 15 | 100 | 625 | 6 | 243.67 | 16.24 |
| 94 | 625 | 625 | 625 | 350 | 533.33 | 1.17 |
Table 3.
Summary of predicted LOELs of all test set queries from the Estate fingerprint-based k-NN model.
| Sr. | Query | Analog-1 | LOEL Analog-2 | Analog-3 | Predicted | Fold_diff |
|---|---|---|---|---|---|---|
| 1 | 30 | 30 | 30 | 20 | 26.67 | 1.13 |
| 2 | 30 | 30 | 1000 | 6 | 345.33 | 11.51 |
| 3 | 1.5 | 200 | 5 | 0.75 | 68.58 | 45.72 |
| 4 | 1250 | 750 | 1000 | 100 | 616.67 | 2.03 |
| 5 | 50 | 781 | 60 | 250 | 363.67 | 7.27 |
| 6 | 0.1 | 30 | 10 | 10 | 16.67 | 166.67 |
| 7 | 1000 | 1000 | 1000 | 11 | 670.33 | 1.49 |
| 8 | 20 | 150 | 200 | 6 | 118.67 | 5.93 |
| 9 | 20 | 5 | 10 | 5 | 6.67 | 3.00 |
| 10 | 100 | 250 | 100 | 500 | 283.33 | 2.83 |
| 11 | 110 | 1000 | 1000 | 11 | 670.33 | 6.09 |
| 12 | 1000 | 1000 | 100 | 750 | 616.67 | 1.62 |
| 13 | 33 | 30 | 10 | 10 | 16.67 | 1.98 |
| 14 | 30 | 3130 | 2500 | 200 | 1943.33 | 64.78 |
| 15 | 10 | 781 | 60 | 30 | 290.33 | 29.03 |
| 16 | 300 | 100 | 300 | 40 | 146.67 | 2.05 |
| 17 | 2 | 30 | 30 | 10 | 23.33 | 11.67 |
| 18 | 200 | 350 | 1000 | 1000 | 783.33 | 3.92 |
| 19 | 125 | 10 | 250 | 40 | 100.00 | 1.25 |
| 20 | 50 | 1000 | 100 | 30 | 376.67 | 7.53 |
| 21 | 100 | 350 | 625 | 6 | 327.00 | 3.27 |
| 22 | 10 | 6 | 15 | 10 | 10.33 | 1.03 |
| 23 | 150 | 1000 | 750 | 200 | 650.00 | 4.33 |
| 24 | 4 | 6 | 625 | 625 | 418.67 | 104.67 |
In the case of internal prediction, a comparison of the predicted LOELs for queries with their experimental LOELs revealed that 71 of the 94 queries from the training set have a fold difference less than a factor of 10 (refer to Table 2). A fold difference of more than 100 was observed in only seven cases. Comparison of all queries with their associated nearest three analogs suggests that most often the structural similarity, as reflected in the 79 Estate fingerprints for each query, results in a similar biological response (refer to Tables S5 and S6). Moreover, we have sorted all queries based on correct class prediction by the Estate fingerprints based k-NN model (refer Table S3 for predicted class information); accordingly, two types of categories were identified: (1) Qualified category (in this category, the query class was correctly predicted); and (2) Non-qualified category (in this category, the query class was wrongly predicted).
The Estate fingerprint-based model has found 70 queries in the qualified type and 24 queries in the non-qualified type of category (Table 4).
Table 4.
Training set queries sorted (in qualified and non-qualified categories) based on its k-NN model-based predicted class, and further divided based upon order of magnitude difference.
| Fold_diff# | Number of Queries | Total | |
|---|---|---|---|
| Qualified Category | Non-Qualified Category | ||
| <10 | 54 | 17 | 71 |
| 10–100 | 12 | 4 | 16 |
| >100 | 4 | 3 | 7 |
| Total | 70 | 24 | 94 |
# over of magnitude, fold differences (Fold_diff) < 10, 10–100 and >100.
The comparison of the predicted LOELs and the experimental LOELs of queries showed that 54 of the 70 queries from the qualified type of category and 17 of the 24 queries from the non-qualified type of category have less than one order of magnitude difference (fold_diff < 10).
The LOEL values for 17 of 24 external test set queries were predicted within a factor of 10 from that of the experimental values. Only two of the 24 queries were predicted to have LOEL values that differed by more than 100-fold (Table 3).
Additionally, we have performed an analysis of categories by sorting queries into the two types of categories, i.e., qualified types and non-qualified types. The comparison of the predicted and experimental LOEL of test set queries has shown that 14 of the 19 queries (74%) from the qualified type of category and 3 of the 5 queries (60%) from the non-qualified type of category have a fold difference less than 10 (Table 5).
