Abstract
Abstract
Aim
To summarize findings from studies reporting the prevalence and incidence of diabetic retinopathy and diabetic maculopathy in African countries in light of the rising prevalence of diabetes mellitus.
Methods
Using a predefined search strategy, we systematically searched MEDLINE, EMBASE, Science Citation index and Conference Proceedings Citation index, African Index Medicus and the grey literature database ‘OpenSIGLE’ for studies published between January 1990 and February 2011. Included studies reported prevalence or incidence of diabetic retinopathy or diabetic maculopathy of subjects with diabetes resident in African countries.
Results
Sixty-two studies from 21 countries were included: three population-based surveys; two cohort studies; five case–control studies; 32 diabetes clinic-based, nine eye clinic-based and 11 other hospital-based surveys. Included studies varied considerably in terms of patient selection, method of assessing the eye and retinopathy classification. In population-based studies, the reported prevalence range in patients with diabetes for diabetic retinopathy was 30.2 to 31.6%, proliferative diabetic retinopathy 0.9 to 1.3%, and any maculopathy 1.2 to 4.5%. In diabetes clinic-based surveys, the reported prevalence range for diabetic retinopathy was 7.0 to 62.4%, proliferative diabetic retinopathy 0 to 6.9%, and any maculopathy 1.2 to 31.1%. No obvious association between prevalence and income level of the country was detected.
Conclusions
Large, community-based cross-sectional and cohort studies are needed to investigate rates and determinants of prevalence of diabetic retinopathy, incidence and progression in Africa. Consensus is needed on the most appropriate methods of identification and classification of retinopathy for research and clinical practice. Estimates of prevalence of diabetic retinopathy, proliferative diabetic retinopathy and maculopathy are comparable with recent European and American studies.
Introduction
The International Diabetes Federation (IDF) has estimated that the number of adults with diabetes in Africa will expand by 98%, from 12.1 million in 2010 to 23·9 million in 2030 [1]—a consequence of urbanization, sedentary lifestyles, obesity, and population growth and ageing (in part as a result of successes in combating communicable diseases) [2]. Thirty-one of the 48 least economically developed countries, as defined by the United Nations, are situated in Africa [3]. The epidemic rise in diabetes therefore poses significant public health and socio-economic challenges for the continent.
Diabetes causes visual impairment through cataract and diabetic retinopathy, a progressive disease of the retinal microvasculature. Diabetic retinopathy can be broadly divided into two clinical categories: non-proliferative and proliferative diabetic retinopathy. The pathophysiology of non-proliferative diabetic retinopathy is characterized by abnormal permeability of retinal capillaries leading to retinal oedema, and closure of capillaries leading to retinal non-perfusion and ischaemia. Diabetic maculopathy occurs when these processes affect the macula and are therefore a threat to visual functioning. Clinically significant macular oedema (CSMO) is a term from the Early Treatment of Diabetic Retinopathy Study (ETDRS) [4] and is an evidence-based threshold for laser photocoagulation treatment.
Proliferative diabetic retinopathy occurs when retinal ischaemia is sufficiently severe to lead to the formation of new vessels. Visual loss occurs in proliferative diabetic retinopathy when these vessels bleed, or tractional retinal detachment ensues from fibrovascular proliferation. Without treatment, 50% of patients with proliferative diabetic retinopathy will become blind within 5 years [5]. Diabetic retinopathy can be graded on the basis of the clinical features. The grades of retinopathy correlate with likelihood of development of proliferative diabetic retinopathy and can be standardized by standard retinal photographs, as used in the Early Treatment of Diabetic Retinopathy Study [4]. The aim of this systematic review was to summarize findings from reliable research studies of estimates of the prevalence and incidence of diabetic retinopathy and maculopathy in African countries.
Methods
Data sources and search strategy
A systematic narrative review of published literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6]. Relevant studies published between 1948 and February 2011 were identified by searching, using a predefined strategy, the following electronic databases: MEDLINE (PubMed), EMBASE (OVID) and EMBASE Classic, Science Citation index and Conference Proceedings Citation index (ISI Web of Science). The following were also searched: the African regional database ‘African Index Medicus’, the grey literature database ‘OpenSIGLE’, the World Health Organization (WHO) International Clinical Trials Registry and the meta-Register of Controlled Trials (mRCT). Customized searches were developed by one of the authors (PIB) in conjunction with a Cochrane Collaboration-trained trials coordinator. Search histories are reproduced in the Supporting Information (Appendix S1). No language, publication status, time limits or language restrictions were applied to electronic searches. Search results were merged using reference management software (Endnote, Thomson Reuters) and duplicate records removed. The reference lists of articles identified, including existing reviews, were hand-searched.
Selection criteria
The following were included: studies reporting prevalence or incidence or progression of diabetic retinopathy or diabetic maculopathy; cross-sectional or cohort study design; studies of subjects with diabetes mellitus resident in African countries. Exclusion criteria were: studies with fewer than 50 subjects; studies of populations of African origin residing outside the continent; reports not published in English; case series and conference abstracts. To improve the current relevance of the review those reports published before 1990 were excluded.
The method used to apply selection criteria was as follows. Titles and abstracts were examined by one investigator (PIB) and obviously irrelevant reports removed. Full text copies of the potentially relevant reports were retrieved. Multiple reports of the same study were linked together. Full-text reports were examined independently by two investigators (PIB and IJCM) for compliance with eligibility criteria. Disagreements were resolved by discussion.
Data extraction and assessment of risk of bias
Major outcome variables were extracted independently by two investigators (PIB and IJCM) into a spreadsheet (Excel, Microsoft) with a standardized approach. Any disagreement was resolved by discussion. The main outcome variables extracted were the prevalence of diabetic retinopathy, proliferative diabetic retinopathy and diabetic maculopathy and the incidence of diabetic retinopathy, proliferative diabetic retinopathy and diabetic maculopathy. Prevalence of grades of retinopathy were recorded by patient according to the worse eye and, unless stated, are presented as such below. Studies were stratified by the source of the population sample (with community studies more likely to give a more accurate population-based assessment of prevalence); and risk of bias was assessed by seeking evidence of incomplete outcome data (missing data, patients excluded from report, patients lost to follow-up in cohort studies).
Results
The literature search yielded 380 citations, of which 142 were reviewed in full text; 71 met the inclusion criteria and reported on a total of 62 studies (Fig. 1) [7–60], and see also Supporting Information (Appendix S2 [82–98]). Literature search report reproduced in the Supporting Information (Table S1).
Figure 1.
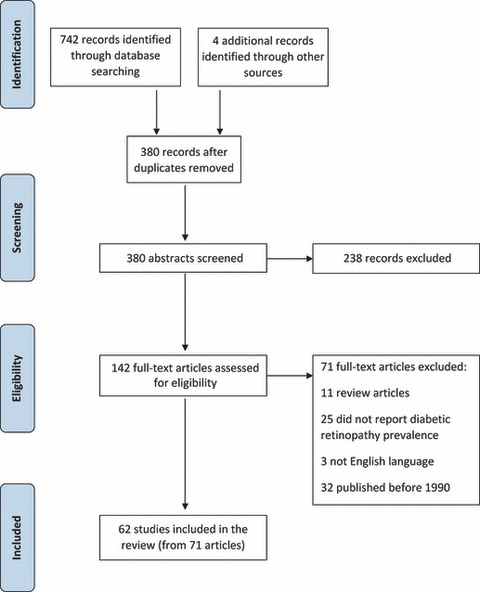
Identification process for eligible studies. Format reproduced from the PRISMA statement [6].
