Abstract
Background
The aim of this study was to investigate the anticancer effect and related mechanisms of gambogic acid (GA), a traditional Chinese medicine, on human leukemia cell line K562, together with the effect on bone marrow mononuclear cells (MNCs).
Material/Methods
K562 cells and MNCs were treated with various concentrations and treatment times of GA. Inhibitory rate was detected by use of the Cell Counting Kit-8 (CCK-8) assay. Apoptosis was analyzed by morphological detection, Annexin-V/PI doubling staining, and TUNEL assays. The expression changes of pivotal proteins were evaluated by Western blotting.
Results
GA not only suppressed cell proliferation, but also induced apoptosis of K562 cells in a dose-dependent manner. While it did not significantly inhibit cell proliferation of MNCs, it did induce apoptosis in a dose-dependent manner. CCK-8 assay revealed that the proliferation of K562 cells was significantly inhibited when the concentration of GA was more than 0.5 μM. Morphological detection showed the nuclei became denser and more intense orange in K562 cells after GA treatment compared with the untreated group. The expression levels of BCL-2, nuclear factor-κB (NF-κB), c-myc, phosphatidylinositol3-kinase (PI3K), and phosphorylation of serine-threonine kinase (p-AKT) were down-regulated by GA.
Conclusions
GA significantly suppressed the proliferation of K562 cells, but has less effect on MNCs. The inhibition of K562 cells proliferation and apoptosis induced by GA might be related to the down-regulation of BCL-2, NF-κB, c-myc, PI3K, and p-AKT.
MeSH Keywords: Apoptosis, Bone Marrow Mononuclear Cells, Gambogic Acid, Gene Expression, Human Leukemia Cell Line K562
Background
Gambogic acid (GA, C38H44O8, mol. wt 628), the main active compound of gamboge, is an orange or brownish resin obtained from Gambogehanburyi HOOKF (genus Garcinia, family Guttiferae) [1]. It has a long history of medicinal use in Southeast Asia, and it is also used as detoxification, homeostasis, anti-inflammatory, parasiticide medicines, and even coloring agent for thousands of years in China [2]. In recent years GA, as a new anticancer drug, has attracted more and more attention, and its anticancer effects are being gradually confirmed [3–5].
Although plant-derived products have served humans as treatments of various ailments for centuries, their objective safety and molecular targets are not fully understood. Identifying the safety and their molecular targets can lead to discovering new clinical uses of such products, as in the cases of vincristine, vinblastine, and others [6]. As many as 70% of all drugs approved by the US Food and Drug Administration between 1980 and 2000 for treating cancer were based on natural sources [7,8]. Previous studies reported that GA activated apoptosis in many cancer cell lines and inhibited human hepatoma cells proliferation [1,3,5,9,10]. However, there are relatively few report on the safety and molecular mechanism in GA on human leukemia cell line K562 and bone marrow mononuclear cells (MNCs) together.
Therefore, the objective of this study was to investigate the anticancer effect and related mechanisms of GA on human leukemia cell line K562, together with the effect on MNCs. We suggest that GA might be an effective therapeutic modality for treating leukemia.
Material and Methods
Medicine
GA was purchased from BIOMOL International LP (Plymouth Meeting, PA, USA) and dissolved in DMSO (Sigma; St. Louis, MO, USA) at a stock concentration of 100 μM, lucifugal and stored at −20°C.
Cell culture
The human leukemia cell line K562 was donated by the Blood Institute in Suzhou, China. Bone MNCs were donated by healthy volunteers. K562 cells and MNCs were cultured in complete RPMI-1640 medium (Gibco, USA) supplemented with 10% and 20%, respectively, heat-inactivated fetal bovine serum (FBS, HyClone, USA), 100 μg/ml penicillin (Gibco, USA), and 100 μg/ml streptomycin (Gibco, USA), in a humidified incubator containing 5% CO2 at 37°C.
All human studies were approved by the China Ethics Committee and performed in accordance with ethics standards. The study design was approved by the local Ethics Committee.