Table 5.
Test set query categorization (qualified and non-qualified) based on k-NN model-based predicted class, and further divided based upon order of magnitude difference.
| Fold_diff# | Number of Queries | Total | |
|---|---|---|---|
| Qualified Category | Non-Qualified Category | ||
| <10 | 14 | 3 | 17 |
| 10–100 | 4 | 1 | 5 |
| >100 | 1 | 1 | 2 |
| Total | 19 | 5 | 24 |
# over of magnitude, fold differences (Fold_diff) <10, 10–100 and >100.
The 77% (54 of the 70 queries) success rate for training set queries and 74% (14 of the 19 queries) success rate for test set queries, shows that our approach is capable of finding qualified categories from the k-NN classification method to perform a read-across study for a LOEL prediction within an order of magnitude.
Our study revealed that the Estate fingerprint-based k-NN classification model performed well predicting LD50 classes for training and test set queries. The model has predicted correct classes of 89 of 118 queries from the training and the test sets. Moreover, our results showed that if the LD50 query class was predicted correctly by the classification method, then it is more likely that its LOEL would be predicted to within an order of magnitude. Our study well establishes that 68 of 89 (76%) queries (of training and test sets) from the qualified type of category were found to have their LOEL prediction with a fold difference of less than 10.
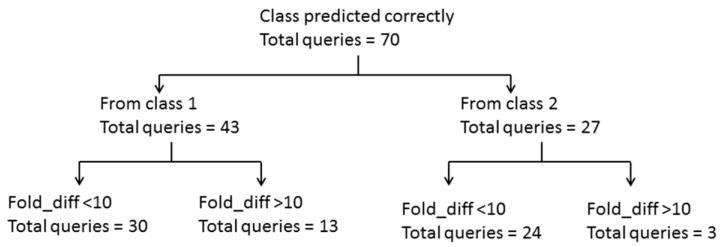
Comparing the predictive power of this model for toxic queries (class 1) from the qualified categories revealed that 43 queries were predicted correctly (Figure 1). The LOEL prediction for 30 of 43 queries was within an order of magnitude. Of the remaining 13 queries, 10 had their LOELs predicted to within 10–100-fold of the experimental value, and the remaining 3 had >100-fold difference. Out of these 13, ten queries (i.e., entries 7, 12, 46, 47, 49, 52, 57, 76, 81 and 87) (Table 2) were extrapolated. Extrapolation is the procedure in read-across where endpoint information from category members at one end of the category is used to predict the endpoint of those members at the other end. These ten queries had the lowest LOEL in their particular categories. Thus, their predicted LOEL was calculated using members of the other side (i.e., the higher LOEL side) in their respective categories, which resulted in values that were too large. The remaining three queries had their LOELs predicted between 10–20 times the experimental values: for entries 61, 79 and 93 (Table 2) ≈ 11, 13 and 16, respectively. Among the 30 queries whose LOELs were predicted within an order of magnitude, entry 55 (in Table 2) was extrapolated, but the LOEL differences among all its analogs were less than 10-fold, and, thus, this query was predicted within an order of magnitude.
Figure 1.
Summary of LOEL prediction for the training set queries from the qualified category.
There were 27 queries in the qualified category that belonged to class 2. Only three queries were predicted with more than a 10-fold difference. The LOEL of entry 53 (in Table 2) was predicted to within a factor of 20 from the experimental value, while the remaining two queries, entries 56 and 69 (Table 2), were extrapolated for their LOEL predictions. As their predicted LOELs were calculated using category analogs of higher LOELs, LOELs of both these entries were thus predicted with more than a 10-fold difference.
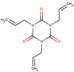
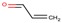
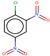
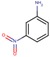
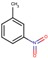
In the case of the test set, out of 19 qualified category queries, ten belonged to class 1 and nine were from class 2. Six out of ten toxic queries (class 1) and eight out of nine non-harmful (class 2) queries were predicted to within an order of magnitude. A total of five queries (four from class 1 and one from class 2) were predicted with more than 10-fold differences, three of them (i.e., entry 15, 17 and 24 (Table 3)) were extrapolated and, thus, their LOELs were predicted with more than 10-fold difference. While the remaining two queries (i.e., entries 2 and 3 (Table 3)) were predicted with a fold difference of 12 and 46, respectively. Further analysis of entry 3 revealed that, in this category, analog 3 (acrolein) is its own metabolite. The entry 3 and its metabolite (acrolein) act mainly by Michael addition to exhibit their toxicity (Table 6). While, analog 2 (triallyl isocynurate) forms iminium ion that acts by SN1 mechanism, whereas analog 1 (1,4-butanediol) forms active metabolite gamma-hydroxy butyric acid, which is CNS depressant. As per toxic hazard classification by Crammer (with extension) [22], the class of hazard for 1,4-butanediol is low while acrolein and triallyl isocynurate have been indicated in the high toxicity class. This explains why this category fails to predict LOEL of entry 3. Our study has correctly predicted entry 22 from test set (Table 6), where all three analogs act with similar mechanism of actions by forming reactive oxygen species [23,24]. The entry 24 was predicted wrongly as LOEL is extrapolated.