Characteristics of included studies
Characteristics of the included studies are summarized in the Supporting Information (Table S2).
Design
Only three community-based studies were identified [7–9]. In Mauritius, in 1998, researchers followed up the population-based study performed in 1992 [7] with a survey of the same cohort 6 years later [10]. An additional cohort study followed a group of patients with Type 1 diabetes identified from a hospital clinic [11–13]. All other studies were clinic-based surveys or case–control studies; the majority were undertaken in diabetes clinics (hospital or primary care) or hospital ophthalmology clinics.
Distribution
The 62 studies were performed in 21 countries. Geographical distribution of studies was uneven and, within geographical regions, certain countries were over-represented. All of 19 studies undertaken in Western Africa took place in Nigeria, except one that covered Nigeria and Ghana [14] and one from Mali [15]. Within East Africa, two studies were conducted in the Seychelles [16,17] and two in Mauritius [7,10]: relatively wealthy, ethnically diverse, small island nations. There was no clear correlation between the average standard of living in a country, as measured by per capita gross domestic product (GDP) and reported prevalence of diabetic retinopathy (Fig. 2) or proliferative diabetic retinopathy (see also Supporting Information, Fig. S1). Only five studies specifically reported data from rural populations [7–9,18,19].
Figure 2.
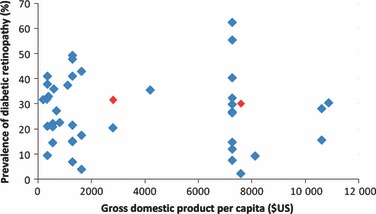
Prevalence of diabetic retinopathy in patients with diabetes according to national per capita gross domestic product. Red markers: population-based studies. Blue markers: cohort and clinic-based studies. For cohort studies prevalence in baseline survey shown. Gross domestic product per capita figures: International Monetary Fund (2011) [79].
Patient selection
Clinic-based studies were highly heterogeneous in patient selection in relation to age range, gender, ethnicity, duration and type of diabetes and co-morbidity. Of those studies conducted in diabetes clinics, 18 included all patients with diabetes attending the clinic, while 14 confined their study to a subgroup; for example, subjects with Type 2 diabetes [20], children 5–18 years [21] or patients with duration of diabetes > 5 years [22]. Of the nine studies conducted in ophthalmology clinics, four studied patients with a particular diagnosis (neovascular glaucoma [23], retinal disease [24,25], blindness [26]), one studied patients attending specific diabetes eye clinics [17] and four studied a cross section of all eye patients [27–30]. In those studies that differentiated Type 1 and Type 2 diabetes (30 studies), most used study-specific definitions, making inter-study comparisons problematic.
Assessment of retinopathy
Methods of assessment and classification of retinopathy varied widely. Only nine studies used retinal photography [77,10,31–31] and six of these were conducted in South Africa [31–33,35–37]. Thirty studies classified retinopathy simply as present or absent; 32 used a recognized grading system. Most used an adaptation of the Early Treatment of Diabetic Retinopathy Study grading system [4]. However, the application and its reporting varied widely. In no study was an external validation of the practitioner’s grading reported.
Evidence of bias
There was evidence of incomplete outcome data in a number of studies. In the majority of clinic-based studies, the number of patients approached to participate was not reported, making selection bias difficult to assess. Many studies reported prevalence of a number of diabetic complications. In some studies, a low proportion of patients were examined for retinopathy. For example, in Harzallah et al. [38] only 19% of 593 patients underwent retinal examination. Many studies excluded patients with significant cornea or media opacities [39,40] or with ungradeable photographs [31].
Community-based studies
We identified three community-based studies (Table 1). In Egypt, in 1991–1994, researchers examined the prevalence of diabetes and the relationship between HbA1c and retinopathy [8,34,41,42]. Articles by Herman et al. [34] and Penman et al. [41] report different prevalence of diabetic retinopathy as graded from retinal photography: 35.4% in 376 subjects in the former and 31.6% in 335 subjects in the latter. No explanation for this difference between the two reports is offered, suggesting missing data in one or both analyses. Herman et al. [34] demonstrated in multivariate analysis that diabetic retinopathy was associated with longer duration of diabetes (per 10 years) (odds ratio = 1.37, 95% CI 1.09–1.73) and higher HbA1c (per unit) (odds ratio = 1.15, 95% CI 1.03–1.27).
Table 1.
Community-based cross-sectional studies reporting prevalence of diabetic retinopathy in Africa
| Study | Methods | Subjects and subgroups | n | Outcome |
||
|---|---|---|---|---|---|---|
| Any diabetic retinopathy, % (95% CI) | Proliferative diabetic retinopathy (%) | Maculopathy (%) | ||||
| Studies reporting prevalence of diabetes and diabetic retinopathy in the general population | ||||||
| Egypt, 1991–1994 [8,34,41,42] | Stratified random sampling of persons ≥ 20 years in urban and rural areas near Cairo. 4620 adults underwent random glucose testing. Those at high risk of diabetes and a sample of those at low risk (total 1451) had a fasting glucose test. Diabetes diagnosed by World Health Organization criteria (see also Supporting Information, Appendix S1 [101]). Retinal photography graded according to Airlie House Classification and binocular indirect ophthalmoscopy examination by ophthalmologist. Those ungradeable on photography and binocular indirect ophthalmoscopy excluded from analysis of retinal photography and binocular indirect ophthalmoscopy, respectively | Subjects with diabetes (retinal photography)* | 335 | 31.6 | 0.9 | 4.5‡ |
| Subjects with diabetes (binocular indirect ophthalmoscopy)* | 404 | 20.3 | 0 | 1.2‡ | ||
| Known diabetes (retinal photography)† | 287 | 41.5 (35.8–47.2) | ||||
| Newly diagnosed diabetes (retinal photography)† | 89 | 15.7 (8.2–23.3) | ||||
| Impaired glucose tolerance (retinal photography)† | 103 | 1.9 (0–4.6) | ||||
| Mauritius, 1992 [7] | 6553 persons in 14 geographically defined clusters underwent glucose tolerance test. In 11 clusters, all adults aged 25–74 years were invited to attend; in three clusters, age-stratified sampling of adults aged 35–64 years performed. Those with diabetes and 25% of those with impaired glucose tolerance [World Health Organization criteria (115)] had 3-field, 45° stereoscopic retinal photography of the right eye. Grading by certified assessor according to modified Airlie House criteria. Those with ungradeable photographs were excluded from analysis | All subjects with diabetes | 746 | 30.2 (26.9–33.5) | 1.3 | |
| Known diabetes | 388 | 44.3 (39.4–49.2) | 2.3 | |||
| Newly diagnosed diabetes | 358 | 14.8 (11.1–18.5) | 0.3 | |||
| Impaired glucose tolerance | 165 | 9.1 (4.7–13.5) | 0 | |||
| Muslim Indian race with diabetes | 186 | 22.8 (18.7–28.9) | 1.1 | |||
| Creole race with diabetes | 160 | 35.7 (28.1–43.3) | 1.3 | |||
| Study of cause of visual impairment in the general population | ||||||
| Nigeria, 2005–2007 [9] | National multistage, stratified cluster sampling of persons ≥ 40 years to determine cause of visual impairment. 13 591 visual acuity tested; 3129 had uncorrected visual acuity < 6/12 in better eye examined by ophthalmologist. Primary cause of visual impairment recorded | Subjects with visual acuity < 6/12 better eye | 3129 | 0.29 | ||
In 1992, researchers in Mauritius [7] investigated prevalence of and risk factors for diabetic retinopathy in Asian Indian, Chinese and Creole Mauritians. This high-quality study demonstrated a high prevalence of diabetic retinopathy in all major ethnic groups in Mauritius. The prevalence of diabetic retinopathy and proliferative diabetic retinopathy were particularly high in known diabetes: 44.3 and 2.3%, respectively. Muslim Indians had the lowest prevalence of retinopathy (10.8 and 34.0% for new and known diabetes, respectively); significantly lower than Creoles (18.8 and 53.8%, respectively). The following were independently associated with retinopathy: duration of diabetes, fasting plasma glucose, systolic blood pressure, albuminuria and decreasing BMI.