Cytotoxicity assay
A Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Gaithersburg, MD, USA) was used to evaluate the cytotoxicity of GA. Cells were seeded in 96-well culture plates (Costar) at a density of 1–2×105/ml cells and a volume of 100 μl per well, added to 0, 0.50, 0.75, and 1.00 μM GA, respectively, followed by incubation for various periods of time. At the end of incubation, 10 μl of CCK-8 reagents was added to each well and incubated at 37°C for another 4 h. The number of viable cells was assessed by measurement of absorbance at 450 nm using Multiskan MS (Labsystems). Cytotoxicity assay was calculated as the following equation:
| [11,12]. |
Cell morphology examination
K562 cells (about 6×105/well) were incubated with 1.0 μM GA for 24 h. After treatment, cells were collected and smeared. Some films were fixed with methanol and stained with Giemsa to observe the morphological changes of apoptosis cells by light microscopy (Merck, Darmstadt, Germany), and others were directly examined with an OLYMPUS inverted microscope.
Annex in V/PI assay
Cells seeded in 6-well plates were exposed to 0, 0.50, 0.75, and 1.00 μM of GA, respectively, for 24 h. Cells undergoing apoptosis were detected using an Annexin V-FITC apoptosis detection kit (Keygen, Nanjing, China). Briefly, 2×106 cells were digested into cell suspension with EDTA-free trypsin and resuspended in cold binding buffer and incubated for 15 min in the dark at room temperature following addition of 5 μl of Annexin V-FITC and 5 μl of propidium iodide (PI, Keygen, Nanjing, China) solutions [13]. Flow cytometry analysis was performed using an FACS-Calibur cytometer (Becton Dickinson, San Jose, CA, USA).
Detection of apoptosis
The K562 cells (2.5×105 cells/cm2) were cultured on a chamber slide, fixed in 4% paraformaldehyde, and membrane-permeabilized by exposure for 30 min to 0.1% Triton X-100 in phosphate-buffered saline at room temperature. Then terminal deoxy-nucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining was performed. For 4,-6-diamidino-2-phenylindole (DAPI) staining, slides were incubated for 30 min at room temperature in the dark with mounting medium for fluorescence containing DAPI (Vectoer Laboratories, Inc., Burlingame, CA, USA). The cells were then observed through a fluorescence microscope (Leica Microsystems AG, Wetzlar, Germany).
Western blotting
Western blotting was done according to the published method with some modifications [14]. Briefly, proteins were extracted from the harvested cells using RIPA lysis buffer (Beyotime, Jiangsu, China) and then quantitated using the Bio-Rad Detergent Compatible Protein Assay kit (Keygen, Nanjing, China). An equal amount of protein (50 μg) was resolved on a 100 g/L minigel by SDS-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) using the Multiphor Novoblot electrophoresis transfer system. Membranes were blocked with 5% skim milk for 1 h and then subjected to immunoblot analysis with antibody against BCL-2 (1:500, Cambridge, MA, USA), phosphatidylinositol3-kinase (PI3K, 1:1000, Beverly, MA, USA), serine-threonine kinase (AKT, 1:1000, Beverly, MA, USA), P-AKT (1:1000, Beverly, MA, USA), c-myc (1:500, Beverly, MA, USA) and nuclear factor-κB (NF-κB, 1:1000, Beverly, MA, USA) at 4°C staying overnight, respectively. Then the membranes were washed with 0.1% Tween 20 in Tris-saline three times. A horseradish peroxidase-conjugated secondary antibody (Amersham, Arlington Heights, IL) was used at a dilution of 1:10000 for 1 h at room temperature. The membranes were subsequently developed using Enhanced chemiluminescence (ECL, Thermo scientific, Rockford, IL, USA) detection system. As a loading control, GAPDH levels were detected using 1:2000 anti-GAPDH antibody (San Diego, CA, USA) followed by 1:10000 anti-mouse secondary antibody (Jackson Immuno Research).
Statistical evaluation
Data from three independent experiments were expressed as mean ±SD. Student’s t test was used to assess variables in this study. Statistical analyses were performed with SPSS 15.0 software package (SPSS Inc, Chicago, IL). Values of p<0.05 were considered to be statistically significant.