Table 6.
Test set query categories with their 3 respective analogs.
| Entry | Data | Query | Analog 1 | Analog 2 | Analog 3 | LOEL Predicted | Fold_diff |
|---|---|---|---|---|---|---|---|
| 3 | Structure | 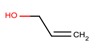 |
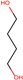 |
 |
 |
||
| LD50 | 64 | 1525 | 1000 | 26 | |||
| LOEL | 1.5 | 200 | 5 | 0.75 | 68.58 | 45.72 | |
| 22 | Structure | 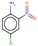 |
 |
 |
 |
||
| LD50 | 400 | 640 | 535 | 256 | |||
| LOEL | 10 | 6 | 15 | 10 | 10.33 | 1.03 | |
| 24 | Structure |  |
 |
 |
 |
||
| LD50 | 953 | 640 | 891 | 1072 | |||
| LOEL | 4 | 6 | 625 | 625 | 418.67 | 104.67 |
2.3. Mechanistic Interpretation
Our model has correctly predicted the classes of queries of specific structural scaffolds such as nitrobenzene, aniline, halogenated hydrocarbons from class 1 (toxic). The influence of substituent electronic effects is represented by the Estate fingerprints [25]. The Estate fingerprint “ddsN” represents the nitro group, “aaCH” represents aromatic carbons, fingerprint “sNH2”, “aaCH:, and “sCl” collectively represent aniline derivatives and fingerprints “ssCH2”, “sCH3”, “sF”, “sCl”, “sBr” and “sI” collectively represent halogenated hydrocarbons. The nitro aromatics and aniline derivatives are known to form reactive oxygen species (ROS) which can lead to oxidative stress and electrophilic adduct formation with tissue proteins [23]. Halogenated hydrocarbons act by SN2 electrophilic reaction to form adduct with DNA or proteins [26].
The correctly predicted class 2 queries are aliphatic alcohols and methacrylate esters. The Estate fingerprints “sOH”, “ssCH2”and “sCH3” collectively represent aliphatic alcohols and fingerprints “dCH2”, “dO” and “ssO” collectively represent methacrylate esters. Most of the alcohols are metabolized by the enzyme alcohol dehydrogenase to form either inactive or active metabolites. It has been shown in the literature that LD50 of methacrylates was related to lipophilicity [27], and they act as Michael acceptors [28].
2.4. Comparison with Previously Published Models for Repeated Dose Toxicity Prediction
Other models for repeated dose toxicity endpoints are listed in Table 7. Comparing our study results with previous published models for LOEL endpoints, our model has shown better predictive power than studies published by De Julian-Ortiz et al. [29], Mazzatorta et al. [30] and Gadaleta et al. [24]. The Sakuratani et al. [31] study had only categorized chemicals into 33 chemical categories, while in our study we formed new categories for each of the chemicals to facilitate better prediction of their LOELs. The study performed by Mumtaz et al. [32] used 234 chemicals for construction of the QSAR model, but authors did not confirm the predictive power of this model using an external test set, thus it is not possible to compare our results with this model. The Garcia-Domenech et al. [33] study has shown slightly better predictive power than our model, but authors have used Integrated Testing Strategy (ITS), which is computationally time expensive. Our study is advantageous in comparison to other previous studies, since we have used 2D fingerprints that are fast and easy to calculate by a freely available computer program [34]. Our study has also not incorporated any difficult methods of descriptor selection that would have made this task more cumbersome and time consuming.
Table 7.