Cohort studies
Table 2 summarizes cohort studies of diabetic retinopathy conducted in Africa. In Mauritius, in 1998, researchers followed up the population-based study performed in 1992 [7] with a survey of diabetes complications [10]. Of subjects with diabetes in the initial survey, 40.5% were re-examined. The 6-year incidence of diabetic retinopathy and proliferative diabetic retinopathy in subjects with diabetes but no diabetic retinopathy in the first survey was 23.8 and 0.4%, respectively. The incidence of proliferative diabetic retinopathy was much higher in subjects with mild non-proliferative diabetic retinopathy (5.2%) and moderate non-proliferative diabetic retinopathy (29.4%) in the first survey. Duration of diabetes and fasting blood glucose were independently associated with incidence of retinopathy. In South Africa, in 1982–2002, Gill and co-workers identified a cohort of patients with diabetes requiring insulin therapy diagnosed before age 30 years [11]. In those subjects seen at 10 years, prevalence of diabetic retinopathy had increased from 6 to 52% and proliferative diabetic retinopathy from 0 to 3% [12]. In subjects seen at 20 years, prevalence of diabetic retinopathy had increased from 12 to 59% [13].
Table 2.
Cohort studies reporting prevalence and incidence of diabetic retinopathy in Africa
| Study | Methods | Subjects and subgroups | n | Outcome |
||
|---|---|---|---|---|---|---|
| Any diabetic retinopathy, % (95% CI) | Proliferative diabetic retinopathy, % | Percentage of subjects progressing | ||||
| 1. Mauritius 1992–1998 | ||||||
| Initial population-based study, 1992 [7] | Population-based study of prevalence of diabetes and diabetic retinopathy: methodology outlined in Table 1 | All subjects with diabetes | 746 | 30.2 (26.9–33.5) | 1.3 | |
| Known diabetes | 388 | 44.3 (39.4–49.2) | 2.3 | |||
| Newly diagnosed diabetes | 358 | 14.8 (11.1–18.5) | 0.3 | |||
| Second survey, 1998 [10] | Of those assessed for complications in 1992, 528 attended the follow-up survey. Grading of retinopathy as in first assessment | Subjects with diabetes | 302 | 33.8 | 3.0 | 25.2 |
| Diabetes with no diabetic retinopathy at baseline | 227 | 23.8* | 0.4† | 23.8 | ||
| Diabetes, mild non-proliferative diabetic retinopathy at baseline | 58 | 5.2† | 27.7 | |||
| Diabetes, moderate non-proliferative diabetic retinopathy at baseline | 17 | 29.4† | 35.3 | |||
| 2. South Africa 1982–2002 | ||||||
| Baseline assessment, 1982 [11] | 88 black South Africans with diabetes requiring insulin therapy diagnosed < 30 years attending the diabetes clinic at Baragwanath Hospital, Soweto were screened for diabetic complications. 66 were examined for retinopathy by a physician using direct ophthalmoscope | Subjects with diabetes requiring insulin therapy diagnosed < 30 years | 66 | 12.1 | 0 | |
| Subgroup subsequently seen at 10 years | 33 | 6 | 0 | |||
| Subgroup subsequently seen at 20 years | 17 | 12 | ||||
| 10-year follow-up, 1992 [12] | Of the original cohort, 24 were lost to follow-up, 10 had died. Of 54 still attending clinic, 36 were examined. In three patients cataracts prevented fundal view | Subjects with diabetes requiring insulin therapy diagnosed < 30 years | 33 | 52 | 3 | |
| 20-year follow-up, 2002 [13] | Of the original cohort, 21 died, 39 were lost to follow-up, 28 were still attending clinic, of which 17 were assessed for complications | Subjects with diabetes requiring insulin therapy diagnosed < 30 years | 17 | 59 | ||
Incidence of diabetic retinopathy at 6 years.
Incidence of proliferative diabetic retinopathy at 6 years.
No other prospective cohort studies were identified. However, studies reflecting cumulative incidence of diabetic retinopathy are available. In South Africa, Distiller et al. [32] reported on 1520 patients with Type 1 diabetes and 8026 patients with Type 2 who had maintained membership for ≥ 5 years of a community-based, privately funded diabetes management programme. In subjects with Type 1 diabetes, prevalence of any retinopathy at baseline and at 5 years was 22.3 and 28%, respectively, and in subjects with Type 2 diabetes was 20.5 and 26.6%, respectively. In retrospective studies of patients with diabetes of long duration, Lester [43] showed a prevalence of diabetic retinopathy of 45.5% in 121 Ethiopian patients with duration of diabetes > 20 years, while Distiller et al. [33] reported presence of diabetic retinopathy in 14.8% of 148 South African Caucasian patients with Type 1 diabetes of > 18 years duration.
Hospital-based and primary care-based surveys
Tables 3,4 and 5 summarize hospital-based and primary care-based surveys reporting prevalence of diabetic retinopathy using a recognized grading system. The most recent large study from Northern Africa was conducted in Cairo during 2007–2008 in endocrinology clinics in two major teaching hospitals [39]. Prevalence of proliferative diabetic retinopathy (2.3%) and clinically significant macular oedema (11.5%) reported in this study was high. Of four studies from Western Africa [44–47], none reported the prevalence of maculopathy (Table 3). Only three were identified from Middle Africa [22,48,49]. Longo-Mbenza et al. [48] studied 3010 patients with diabetes attending diabetes primary care facilities using retinal photography; prevalence of diabetic retinopathy was 31.6%.
Table 3.
Hospital-based surveys of patients with diabetes reporting prevalence of diabetic retinopathy using a recognized grading system in Northern, Western and Middle Africa
| Study | Country | Methods | n | Any diabetic retinopathy (%) | Proliferative diabetic retinopathy (%) | Clinically significant macular oedema (%) | Statistically significant associations of diabetic retinopathy |
|---|---|---|---|---|---|---|---|
| Northern Africa | |||||||
| Elbagir et al., 1995 [53] | Sudan | Patients with diabetes requiring insulin (duration > 1 year) aged 15–75 years attending medical outpatient department examined with direct ophthalmoscope by a physician | 91 | 43 | 10* | Not reported | Current age of patient, duration of diabetes, systolic blood pressure, diastolic blood pressure, cholesterol, BMI (univariate analysis) |
| Macky et al., 2011 [39] | Egypt | Patients > 18 years of age attending a diabetes clinic examined with slit-lamp biomicroscopy by ophthalmologist. Excluded 47 patients because of media opacities | 1325 | 20.5 | 2.3 | 11.5 | Duration of diabetes, hypertension, female gender (univariate analysis) |
| Western Africa | |||||||
| Ikem and Akinola, 2001 [47] | Nigeria | Consecutive patients with Type 2 diabetes seen at medical outpatient department. Examined by physician; instrument not stated | 132 | 41.1 | 1.0 | Not reported | Hypertension |
| Alebiosu et al., 2003 [44] | Nigeria | Hospitalized subjects with Type 2 diabetes and nephropathy. Examined with direct ophthalmoscope by physician | 191 | 47.1 | 12.6 | Not reported | Not reported |
| Omolase et al., 2010 [45] | Nigeria | Patients with diabetes attending medical outpatient department. Examined with direct ophthalmoscope by ophthalmologist | 100 | 15.0 | 2.0 | Not reported | Duration of diabetes (univariate analysis) |
| Onakpoya et al., 2010 [46] | Nigeria | Patients with Type 2 diabetes attending a 3° centre diabetes clinic; invited for screening by ophthalmologist with direct ophthalmoscope. 3.6% no fundal view | 80 | 21.6 | 1.2 | Not reported | Not reported |
| Middle Africa | |||||||
| Sobngwi et al., 1999 [49] | Cameroon | Adults attending diabetes clinic. Excluded patients with renal disease. Slit lamp biomicroscopy examination by ophthalmologist | 64 | 37.5 | 1.6 | Not reported | Univariate analysis: current age of patient, systolic blood pressure, microalbuminuria Multivariate analysis: micro-albuminuira |
’Severe retinopathy’ by World Health Organization multinational study criteria [80].