Results
Growth inhibition induction by GA in K562 cells
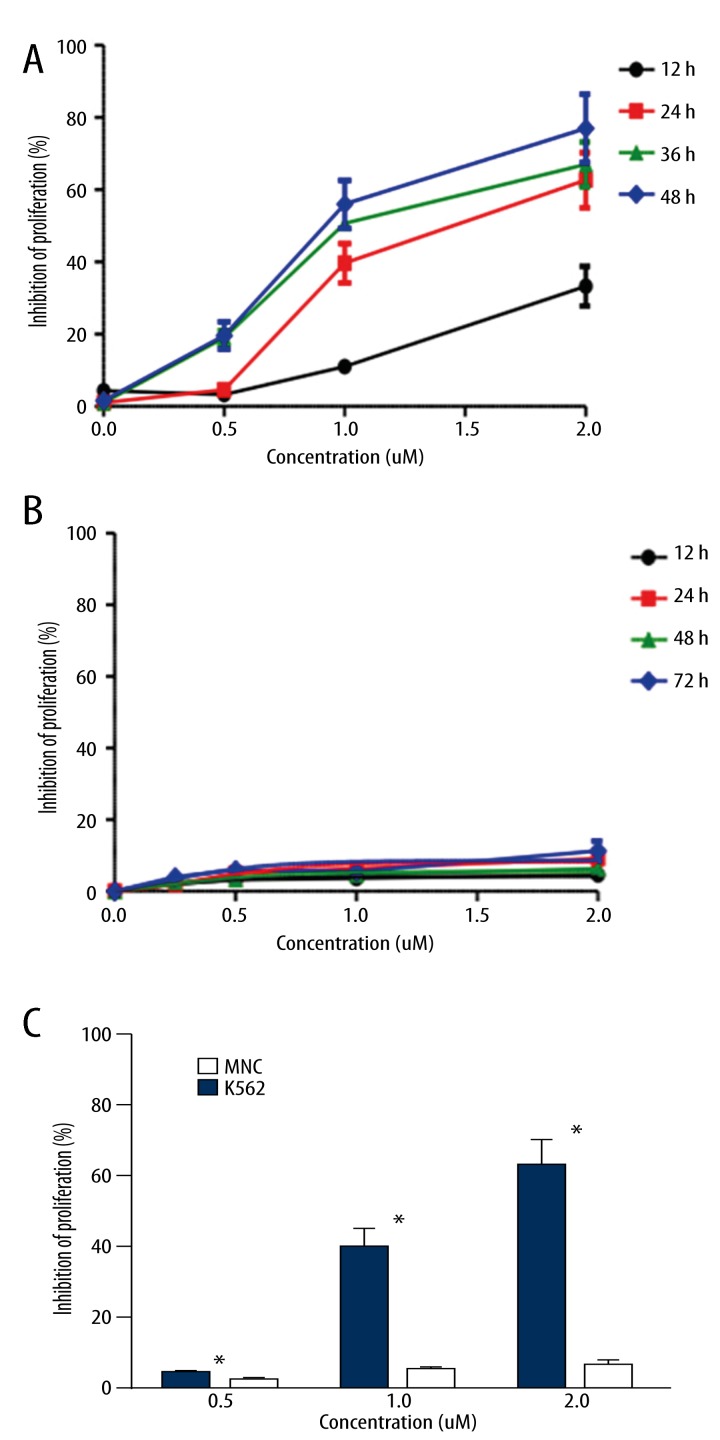
As shown in Figure 1A, treatment with GA resulted in a significant reduction in K562 cell proliferation and viability, and the degree of inhibition depended on both the concentration and the length of treatment. At a dose of 2.0 μM and 24 h treatment on K562 cells, the inhibitory rate of GA was 63%. However, those changes were not found in MNCs as shown in Figure 1B. It was clear that 72 h treatment of GA, even at a concentration as high as 4 μM, had little cytotoxicity to the MNCs. In addition, Figure 1C provided a comparison of inhibition between K562 cells and MNCs with the same treatment of GA. The inhibition of proliferation (%) of GA on K562 cells was significantly higher than that on MNCs (p<0.05).
Figure 1.
GA inhibition of proliferation of K562 cells (A) and MNCs (B). The results shown were the mean of 3 parallel experiments for each concentration point. Student’s t test was used in the comparison of inhibition ratio (%) between K562 cells and MNCs (C), * p<0.05.