Literature survey of QSAR models for prediction of repeated dose toxicity endpoint.
| Method | Training Set Chemicals | Test Set Chemicals | Training Set Prediction | Test Set Prediction | Comment | Reference |
|---|---|---|---|---|---|---|
| Multivariate analysis | 234 | none | 95% within factor of 5 | none | No external prediction | [32] |
| MLR | 234 | none | R2 = 0.52 | none | [29] | |
| MLR | 86 | 16 | R2 = 0.79 | R2 = 0.71 | [33] | |
| PLS | 445 | none | R2 = 0.54 | none | No external prediction | [30] |
| Read-across | 500 | none | none | none | 33 chemical categories formed | [31] |
| k-NN | 254 | 179 | q2 = 0.63 | R2 = 0.54 | [24] |
Furthermore, this is the novel category-approach that has taken into consideration the acute toxicity information (LD50 based classes) for predicting LOELs of queries in their respective categories. There are published models that have used acute toxicity data for the prediction of chronic toxicity data. Kenega [35] introduced the concept of acute/chronic ratios (ACRs). Subsequently, Rand et al. [36] derived ACRs by dividing the acute measure for a particular organism by its chronic measure. Kumar et al. [37] have developed linear regression of LogLC50 against inverse of exposure time (log-inverse method), the intercept of the regression was then used to estimate chronic toxicity. All these approaches only relied on biological endpoints and no theoretical information (description of chemicals) was taken into account for predicting chronic toxicity data.
While in our study we have not directly used LD50 to predict LOELs of chemicals, we have instead formed LD50-based classes to identify k-neighbors for each chemical using k-NN method. Then, we have incorporated fingerprints that describe the molecular structure of chemicals. Subsequently, quantitative structural activity relationships were found among all the training set chemicals with the two classes (i.e., toxic and non-harmful) by means of k-NN algorithms. Finally, LOELs of chemicals have been calculated by taking arithmetic mean of LOELs of their respective k-analogs, provided that their LD50 based classes have been correctly predicted.
2.5. Toxicological Significance
The significance of this study is supported by the notable relationship found between different mechanisms of acute (LD50) and chronic toxicity (LOEL), e.g., the acute toxicity effect of liver toxicity is well explained by some of the chronic toxicity effects such as liver serum indicator and liver hypertrophy. Similarly, the mitochondrial toxicity is explained by hypothermia; the kidney toxicity is explained by creatinine, chloride, and serum protein levels as well as urine volume; the locomotor activity is explained by choline esterase level, etc.
It has been observed that the Estate fingerprints-based model has identified structurally similar k-analogs for queries. The comparison of their structures revealed that they could exhibit similar modes of actions, e.g., the category for entry 22 has revealed that the query along with three analogs could possibly form reactive oxygen species, and it is very likely that they will react towards similar receptors for exhibiting their toxic actions, while in some cases, our approach has failed to derive structurally similar k-analogs for the query. In those categories, all members do not follow similar modes of actions (e.g., entry 3), and thus LOEL predictions can’t be performed.
3. Experimental Section
3.1. Software and Modules
The classification_toolbox Matlab module developed at the Milano Chemometrics and QSAR Research Group, University of Milan, Italy [38] was employed for the development of k-NN classification model. The classification_toolbox Matlab module is freely available at: http://michem.disat.unimib.it/chm/download/classificationinfo.htm.
3.2. Setting of the Dataset
The New Energy and Industrial Technology Development Organization (NEDO) 2007–2010 employed a database of chemicals for repeated dose toxicity endpoint in the development of the Hazard Evaluation Support System (HESS) integrated platform [39]. This database was incorporated in the OECD QSAR toolbox version 2.2 [40,41]. The 279 substances were retrieved from the RDT NEDO database using the OECD QSAR toolbox 2.2. These substances were each authenticated with respect to structure, IUPAC name and CAS registry number (RN). The SMILES notations of incorrectly assigned substances were corrected and missing SMILES notations were retrieved by using ChemSpider (http://www.chemspider.com/) [42], PubChem (http://pubchem.ncbi.nlm.nih.gov) [43] and SigmaAldrich (http://www.sigmaaldrich.com) [44]. Salts and mixtures were excluded from the dataset, as was a single chemical containing a fluorenone ring due to the lack of bulky polycyclic structures in our dataset. The resulting data set was comprised of 224 chemicals and their respective LOEL values (organism-rat, route-oral).
Acute toxicity (LD50) values (organism-rat, route-oral) for 134 of the 224 chemicals were found using the Toxnet (http://toxnet.nlm.nih.gov/index.html) [45] web server. Among those 134 chemicals, 16 were found to have LOEL values larger than LD50 values and were thus discarded from the dataset, as this implied the presence of a fundamental problem with the data underlying these 16 particular chemicals. The LOEL values for 118 chemicals were obtained by assays of varied duration (such as 28, 42, 44, 46, 49, 56, 90, 91 and 98 days, as summarized in Table S2). We included data from all assays for completeness. The selected 118 chemicals (refer to Table S2) were then classified into one of the two classes (toxic and non-harmful) using the Globally Harmonized Scheme (GHS) [46], see Table 8. These 118 chemicals were randomly divided into a training set (94 chemicals, ≈80%) and test set (24 chemicals, ≈20%) based on the principle of keeping 80% chemicals from each class in to a training and 20% chemicals from each class in to a test set.