Table 4.
Hospital-based surveys of patients with diabetes reporting prevalence of diabetic retinopathy using a recognized grading system in Eastern Africa
| Study | Country | Methods | n | Any diabetic retinopathy (%) | Proliferative diabetic retinopathy (%) | Any maculopathy (%) | Statistically significant associations of diabetic retinopathy |
|---|---|---|---|---|---|---|---|
| Sulivan et al., 1990 [16] | Seychelles | Patients with diabetes requiring insulin therapy attending diabetic clinic examined by a physician. Instrument not reported | 108 | 15.7 | 2.8 | Not reported | Not reported |
| Lester, 1992§ | Ethiopia | Type 1 diabetes seen 1976–1990. Examination by physician. Instrument not reported | 431 | 9.5 | 2.6 | 1.2 | Not reported |
| Lester 1993§ | Ethiopia | Type 2 diabetes seen 1976–1991. Examination by physician. Instrument not reported | 503 | 41.1 | 6.9 | 4.0 | Not reported |
| Taylor et al., 1997 [17] | Seychelles | Type 2 diabetes: 184 attending an eye clinic, 199 invited for screening. Ophthalmologist slit-lamp biomicroscopy examination | 383 | 28 | 4 | 19 | Insulin therapy, duration |
| Seyoum and Mengistu, 2001 [54] | Ethiopia | Patients attending a diabetes clinic. Direct ophthalmoscope examination by ophthalmologist. Three patients excludedas no fundal view | 302 | 37.8 | 1.7 | Not reported | Current age of patient, duration of diabetes, systolic blood pressure, diastolic blood pressure, proteinuria |
| Teshome and Melaku, 2004 [25] | Ethiopia | Consecutive patients seen at a retinal clinic (not all had diabetes). Slit-lamp biomicroscopy examination by ophthalmologist | 1390 | 28.7 | 9.9 | 11.1‡ | Not reported |
| Mumba et al., 2007 [55] | Tanzania | Patients > 18 years attending diabetes clinic. No previous fundus examination. Slit-lamp biomicroscopy examination by ophthalmologist | 86 | 20.9 | 1.2 | Not reported | Not reported |
| Mwale et al., 2007 [40] | Kenya | Clinic patients with Type 2 diabetes. Slit-lamp biomicroscopy examination by ophthalmologist. Excluded cornea or media opacity | 96 | 22.6 | 0 | Not reported | Not reported |
| Gill et al., 2008 [18] | Ethiopia | Consecutive patients attending hospital diabetes clinic in a remote region. Slit-lamp biomicroscopy examination by ophthalmologist | 105 | 21 | 1.9 | Not reported | Not reported |
| Glover et al., 2011 [52] | Malawi | Consecutive adults attending a hospital diabetes clinic. Slit-lamp biomicroscopy examination by ophthalmologist | 281 | 32.0 | 5.7 | 15.0* | Albuminuria, neuropathy, insulin therapy† |
Sight-threatening maculopathy according to Liverpool Diabetic Eye Study adaptation of the Early Treatment Diabetic Retinopathy Study (ETDRS) grading [81].
Multivariate associations of sight-threatening diabetic retinopathy for patients with Type 2 diabetes.
Clinically significant macular oedema.
Table 5.
Hospital-based and primary care-based surveys of patients with diabetes reporting prevalence of diabetic retinopathy using a recognized grading system in Southern Africa
| Study | Country | Methods | n | Any diabetic retinopathy (%) | Proliferative diabetic retinopathy (%) | Clinically significant macular oedema (%) | Statistically significant associations of diabetic retinopathy |
|---|---|---|---|---|---|---|---|
| Mollentz et al., 1990 [37] | South Africa | Black patients with diabetes > 5 years duration attending diabetes clinic. Retinal photography graded by ophthalmologist | 86 | 29.7‡ | 1.2‡ | Not reported | Not reported |
| Levitt et al., 1997 [50] | South Africa | Black Africans attending diabetes primary care service. Examined by a physician with direct ophthalmoscope | 243 | 55.4 | 4.3 | 31.1† | Not reported |
| Rotchford and Rotchford, 2002 [19] | South Africa | Adults attending a nurse-led primary care diabetes service in rural KwaZulu-Natal. Examined with slit-lamp biomicroscopy by an ophthalmologist | 253 | 40.3 | 5.6 | 10.3 | Albuminuria, duration of diabetes, current age of patient, HbA1c, BMI (inverse) |
| Huddle, 2005 [58] | South Africa | Pregnant women with diabetes attending a clinic: Type 1, Type 2 and gestational diabetes. Direct ophthalmoscope examination; practitioner grading retinopathy not reported | 733 | 7.6 | 0.1 | Not reported | Type 1 diabetes |
| Carmichael et al., 2005* [31] | South Africa | Patients attending an urban diabetes clinic: 588 black, 739 white, 180 indian. Retinal photography graded by ophthalmologist. Ungradeable photographs excluded | 1517 | 26.5 | Not reported | Not reported | Duration of diabetes, insulin therapy, albumin–creatinine ratio, systolic blood pressure |
| Mengesha, 2006 [59] | Botswana | Patients with diabetes attending government health facilities. Slit-lamp biomicroscopy examination by ophthalmologist | 401 | 9.2 | 3.0 | Not reported | Not reported |
| Mash et al., 2007 [36] | South Africa | Patients attending primary care diabetes service: 44%‘black’; 56%‘coloured’. Retinal photography graded by ophthalmologist. 17.5% of photographs ungradeable | 400 | 62.4 | 6.1 | 15.2† | Not reported |
| Read and Cook, 2007 [51] | South Africa | Patients with Type 2 diabetes attending a primary care diabetes clinic (124 ‘Black’; 119 ‘Coloured’; 5 ‘White’; 1 ‘Asian’). Direct ophthalmoscope examination by ophthalmologist | 248 | 32.3 | 2.4 | 8.5 | Not reported |
Three reports [31,35,60] described grades of diabetic retinopathy in overlapping populations. Figure for any diabetic retinopathy taken from the largest report [31] (n = 1517); associations of diabetic retinopathy taken from smaller report (n = 507) [35].
Any maculopathy.
‡Percentage of eyes (not patients) with specified grade of diabetic retinopathy.