Apoptosis induction by GA in K562 cells
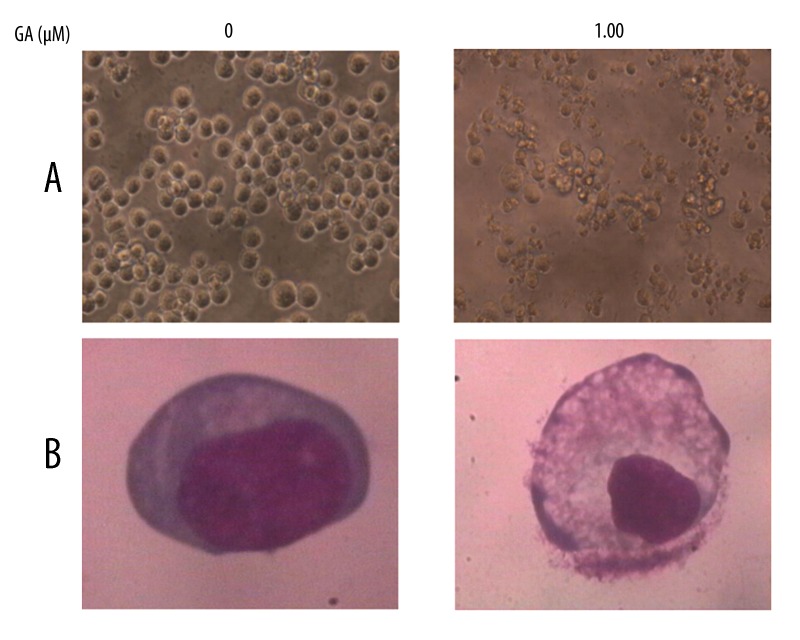
Direct observation using an inverted microscope showed many distinct morphological changes in cells treated with GA, compared with untreated cells (Figure 2A). In particular, cell shrinkage, cytoplasm condensation, and formation of cytoplasmic filaments with protuberances resulted in more of a spindle shape, membrane shrinkage. In addition, using morphological analysis with Giemsa staining, nuclei with chromatin condensation and formation of apoptotic bodies were observed in cells cultured with 1.00 μM GA for 24 h. In contrast, very few were observed in the control culture (Figure 2B).
Figure 2.
Morphological changes in K562cells observed by inverted microscope (A, magnification ×200) and by light microscope (B, stained with Giemsa, magnification ×1000).
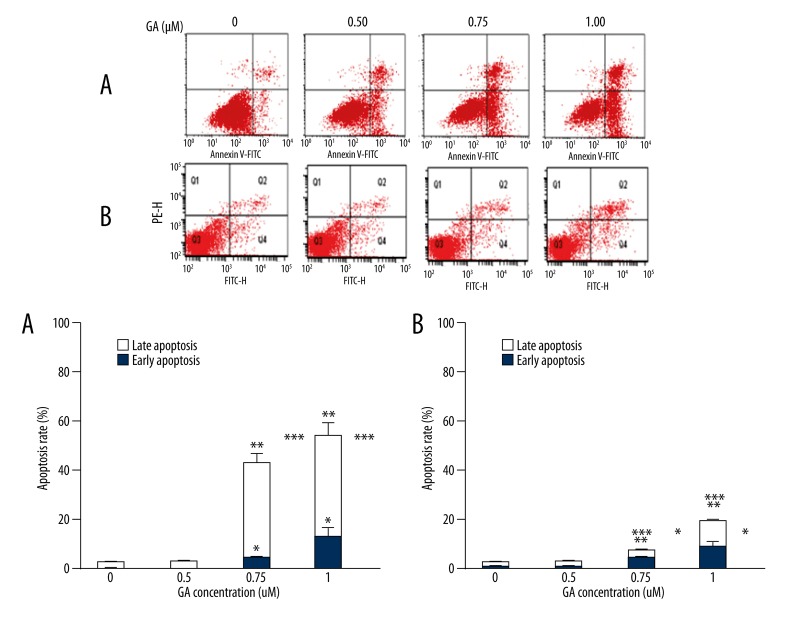
After K562 cells were treated with GA in different concentrations for 24 h, cell populations in both early and late apoptotic phases increased comparing with apoptotic cells in vehicle-treated control (p<0.05, Figure 3A). Apoptosis was also observed in MNCs but to a much less extent than in K562 cells (p<0.05, Figure 3B).