Table 8.
Classifications of the 118 chemicals in the training and test sets prior to k-NN model construction.
| Description | LD50 (mg/kg/day) | Number of Entries | Training Set Entries | Test Set Entries | |
|---|---|---|---|---|---|
| Class 1 | Highly toxic, toxic and harmful | ≤2000 | 70 | 56 | 14 |
| Class 2 | Non-harmful | >2000 | 48 | 38 | 10 |
3.3. Fingerprint Calculations
Eight types of fingerprints were employed for the development of classification models. These fingerprints were calculated using the PaDEL software [34]. The PaDEL software calculates fingerprints mainly using the Chemistry Development Kit [47]. In addition, it has incorporated additional fingerprints that include atom type electro-topological state descriptors, binary fingerprints and chemical substructures count identified by Klekotha and Roth. We considered eight types of fingerprints and those are: Estate (length-79), CDK (length-1024), Extended CDK (length-1024), CDK Graph (length-1024), Pubchem (length-881), MACCS (length-166), Substructural (length-307) and Klekotha-Roth (length-4860). Each of the eight types of fingerprints was used separately to construct a classification model.
3.4. Development of the Classification Model
The “Jaccard-Tanimoto” distance method for calculation of distance matrices was employed for chemical classification [21]. In k-NN, the k stands for the number of neighbors to be considered. Thus, while applying k-NN algorithm, the optimal value of k needs to be determined. We have used cross validation to determine the optimal number of nearest neighbors (k), where a series of k values was assigned (from k = 1 to 10); based on lowest class error, an optimal k value was identified. The fivefold cross validation was implemented. Four groups were used for testing the class membership of the omitted group, where the class of the majority of k neighbors was assigned to the member of the omitted group. The k-NN method provided a final output for all eight types of fingerprints. All these models were later validated using the external test set.
3.5. External Validation
An external validation demonstrates the true predictability of a model. The test set of 24 chemicals, which were not considered for the model calibration, was used for an external validation of the model. Several validation parameters were studied to evaluate an optimum model such as non-error rate (NER), sensitivity, specificity and class error.
3.6. Model Selection and Read-Across
The parameters for the internal and external validations were used in order to identify the most robust model, which was used in subsequent read-across studies. We have considered all training and test set chemicals as “queries”. By applying the k-NN approach, k-neighbors were identified for every query; each was called as its “analog”. A particular query with its corresponding k-analogs was considered as a single category. To predict the LOELs of each query in its category, we took the arithmetic mean of the LOELs of all the k-neighbors of each query.
4. Conclusions
A recent report from the European Chemical Agency (ECHA) has highlighted the potential of the “read-across” method to fill toxicological information data gaps [48]. At present, there is no existing rule or criteria for the acceptance or elimination of analogs from a category that is needed for read-across studies. There are also no rules for the validation of a category [9] since LD50 data can be used in the setting of dose levels for chronic toxicity studies [49]. Both endpoints are also influenced by multiple mechanisms including off-target and non-specific effects. Thus, we suggest a new approach for supporting the acceptance of a category for the execution of read-across, i.e., if the classification model could predict correctly the class of query (toxic or non-harmful, based on LD50 values) by means of a k-NN approach, then such a correctly predicted query and its corresponding k-analogs can be used to perform a read-across study for the prediction of LOEL of a query.
Thus, we have successfully demonstrated the applicability of a read-across–k-NN coupled strategy for the prediction of repeated dose toxicity (LOEL) using acute toxicity (LD50) based classes. This approach should provide researchers with a tool to fill data gaps and allow the prediction of sub-chronic or chronic toxicity. This study should benefit computational toxicology, pharmacologists and risk assessors for carrying out read-across studies for the prediction of toxicological endpoints. Ultimately, this novel read-across–k-NN coupled strategy should contribute to a reduction in the number of animals used for chronic toxicity testing.
Acknowledgments
This work has been supported by the EU FP-7 Environmental Chemoinformatics project (Grant number 238701) and Linnaeus University-Sweden.