Hospital-based surveys from Eastern Africa cover nine countries showing a general trend of increasing prevalence of diabetic retinopathy from earlier to more recent studies (Table 4). Diabetes clinic-based surveys from Southern Africa in general report higher prevalence of diabetic retinopathy and proliferative diabetic retinopathy than comparable clinics in other regions of Africa (Table 5). Proliferative diabetic retinopathy prevalence > 4% was recorded in three studies of unselected diabetes clinic attendees from South Africa [19,36,50]. Data on prevalence of diabetic maculopathy were limited from all regions. However, eight studies suggest high prevalence [17,19,25,36,39,50–52]. Of note, three South African, primary care-based studies were identified. Levitt et al. [50], Mash et al. [36] and Read and Cook [51] reported high prevalence of proliferative diabetic retinopathy and maculopathy (Table 5), comparable with hospital-based surveys in the same country and higher than hospital-based surveys elsewhere in Africa.
Two studies from South Africa compared prevalence of diabetic retinopathy in different ethnic groups [35,51]. The authors acknowledge the effect of environmental factors on different racial communities, even in the post-apartheid era. Kalk et al. [35] studied 507 ‘poor or indigent’ patients attending a free hospital diabetes clinic. Prevalence of diabetic retinopathy was similar in patients of African (37%), European (41%) or Indian (37%) heritage. However, ‘severe diabetic retinopathy’ (study-specific classification) was significantly more frequent in Africans (52%) and Indians (41%) compared with Europeans (26%). Read and Cook [51] found no relationship between ethnicity and diabetic retinopathy prevalence.
Studies reporting visual acuity
Nineteen studies reported visual acuity in subjects with diabetes; parameters reported varied widely between studies. Only the Nigerian national blindness and visual impairment survey [9] tested logarithm of the minimum angle of resolution (logMAR) acuity. The population-based Mauritius diabetes complication study [7] reported best-corrected visual acuity < 6/12 in 7.1% of subjects with diabetes at baseline. There was no difference in this figure for subjects with and without retinopathy. The Diabetes in Egypt project [41] reported visual acuity in 427 subjects with diabetes. Of these, 31 (7.3%) were blind (defined as best-corrected visual acuity in the better eye less than 6/60); 239 (56%) had a best-corrected visual acuity between 6/12 and 6/60. It is likely that media opacities accounted for a proportion of this visual impairment: 123 eyes had cataract; 11 had corneal opacity; 17 had both.
The Nigerian national blindness and visual impairment survey was conducted between 2005 and 2007 [9]. Diabetic retinopathy was identified as the primary cause of visual impairment in 0.29% of 3129 subjects with uncorrected visual acuity worse than 6/12 and in 0.5% of those with acuity less than 3/60. This study is likely to underestimate the visual impact of diabetic retinopathy as examiners were instructed to preferentially record treatable, then preventable causes of visual impairment; i.e. cataract would be recorded in preference to diabetic retinopathy if both were affecting visual acuity to similar degrees.
Discussion
This systematic narrative review describes 62 studies reporting the prevalence and incidence of diabetic retinopathy and maculopathy in Africa. The methodological approach used standard inclusion, appraisal and data extraction approaches. Few high-quality, population-based studies were identified: the majority of studies were surveys of hospital clinic attendees. Identified studies were highly heterogeneous in terms of patient selection and method of assessment and classification of retinopathy. Despite these inconsistencies between studies, the review identified rates of prevalence of diabetic retinopathy in many areas of Africa comparable with high-income countries. Prevalence of proliferative diabetic retinopathy and maculopathy was high in recent studies, particularly those from Southern and Eastern Africa. Common themes were identified in the associations of diabetic retinopathy and impact on vision.
Methodology of included studies
The review identified three high-quality, population-based, cross-sectional studies of diabetic retinopathy epidemiology [7–9]. Only two cohort studies were identified. Large epidemiological studies are expensive; the population-based studies were conducted in states with relatively greater resources: Nigeria, Mauritius and Egypt. The lack of studies from Middle Africa is likely to reflect lack of resources, poor health infrastructure and deficiency of trained medical professionals. The relatively small number of studies identified from Northern Africa is partially explained by the tendency of francophone countries to publish in French.
The literature is dominated by studies of urban populations reflecting the distribution of major health facilities. Urbanization is seen as an important factor driving the diabetes epidemic [61]; studies of urban populations may overestimate diabetic retinopathy prevalence. A caveat is that, in resource-poor settings, patients travel long distances to health facilities and rural patients may therefore be included. The majority of studies identified were hospital clinic-based surveys; selection bias is a major issue and the findings should be generalized to other settings with caution. Another bias is that clinics are seen by many as a point to collect medication; patients with diet-controlled diabetes may be under-represented. The classification of diabetes in Africa is problematic, particularly where investigations are limited. Disease characteristics differ from Caucasian populations. For example, peak age of onset of Type 1 diabetes is later in African communities, typically 22–29 years [62]. Other phenotypes of diabetes are recognized in patients of African origin, including ‘atypical African diabetes’ and ‘malnutrition-related diabetes’ [63].
Adaptations of the Early Treatment of Diabetic Retinopathy Study grading system have become the accepted reference standard for classifying retinopathy in research settings. Despite this, its use in everyday clinical practice is difficult because of a large number of levels requiring correlations with standard photographs and grading rules that must be remembered. General ophthalmologists and physicians in resource-poor settings may not be able to use this system to a reproducible level. Stereoscopic photography with validated grading is rapidly becoming the reference standard for assessing retinopathy. Digital photography allows transfer of images to distant reading centres, as was used in the Diabetes in Egypt project [8,34,41,42]. While expensive, this may be the direction of future research.
Prevalence and incidence of diabetic retinopathy and diabetic maculopathy
Community-based studies identified in this review reported prevalence rates of diabetic retinopathy and proliferative diabetic retinopathy comparable with American and European populations with diabetes. The Diabetes in Egypt project [8,34,41,42] reported a prevalence of diabetic retinopathy and proliferative diabetic retinopathy in subjects with diabetes of 31.6 and 0.9%, respectively. The Mauritius diabetes complication study [7] reported 30.2% diabetic retinopathy and 1.3% proliferative diabetic retinopathy; the prevalence of proliferative diabetic retinopathy in subjects with known diabetes was 2.3%. In comparison, a 2005–2008 cross-sectional sample of US adults with diabetes aged 40 years and older estimated prevalence of diabetic retinopathy and proliferative diabetic retinopathy as 28.5% and 1.5%, respectively [64]. Recent population-based studies in Europe have reported similar rates [65–69]. Younis et al. [65] studied 8062 patients with diabetes entering an English primary care-based screening programme. The prevalences of any retinopathy and proliferative diabetic retinopathy in Type 1 diabetes were 45.7 and 3.7%, respectively, and in Type 2 diabetes were 25.3 and 0.5%, respectively.
The lack of community-based studies from Sub-Saharan Africa is important. Very high prevalence of diabetic retinopathy, proliferative diabetic retinopathy and maculopathy has been reported in notable high-quality, clinic-based surveys in the last decade: in Eastern Africa by Glover et al. [52] (32.0% diabetic retinopathy, 5.7% proliferative diabetic retinopathy, 15% sight-threatening maculopathy), and in South Africa by Mash et al. [36] (62.4% diabetic retinopathy, 6.1% proliferative diabetic retinopathy, 15.2% any maculopathy) and Rotchford and Rotchford [19] (40.3% diabetic retinopathy, 5.6% proliferative diabetic retinopathy, 10.3% clinically significant macular oedema). These figures are likely to reflect factors including ethnicity, poor access to medical services, late diagnosis, and co-pathology including infection (importantly HIV and malaria), hypertension, malnutrition, and anaemia. We found no clear relationship between per capita gross domestic product and prevalence of diabetic retinopathy or proliferative diabetic retinopathy. However, the increased infrastructure to detect disease in states with greater resources is an important confounding factor.