Figure 3.
Fluorescence-activated cell sorter analysis for Annexin-V and PI staining of K562 cells (A) and MNCs (B); early apoptotic cells were localized in the lower right quadrant of the dot-plot graph,* p<0.05 vs. 0 μM group, early apoptotic rate; ** p<0.05 vs. 0 μM group, late apoptotic rate;*** p<0.05 vs. 0 μM group, total apoptotic rate.
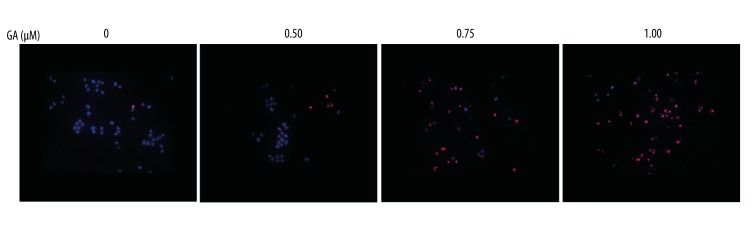
As displayed in Figure 4, apoptotic cells number (DAPI-stained TUNEL-positive cells) of K562 cells was increased compared with untreated group. Apoptosis determined after 24 h treatment was increased by 0.5 μM GA in K562 cells, and the cell death was further increased in the presence of 0.75 μM GA treatment which cause about some of apoptotic cell death itself; this trend continues at 1.00 μl GA.
Figure 4.
Apoptosis analysis of TUNEL and DAPI for K562 cells treated with the indicated concentrations of GA for 24 h.
Western blotting
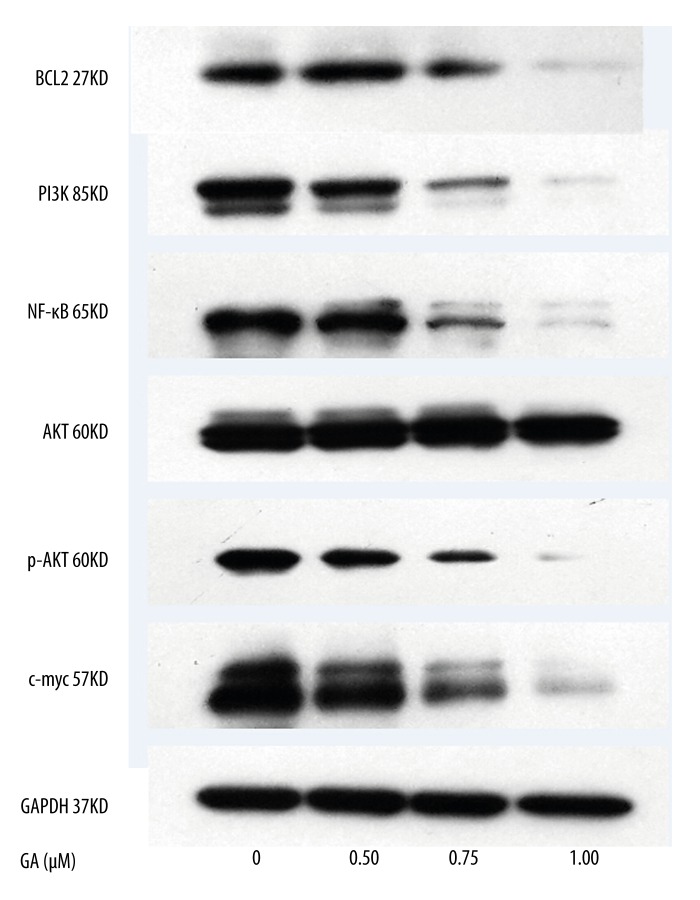
To investigate the apoptotic mechanisms activated by GA, we used western blotting to measure the expression of the death receptors and corresponding pro-apoptotic ligands, as well as the expression of the BCL2. As shown in Figure 5, the protein levels of PI3K, P-AKT, c-myc and NF-κB expression were markedly decreased in the GA-treated K562 cells, similarly the levels of anti-apoptotic BCL-2 expression were significantly inhibited in response to the GA treatment in a concentration-dependent manner. Besides, the level of AKT expression was relatively unchanged in response to GA treatment.
Figure 5.