We sincerely thank Roberto Todeschini (Chemometrics and QSAR research group, University of Milan, Italy) for sharing the classification_toolbox Matlab routines for k-NN model development. The authors are thankful to Vijay More (National Institute of Environmental Health Sciences, NC, USA), Murli Mishra (University of Kentucky, USA) and Abhijit Shinde (Katholieke Universiteit Leuven, Belgium) for their valuable comments and explanations about off-target non-specific toxicity.
Abbreviations
- CAS
Chemical Abstracts Service
- CDK
Chemistry Developmental Kit
- CV
Cross Validation
- GHS
Globally Harmonized Scheme
- IUPAC
International Union of Pure and Applied Chemistry
- k-NN
k Nearest Neighbor
- LOEL
Lowest Observed Effect Level
- MACCS
Molecular ACCess System
- NEDO
New Energy and industrial technology Development Organization
- NER
Non-Error Rate
- OECD
Organization for Economic Cooperation and Development
- QSAR
Quantitative Structure-Activity Relationship
- RDT
Repeated Dose Toxicity
- SMILES
Simplified Molecular-Input Line-Entry System
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/16/05/11659/s1.
Author Contributions
The study design was performed by Swapnil Chavan and Ian A. Nicholls. Swapnil Chavan was responsible for data collection and model development. All authors contributed to the data analysis and towards preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Anighoro A., Bajorath J.R., Rastelli G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014;57:7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 2.Stegmeier F., Warmuth M., Sellers W., Dorsch M. Targeted cancer therapies in the twenty-first century: Lessons from imatinib. Clin. Pharmacol. Ther. 2010;87:543–552. doi: 10.1038/clpt.2009.297. [DOI] [PubMed] [Google Scholar]
- 3.Abassi Y.A., Xi B., Zhang W., Ye P., Kirstein S.L., Gaylord M.R., Feinstein S.C., Wang X., Xu X. Kinetic cell-based morphological screening: Prediction of mechanism of compound action and off-target effects. Chem. Biol. 2009;16:712–723. doi: 10.1016/j.chembiol.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keiser M.J., Setola V., Irwin J.J., Laggner C., Abbas A.I., Hufeisen S.J., Jensen N.H., Kuijer M.B., Matos R.C., Tran T.B. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enoch S. Springer; Dordrecht, The Netherlands: 2010. Chemical Category Formation and Read-Across for the Prediction of Toxicity; pp. 209–219. [Google Scholar]
- 6.Patlewicz G., Simon T.W., Rowlands J.C., Budinsky R.A., Becker R.A. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regul. Toxicol. Pharmacol. 2015;71:463–477. doi: 10.1016/j.yrtph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 7.European Chemical Agency. (Helsinki, Finland). Grouping of substances and read-across approach. [(accessed on 17 March 2015)]. Available online: http://echa.europa.eu/documents/10162/13628/read_across_introductory_note_en.pdf.
- 8.Patlewicz G., Ball N., Booth E.D., Hulzebos E., Zvinavashe E., Hennes C. Use of category approaches, read-across and (Q)SAR: General considerations. Regul. Toxicol. Pharmacol. 2013;67:1–12. doi: 10.1016/j.yrtph.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.De Raat K., Netzava T. Assessment of read-across in ECHA, European Chemical Agency. [(accessed on 18 February 2015)]. Available online: http://echa.europa.eu/documents/10162/5649897/ws_raa_20121003_assessment_of_read-across_in_echa_de_raat_en.pdf.