The influence of ethnicity on diabetic retinopathy prevalence in populations of African origin has yet to be determined. In the USA, Zhang et al. [64] reported prevalence of both diabetic retinopathy and vision-threatening retinopathy (defined as Early Treatment of Diabetic Retinopathy Study severe non-proliferative diabetic retinopathy, proliferative diabetic retinopathy, or clinically significant macular oedema) to be higher in non-Hispanic black subjects (38.8 and 9.3%, respectively) compared with non-Hispanic white subjects (26.4 and 3.2%, respectively). Previous studies have shown similar results [70,71]. However, differences were attributable to risk factors for retinopathy [71]. Therefore, while associations between polymorphisms of specific genes and diabetic retinopathy have been described in African populations [72], no ethnic propensity to retinopathy has been identified.
Neither of the two cohort studies identified by this review reported two- or three-step progression on the Early Treatment of Diabetic Retinopathy Study scale, as used in recent European studies [73]. The Mauritius diabetes complication study [10] reported 6-year incidence of diabetic retinopathy (23.8%). Six-year progression to proliferative diabetic retinopathy was reported from no diabetic retinopathy (0.4%), mild non-proliferative diabetic retinopathy (5.2%) and moderate non-proliferative diabetic retinopathy (29.4%). The UK Prospective Diabetes Study (UKPDS) reported similar 6-year incidence of diabetic retinopathy: 22% [74]. However, the UKPDS population were studied from a later time point: clinical diagnosis of diabetes. In the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) 4-year progression of diabetic retinopathy and progression to proliferative diabetic retinopathy was observed in 41.2 and 10.5% of subjects with Type I diabetes, 34 and 7.4% of insulin-treated patients with Type 2 diabetes and 24.9 and 2.3% of non-insulin treated patients, respectively [75].
Impact of diabetic retinopathy on vision
Estimates of the proportion of African patients with diabetes who are visually impaired are high even compared with older European and American studies. Of subjects in the Diabetes in Egypt project [41], 7.3% had best-corrected visual acuity in the better eye < 6/60. In contrast, of the population in the Wisconsin Epidemiological Study of Diabetic Retinopathy, 3.6% of patients aged < 30 years at diagnosis, and 1.6% of patients aged ≥ 30 years at diagnosis were legally blind according to US standards [76,77]. The World Health Organization estimates that, in the USA and Canada, 17% of blindness is attributable to diabetic retinopathy [78]. While data are sparse, the proportion of visual impairment and blindness as a result of diabetic retinopathy in Africa appears to be considerably less. However, the prevalence of visual impairment and blindness is significantly higher in Africa [78], reflecting high prevalence of pathologies including uncorrected refractive error, cataract, corneal opacities and glaucoma.
The findings of this review have important implications for both research and clinical practice. Large, community-based cross-sectional and cohort studies are needed to investigate rates and determinants of diabetic retinopathy prevalence, incidence and progression across Africa. Consensus is needed on standardized data sets and the most appropriate methods of identification and classification of diabetic retinopathy in Africa. In Europe and America, there is strong evidence for the role of poor glycaemic control and co-pathology, including hypertension in development and progression of diabetic retinopathy. Similarly, a strong evidence base exists for the treatment of sight-threatening diabetic retinopathy with laser photo-coagulation and intravitreal agents. This evidence has yet to be accrued in African settings. Management of systemic disease and screening and treatment of retinopathy requires substantial infrastructure, which is currently lacking in many African states. The public health and health economic challenges for policymakers across Africa are significant.
Funding sources
None.
Competing interests
Nothing to declare.
Acknowledgments
Funding for this study came from the Wellcome Trust via a Clinical PhD Fellowship (PIB). The authors acknowledge the help of Vittoria Lutje, Cochrane infectious diseases group, Liverpool School of Tropical Medicine for help in designing customized literature searches.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Prevalence of proliferative diabetic retinopathy in patients with diabetes according to national per capita gross domestic product.
Table S1. Literature search report for articles reporting prevalence, incidence or progression of diabetic retinopathy or diabetic maculopathy in African countries.
Table S2. Characteristics of 62 studies reporting prevalence of diabetic retinopathy and maculopathy in Africa.
Appendix S1. Search histories.
Appendix S2. Supplementary references.
References
- 1.IDF. Diabetes Atlas. 4th edition. International Diabetes Federation; 2009. Brussels: [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Statistics Division. Composition of Macro Geographical (Continental) Regions. Available at http://unstats.un.org/unsd/methods/m49/m49regin.htm#least Last accessed 1 April 2011. [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthal. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 5.Hamilton AMP, Ulbig MW, Polkinghorne P. Management of Diabetic Retinopathy. BMJ Publishing Group; 1996. London: [Google Scholar]
- 6.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Checklist. Available at http://www.prisma-statement.org/ Lasts accessed 1 April 2011. [DOI] [PubMed] [Google Scholar]
- 7.Dowse GK, Humphrey AR, Collins VR, Plehwe W, Gareeboo H, Fareed D, et al. Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol. 1998;147:448–457. doi: 10.1093/oxfordjournals.aje.a009470. [DOI] [PubMed] [Google Scholar]
- 8.Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care. 1997;20:785–791. doi: 10.2337/diacare.20.5.785. [DOI] [PubMed] [Google Scholar]
- 9.Abdull MM, Sivasubramaniam S, Murthy GV, Gilbert C, Abubakar T, Ezelum C, et al. Nigeria National Blindness and Visual Impairment Study Group. Causes of blindness and visual impairment in Nigeria: the Nigeria national blindness and visual impairment survey. Invest Ophthalmol Vis Sci. 2009;50:4114–4120. doi: 10.1167/iovs.09-3507. [DOI] [PubMed] [Google Scholar]
- 10.Tapp RJ, Zimmet PZ, Harper CA, McCarty DJ, Chitson P, Tonkin AM, et al. Six-year incidence and progression of diabetic retinopathy: results from the Mauritius diabetes complication study. Diabetes Res Clin Pract. 2006;73:298–303. doi: 10.1016/j.diabres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Gill GV, Huddle KR, Krige LP. Intensive health screening of young black diabetics. S Afr Med J. 1984;65:815–816. [PubMed] [Google Scholar]
- 12.Gill GV, Huddle KR, Rolfe M. Mortality and outcome of insulin-dependent diabetes in Soweto, South-Africa. Diabet Med. 1995;12:546–550. doi: 10.1111/j.1464-5491.1995.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 13.Gill GV, Huddle KRL, Monkoe G. Long-term (20 years) outcome and mortality of Type 1 diabetic patients in Soweto, South Africa. Diabet Med. 2005;22:1642–1646. doi: 10.1111/j.1464-5491.2005.01712.x. [DOI] [PubMed] [Google Scholar]
- 14.Rotimi C, Daniel H, Zhou J, Obisesan A, Chen G, Chen Y, et al. Prevalence and determinants of diabetic retinopathy and cataracts in West African type 2 diabetes patients. Ethn Dis. 2003;13:S110–S117. [PubMed] [Google Scholar]
- 15.Sidibe AT, Dembele M, Cisse A, Alwata F, Ahmedou Ould MY, Coulibaly T, et al. Diabetic hand infections in hospital practice in Bamako, Mali. Diabetes Metab. 2006;32:89. doi: 10.1016/s1262-3636(07)70253-7. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan FM, Rosalie D, Sivakumar U. Using insulin U 100 conversion to estimate the prevalence and problems of insulin-treated diabetic patients in the Republic of Seychelles. Diabetologia. 1990;33:184–185. doi: 10.1007/BF00404050. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RH, Jones HS, Dodson PM, Hamilton AP, Kritzinger EE. Diabetic eye disease: a natural history study. Eye. 1997;11:547–553. doi: 10.1038/eye.1997.141. [DOI] [PubMed] [Google Scholar]
- 18.Gill GV, Gebrekidan A, English P, Wile D, Tesfaye S. Diabetic complications and glycaemic control in remote North Africa. Q J Med. 2008;101:793–798. doi: 10.1093/qjmed/hcn096. [DOI] [PubMed] [Google Scholar]
- 19.Rotchford AP, Rotchford KM. Diabetes in rural South Africa—an assessment of care and complications. S Afr Med J. 2002;92:536–541. [PubMed] [Google Scholar]
- 20.Elmahdi EM, Kaballo AM, Mukhtar EA. Features of non-insulin-dependent diabetes mellitus (NIDDM) in the Sudan. Diabetes Res Clin Pract. 1991;11:59–63. doi: 10.1016/0168-8227(91)90142-z. [DOI] [PubMed] [Google Scholar]
- 21.Majaliwa ES, Munubhi E, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30:2187–2192. doi: 10.2337/dc07-0594. [DOI] [PubMed] [Google Scholar]
- 22.El-Shazly M, Zaki A, Nicolucci A. Care-related risk factors for chronic diabetic complications in developing countries: a case from Egypt. Public Health. 2002;116:289–296. doi: 10.1038/sj.ph.1900855. [DOI] [PubMed] [Google Scholar]
- 23.Ashaye AO, Adeoti CO. Neovascular glaucoma in a Nigerian African population. East Afr Med J. 2006;83:559–564. doi: 10.4314/eamj.v83i10.9469. [DOI] [PubMed] [Google Scholar]
- 24.Oluleye TS, Ajaiyeoba AI. Retinal diseases in Ibadan. Eye. 2006;20:1461–1463. doi: 10.1038/sj.eye.6702343. [DOI] [PubMed] [Google Scholar]
- 25.Teshome T, Melaku S. Pattern of retinal diseases at a teaching eye department, Addis Ababa, Ethiopia. Ethiop Med J. 2004;42:185–193. [PubMed] [Google Scholar]
- 26.Poole TRG. Causes of blindness in Northern Tanzania: a hospital and rural health centre based study. Int Ophthalmol. 2001;24:195–198. doi: 10.1023/a:1022543230358. [DOI] [PubMed] [Google Scholar]
- 27.Carreras FJ, Rodriguez-Hurtado F, David H. Ophthalmology in Luanda (Angola): a hospital based report. Br J Ophthalmol. 1995;79:926–933. doi: 10.1136/bjo.79.10.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eze BI, Uche JN, Shiweobi JO. The burden and spectrum of vitreo-retinal diseases among ophthalmic outpatients in a resource-deficient tertiary eye care setting in South-eastern Nigeria. Middle East Afr J Ophthalmol. 2010;17:246–249. doi: 10.4103/0974-9233.65491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwosu SNN. Prevalence and pattern of retinal diseases at the Guinness Eye Hospital, Onitsha, Nigeria. Ophthalmic Epidemiol. 2000;7:41–48. [PubMed] [Google Scholar]
- 30.Onakpoya OH, Olateju SO, Ajayi IA. Retinal diseases in a tertiary hospital: the need for establishment of a vitreo-retinal care unit. J Natl Med Assoc. 2008;100:1286–1289. doi: 10.1016/s0027-9684(15)31506-6. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael TR, Carp GI, Welsh ND, Kalk WJ. Effective and accurate screening for diabetic retinopathy using a 60 degree mydriatic fundus camera. S Afr Med J. 2005;95:57–61. [PubMed] [Google Scholar]
- 32.Distiller LA, Brown MA, Joffe BI, Kramer BD. Striving for the impossible dream: a community-based multi-practice collaborative model of diabetes management. Diabet Med. 2010;27:197–202. doi: 10.1111/j.1464-5491.2009.02907.x. [DOI] [PubMed] [Google Scholar]
- 33.Distiller LA, Joffe BI, Melville V, Welman T, Distiller GB. Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complications. 2006;20:280–284. doi: 10.1016/j.jdiacomp.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Herman WH, Aubert RE, Engelgau MM, Thompson TJ, Ali MA, Sous ES, et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complications. Diabet Med. 1998;15:1045–1051. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Kalk WJ, Joannou J, Ntsepo S, Mahomed I, Mahanlal P, Becker PJ. Ethnic differences in the clinical and laboratory associations with retinopathy in adult onset diabetes: studies in patients of African, European and Indian origins. J Intern Med. 1997;241:31–37. doi: 10.1046/j.1365-2796.1997.70892000.x. [DOI] [PubMed] [Google Scholar]
- 36.Mash B, Powell D, du Plessis F, van Vuuren U, Michalowska M, Levitt N. Screening for diabetic retinopathy in primary care with a mobile fundal camera—evaluation of a South African pilot project. S Afr Med J. 2007;97:1284–1288. [PubMed] [Google Scholar]
- 37.Mollentze WF, Stulting AA, Steyn AF. Ophthalmoscopy versus non-mydriatic fundus photography in the detection of diabetic retinopathy in black patients. S Afr Med J. 1990;78:248–250. [PubMed] [Google Scholar]
- 38.Harzallah F, Alberti H, Kanoun F, Elhouch F, Slimane H. Quality of care of patients with type 2 diabetes in a Tunisian university hospital. Diabetes Metab. 2004;30:523–526. doi: 10.1016/s1262-3636(07)70150-7. [DOI] [PubMed] [Google Scholar]
- 39.Macky TA, Khater N, Al-Zamil MA, El Fishawy H, Soliman MM. Epidemiology of Diabetic Retinopathy in Egypt: a hospital-based study. Ophthalmic Res. 2011;45:73–78. doi: 10.1159/000314876. [DOI] [PubMed] [Google Scholar]
- 40.Mwale C, Karimurio J, Njuguna M. Refractive errors in type 2 diabetic patients. East Afr Med J. 2007;84:259–263. doi: 10.4314/eamj.v84i6.9534. [DOI] [PubMed] [Google Scholar]
- 41.Penman AD, Saaddine JB, Hegazy M, Sous ES, Ali MA, Brechner RJ, et al. Screening for diabetic retinopathy: the utility of non-mydriatic retinal photography in Egyptian adults. Diabet Med. 1998;15:783–787. doi: 10.1002/(SICI)1096-9136(199809)15:9<783::AID-DIA634>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Thompson TJ, Engelgau MM. The onset of NIDDM and its relationship to clinical diagnosis in Egyptian adults. Diabet Med. 1996;13:337–340. doi: 10.1002/(SICI)1096-9136(199604)13:4<337::AID-DIA71>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Lester FT. Clinical status of Ethiopian diabetic patients after 20 years of diabetes. Diabet Med. 1991;8:272–276. doi: 10.1111/j.1464-5491.1991.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 44.Alebiosu CO, Odusan O, Jaiyesimi A. Morbidity in relation to stage of diabetic nephropathy in type 2 diabetic patients. J Natl Med Assoc. 2003;95:1042–1047. [PMC free article] [PubMed] [Google Scholar]
- 45.Omolase CO, Adekanle O, Owoeye JF, Omolase BO. Diabetic retinopathy in a Nigerian community. Singapore Med J. 2010;51:56–59. [PubMed] [Google Scholar]