- 10.Russell W.M.S., Burch R.L., Hume C.W. The Principles of Humane Experimental Technique. Methuen; London, UK: 1959. pp. 1–238. [Google Scholar]
- 11.Spielmann H., Sauer U.G., Mekenyan O. A critical evaluation of the 2011 ECHA reports on compliance with the REACH and CLP regulations and on the use of alternatives to testing on animals for compliance with the REACH regulation. Altern. Lab. Anim. 2011;39:481–493. doi: 10.1177/026119291103900509. [DOI] [PubMed] [Google Scholar]
- 12.Lapenna S., Fuart-Gatnik M., Worth A. Joint Research Centre; Ispra, Italy: [(accessed on 17 March 2015)]. Review of QSAR models and software tools for predicting acute and chronic systemic toxicity. Available online: http://s-ihcpiprwb002p.jrc.it/our_labs/eurl-ecvam/laboratories-research/predictive_toxicology/doc/EUR_24639_EN.pdf. [Google Scholar]
- 13.Kowalski B., Bender C. k-Nearest Neighbor Classification Rule (pattern recognition) applied to nuclear magnetic resonance spectral interpretation. Anal. Chem. 1972;44:1405–1411. doi: 10.1021/ac60316a008. [DOI] [Google Scholar]
- 14.Hellman M.E. The nearest neighbor classification rule with a reject option. IEEE Trans. Syst. Sci. Cybern. 1970;6:179–185. doi: 10.1109/TSSC.1970.300339. [DOI] [Google Scholar]
- 15.Patrick E.A., Fischer Iii F.P. A generalized k-nearest neighbor rule. Inf. Control. 1970;16:128–152. doi: 10.1016/S0019-9958(70)90081-1. [DOI] [Google Scholar]
- 16.Jeng-Shyang P., Yu-Long Q., Sheng-He S. A fast k-nearest neighbors classification algorithm. IEICE Trans. Fundam. Electron. 2004;87:961–963. [Google Scholar]
- 17.Chavan S., Nicholls I.A., Karlsson B.C., Rosengren A.M., Ballabio D., Consonni V., Todeschini R. Towards global QSAR model building for acute toxicity: Munro database case study. Int. J. Mol. Sci. 2014;15:18162–18174. doi: 10.3390/ijms151018162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todeschini R. k-nearest neighbour method: The influence of data transformations and metrics. Chemom. Intell. Lab. Syst. 1989;6:213–220. doi: 10.1016/0169-7439(89)80086-3. [DOI] [Google Scholar]
- 19.Cover T., Hart P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory. 1967;13:21–27. doi: 10.1109/TIT.1967.1053964. [DOI] [Google Scholar]
- 20.Zhang B., Srihari S.N. Properties of binary vector dissimilarity measures. [(accessed on 18 February 2015)]. Available online: http://www.cedar.buffalo.edu/papers/articles/CVPRIP03_propbina.pdf.
- 21.Cheetham A.H., Hazel J.E. Binary (presence-absence) similarity coefficients. J. Paleontol. 1969;43:1130–1136. [Google Scholar]
- 22.Cramer G., Ford R., Hall R. Estimation of toxic hazard—A decision tree approach. Food Chem. Toxicol. 1976;16:255–276. doi: 10.1016/S0015-6264(76)80522-6. [DOI] [PubMed] [Google Scholar]
- 23.Nelson S.D. Metabolic activation and drug toxicity. J. Med. Chem. 1982;25:753–765. doi: 10.1021/jm00349a001. [DOI] [PubMed] [Google Scholar]
- 24.Gadaleta D., Pizzo F., Lombardo A., Carotti A., Escher S.E., Nicolotti O., Benfenati E. A k-NN algorithm for predicting oral sub-chronic toxicity in the rat. Altex. 2014;31:423–432. doi: 10.14573/altex.1405091s. [DOI] [PubMed] [Google Scholar]
- 25.Hall L.H., Kier L.B. Electrotopological state indices for atom types: A novel combination of electronic, topological, and valence state information. J. Chem. Inf. Comput. Sci. 1995;35:1039–1045. doi: 10.1021/ci00028a014. [DOI] [Google Scholar]
- 26.Enoch S.J., Cronin M.T.D., Schultz T.W., Madden J.C. Quantitative and mechanistic read across for predicting the skin sensitization potential of alkenes acting via Michael addition. Chem. Res. Toxicol. 2008;21:513–520. doi: 10.1021/tx700322g. [DOI] [PubMed] [Google Scholar]
- 27.Yoshii E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. A. 1997;37:517–524. doi: 10.1002/(SICI)1097-4636(19971215)37:4<517::AID-JBM10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Freidig A.P., Verhaar H.J., Hermens J.L. Comparing the potency of chemicals with multiple modes of action in aquatic toxicology: Acute toxicity due to narcosis versus reactive toxicity of acrylic compounds. Environ. Sci. Technol. 1999;33:3038–3043. doi: 10.1021/es990251b. [DOI] [Google Scholar]
- 29.De Julian-Ortiz J., Garcia-Domenech R., Galvez J., Pogliani L. Predictability and prediction of lowest observed adverse effect levels in a structurally heterogeneous set of chemicals. SAR QSAR Environ. Res. 2005;16:263–272. doi: 10.1080/10659360500036927. [DOI] [PubMed] [Google Scholar]