- 46.Onakpoya OH, Adeoye AO, Kolawole BA. Determinants of previous dilated eye examination among type II diabetics in Southwestern Nigeria. Eur J Intern Med. 2010;21:176–179. doi: 10.1016/j.ejim.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Ikem RT, Akinola NO. What does the presence of hypertension portend in the Nigerian with non-insulin-dependent diabetes mellitus. West Afr J Med. 2001;20:127–130. [PubMed] [Google Scholar]
- 48.Longo-Mbenza B, Nkondi Mbadi ANJ, Mbungu Fuele S. Higher pulse pressure, systolic arterial hypertension, duration of diabetes and family history of diabetes in Central Africans. Int J Diabetes Metab. 2008;16:17–23. [Google Scholar]
- 49.Sobngwi E, Mbanya JC, Moukouri EN, Ngu KB. Microalbuminuria and retinopathy in a diabetic population of Cameroon. Diabetes Res Clin Pract. 1999;44:191–196. doi: 10.1016/s0168-8227(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 50.Levitt NS, Bradshaw D, Zwarenstein MF, Bawa AA, Maphumolo S. Audit of public sector primary diabetes care in Cape Town, South Africa: high prevalence of complications, uncontrolled hyperglycaemia, and hypertension. Diabet Med. 1997;14:1073–1077. doi: 10.1002/(SICI)1096-9136(199712)14:12<1073::AID-DIA498>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Read O, Cook C. Retinopathy in diabetic patients evaluated at a primary care clinic in Cape Town. S Afr Med J. 2007;97:941–944. [PubMed] [Google Scholar]
- 52.Glover SJ, Burgess PI, Cohen DB, Harding SP, Hofland HWC, Zijlstra EE, et al. Prevalence of diabetic retinopathy, cataract and visual impairment in diabetic patients in Sub-Saharan Africa. Br J Ophthalmol. 2012;96:156–161. doi: 10.1136/bjo.2010.196071. [DOI] [PubMed] [Google Scholar]
- 53.Elbagir MN, Eltom MA, Mahadi EO, Berne C. Pattern of long-term complications in Sudanese insulin-treated diabetic patients. Diabetes Res Clin Pract. 1995;30:59–67. doi: 10.1016/0168-8227(95)01146-3. [DOI] [PubMed] [Google Scholar]
- 54.Seyoum B, Mengistu Z. Retinopathy in patients of Tikur Anbessa Hospital diabetic clinic. Ethiop Med J. 2001;39:123–131. [PubMed] [Google Scholar]
- 55.Mumba M, Hall A, Lewallen S. Compliance with eye screening examinations among diabetic patients at a Tanzanian referral hospital. Ophthalmic Epidemiol. 2007;14:306–310. doi: 10.1080/09286580701272079. [DOI] [PubMed] [Google Scholar]
- 56.Lester FT. Clinical features, complications and mortality in type 1 (insulin-dependent) diabetic patients in Addis Ababa, Ethiopia, 1976–1990. Q J Med. 1992;83:389–399. [PubMed] [Google Scholar]
- 57.Lester FT. Clinical features, complications and mortality in type 2 (non-insulin dependent) diabetic patients in Addis Abeba, Ethiopia, 1976–1990. Ethiop Med J. 1993;31:109–126. [PubMed] [Google Scholar]
- 58.Huddle KR. Audit of the outcome of pregnancy in diabetic women in Soweto, South Africa, 1992–2002. J Endocrinol Metab Diabetes S Afr. 2005;10:102–107. [PubMed] [Google Scholar]
- 59.Mengesha AY. Spectrum of eye disorders among diabetes mellitus patients in Gaborone, Botswana. Trop Doct. 2006;36:109–111. doi: 10.1258/004947506776593576. [DOI] [PubMed] [Google Scholar]
- 60.Joannou J, Kalk WJ, Mahomed I, Ntsepo S, Berzin M, Joffe BI, et al. Screening for diabetic retinopathy in South Africa with 60 degrees retinal colour photography. J Intern Med. 1996;239:43–47. doi: 10.1046/j.1365-2796.1996.413755000.x. [DOI] [PubMed] [Google Scholar]
- 61.Levitt NS. Diabetes in Africa: epidemiology, management and health care challenges. Heart. 2008;4:1376–1382. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 62.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 63.Gill GV, Mbanya JC, Ramaiya KL, Tesfaye S. A sub-Saharan African perspective of diabetes. Diabetologia. 2009;52:8–16. doi: 10.1007/s00125-008-1167-9. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. J Am Med Assoc. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Younis N, Broadbent DM, Harding SP, Vora JP. Prevalence of diabetic eye disease in patients entering a systematic primary care-based eye screening programme. Diabet Med. 2002;19:1014–1021. doi: 10.1046/j.1464-5491.2002.00854.x. [DOI] [PubMed] [Google Scholar]
- 66.Henricsson M, Nilsson A, Groop L, Heijl A, Janzon L. Prevalence of diabetic retinopathy in relation to age at onset of the diabetes, treatment, duration and glycaemic control. Acta Ophthalmol Scand. 1996;74:523–527. doi: 10.1111/j.1600-0420.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 67.Kristinsson JK, Stefonsson E, Janasson F, Goslason I, Bjornsson S. Screening for eye disease in type 2 diabetes mellitus. Acta Ophthalmol. 1994;72:341–346. doi: 10.1111/j.1755-3768.1994.tb02770.x. [DOI] [PubMed] [Google Scholar]
- 68.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. UK Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 69.Burnett S, Hurwitz B, Davey C, Ray J, Chaturvedi N, Salzmann J, et al. The implementation of prompted retinal screening for diabetic eye disease by accredited optometrists in an inner-city district of North London: a quality of care study. Diabet Med. 1998;15:S38–S43. doi: 10.1002/(sici)1096-9136(1998110)15:3+<s38::aid-dia729>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 70.Wong TY, Klein R, Islam A. Diabetic retinopathy in a multiethnic cohort in the United states. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris MJ, Klein R, Cowie CC. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? Diabetes Care. 1998;21:1230–1235. doi: 10.2337/diacare.21.8.1230. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Huang H, Zhou J, Doumatey A, Lashley K, Chen G, et al. Polymorphism of the endothelial nitric oxide synthase gene is associated with diabetic retinopathy in a cohort of West Africans. Mol Vis. 2007;13:2142–2147. [PubMed] [Google Scholar]
- 73.ACCORD Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stratton IM, Kohner EM, Aldington SJ. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type 2 diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 75.Klein R, Klein BE, Moss SE. The Wisconsin Epidemiological Study of Diabetic Retinopathy: a review. Diabetes Metab Rev. 1989;5:559–570. doi: 10.1002/dmr.5610050703. [DOI] [PubMed] [Google Scholar]
- 76.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 77.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 78.Resnikoff S, Pascolini D, Etya’ale D. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 79.International Monetary Fund. World Economic Outlook Database. September 2011. Available at http://www.imf.org/external/data.htm Last accessed 4 October 2011. [Google Scholar]
- 80.Diabetes Drafting Group. Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 1985;28:615–640. doi: 10.1007/BF00290267. [DOI] [PubMed] [Google Scholar]
- 81.Broadbent DM, Scott JA, Vora JP, Harding SP. Prevalence of diabetic eye disease in an inner city population: the Liverpool Diabetic Eye Study. Eye. 1999;13:160–165. doi: 10.1038/eye.1999.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Prevalence of proliferative diabetic retinopathy in patients with diabetes according to national per capita gross domestic product.
Table S1. Literature search report for articles reporting prevalence, incidence or progression of diabetic retinopathy or diabetic maculopathy in African countries.
Table S2. Characteristics of 62 studies reporting prevalence of diabetic retinopathy and maculopathy in Africa.
Appendix S1. Search histories.
Appendix S2. Supplementary references.