- 30.Mazzatorta P., Estevez M.D., Coulet M., Schilter B. Modeling oral rat chronic toxicity. J. Chem. Info. Model. 2008;48:1949–1954. doi: 10.1021/ci8001974. [DOI] [PubMed] [Google Scholar]
- 31.Sakuratani Y., Zhang H., Nishikawa S., Yamazaki K., Yamada T., Yamada J., Gerova K., Chankov G., Mekenyan O., Hayashi M. Hazard Evaluation Support System (HESS) for predicting repeated dose toxicity using toxicological categories. SAR QSAR Environ. Res. 2013;24:351–363. doi: 10.1080/1062936X.2013.773375. [DOI] [PubMed] [Google Scholar]
- 32.Mumtaz M., Knauf L., Reisman D., Peirano W., Derosa C., Gombar V., Enslein K., Carter J., Blake B., Huque K. Assessment of effect levels of chemicals from quantitative structure-activity relationship (QSAR) models. I. Chronic lowest-observed-adverse-effect level (LOAEL) Toxicol. Lett. 1995;79:131–143. doi: 10.1016/0378-4274(95)03365-R. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Domenech R., De Julian-Ortiz J., Besalu E. True prediction of lowest observed adverse effect levels. Molec. Divers. 2006;10:159–168. doi: 10.1007/s11030-005-9007-z. [DOI] [PubMed] [Google Scholar]
- 34.Yap C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comp. Chem. 2011;32:1466–1474. doi: 10.1002/jcc.21707. [DOI] [PubMed] [Google Scholar]
- 35.Kenaga E.E. Predictability of chronic toxicity from acute toxicity of chemicals in fish and aquatic invertebrates. Environ. Toxicol. Chem. 1982;1:347–358. doi: 10.1002/etc.5620010410. [DOI] [Google Scholar]
- 36.Rand G.M., Petrocelli S.R. Fundamentals of aquatic toxicology: Methods and applications. FMC Corp., Princeton, NJ; Washington DC, USA: 1985. pp. 1–666. [Google Scholar]
- 37.Kumar A., Correll R., Grocke S., Bajet C. Toxicity of selected pesticides to freshwater shrimp, Paratya australiensis (Decapoda: Atyidae): Use of time series acute toxicity data to predict chronic lethality. Ecotoxicol. Environ. Saf. 2010;73:360–369. doi: 10.1016/j.ecoenv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Ballabio D., Consonni V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods. 2013:3790–3798. doi: 10.1039/c3ay40582f. [DOI] [Google Scholar]
- 39.National Institute of Technology and Evaluation. Hazard Evaluation Support System Integrated Platform. 2012. [(accessed on 13 March 2015)]. http://www.nite.go.jp/en/chem/qsar/hess-e.html.
- 40.The OECD QSAR toolbox. [(accessed on 17 March 2017)]. http://www.qsartoolbox.org/
- 41.The OECD QSAR toolbox version 2.2 release note. [(accessed on 17 March 2017)]. http://www.oecd.org/chemicalsafety/risk-assessment/48413109.pdf.
- 42.Pence H.E., Williams A. ChemSpider: An online chemical information resource. J. Chem. Educ. 2010;87:1123–1124. doi: 10.1021/ed100697w. [DOI] [Google Scholar]
- 43.Wang Y., Xiao J., Suzek T.O., Zhang J., Wang J., Bryant S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucl. Acids Res. 2009;37:623–633. doi: 10.1093/nar/gkp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenga R.E., Votoupal K.L. The Sigma-Aldrich library of regulatory and safety data. [(accessed on 18 February 2015)]. https://www.sigmaaldrich.com/
- 45.Wexler P. TOXNET: An evolving web resource for toxicology and environmental health information. Toxicology. 2001;157:3–10. doi: 10.1016/S0300-483X(00)00337-1. [DOI] [PubMed] [Google Scholar]
- 46.United Nations . Geneva, Switzerland: 2011. [(accessed on 17 March 2015)]. Globally harmonized system of classification and labelling of chemicals (GHS) http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf. [Google Scholar]
- 47.Steinbeck C., Han Y., Kuhn S., Horlacher O., Luttmann E., Willighagen E. The Chemistry Development Kit (CDK): An open-source Java library for chemo-and bioinformatics. J. Chem. Inform. Comput. Sci. 2003;43:493–500. doi: 10.1021/ci025584y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dancet G. European Chemical Agency; [(accessed on 18 February 2015)]. The use of alternatives to testing on animals for the REACH regulation; pp. 1–64. Available online: http://echa.europa.eu/documents/10162/13639/alternatives_test_animals_2014_en.pdf. [Google Scholar]
- 49.Stephens M.L., Fowle J., Boverhof D., Richmond J., Recio L., Zhu H., Alepee N., Curren R., Basketter D., Natsch A. Reducing, Refining and Replacing the Use of Animals in Toxicity Testing. Royal Society of Chemistry; Cambridge, UK: 2013. pp. 1–347. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.