Effects of GA treatment on levels of pivotal proteins in K562 cells. Cells were treated with the indicated concentrations of GA for 24 h. All experiments were repeated 3 times.
Discussion
In this study, we analyzed effects of GA on proliferation and apoptosis of the human leukemia cell line K562 and MNCs. Dramatically growth inhibition of GA on K562 cells was observed by CCK-8 assay and the biggest inhibitory rate was 63% (Figure 1A). Unlike K562 cells (p<0.05), the significant growth inhibition MNCs were not found in MNCs (Figure 1B, 1C). The following experiments demonstrated that cell death was partly caused by the apoptosis induction and down-regulation of pivotal proteins by GA.
Discovery of novel agents with anticancer activity from natural resources has gained significant importance in cancer prevention and therapy. The number of natural compounds with anticancer properties discovered and tested is increasing exponentially [15]. GA, an active compound extracted from the gamboge resin of Garcinia hanburyi, was selected for further study due to its potent antitumor activities [1]. According to previous study, GA has potent antitumor activities against Lewis lung carcinoma, cultured human hepatocellular carcinoma cells, and human gastric adenocarcinoma [1,3,16]. The inhibition of GA on human gastric cancer line BGC-823 was confirmed and the result was also confirmed in vivo [3]. The potential role of GA to reverse docetaxel resistance though down-regulation of surviving made it an attractive new agent for the chemosensitization of cancer cells [17]. A recent study discussed the results of a phase II trial of GA in solid cancer therapy, suggesting an alternative strategy to overcome imatinib resistance [18]. In the present study, we detected whether or not GA could inhibit proliferation of human leukemia cell line K562 and MNCs. Data obtained from CCK-8 assay showed that GA exerted significant inhibition of the proliferation of K562 cells in dose-dependent and time-dependent manners. However, those changes and tendency did not appear in MNCs, which suggests GA could induce tumor cell death selectively with less toxicity to normal cells.
Apoptosis represents a major protective mechanism against cancer [19], and morphologic observation indicated that GA could induce apoptosis on K562 cells (Figure 2). As we all know, an early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface of membrane to the extracellular surface [13]. This loss of membrane asymmetry can be detected by using the binding properties of Annexin V. To identify apoptosis, we used an Annexin V antibody, which was conjugated with a fluorescein isothiocyanate (FITC) fluorescent dye. MNCs were treated as the same methods. The results revealed that GA could accelerate the apoptosis of K562 cells, which was consistent with cellular morphological examination. Besides, GA could also promote the apoptosis of MNCs, but the function was less obvious than that on K562 cells.
Activation of apoptosis pathways is a key mechanism by which cytotoxic drugs kill tumor cells. There are two major apoptosis signaling pathways, mitochondrial and death receptor pathway. The Bcl-2 family is composed of both pro-apoptotic and anti-apoptotic family members. The best characterized anti-apoptotic proteins, BCL-2 and BCL-xL, appear to directly or indirectly preserve the integrity of the outer mitochondrial membrane, thus preventing cytochrome c release and cell death initiation through the mitochondrial pathways [20,21]. Moreover, the anti-apoptotic BCL2 gene is reported to be overexpressed in 65 to 70 percent of Chronic Lymphocytic Leukemia [22,23]. To further discuss the exact molecular mechanism of death induced by GA, some pivotal proteins were detected by western blotting. Our results showed that treatment with GA resulted in down-regulation of BCL-2, which suggested GA might induce cell apoptosis through suppressing mitochondrial pathways. Traditional clinicopathologic factors and several interesting molecules, including phosphatidylinositol 3-kinase (PI3K) [24], oncogenes such as c-myc [25], and nuclear factor-kappa B (NF-κB) have been reported to correlate to the prognosis of leukemia patients. Previous studies have also revealed numerous cellular proteins (e.g., c-myc, PI3K and AKT) were targeted by GA in several cell lines [2,26,27]. We subsequently examined the expression of pivotal genes, which are associated with cell proliferation and apoptosis. As expected, our results supported the hypothesis that the expression levels of NF-κB, c-myc, PI3K and p-AKTkt were all down-regulation by GA in K562 cells.
In the final, we would like to discuss some weak points of our study, of which we are conscious at present. It is also known that anti-sense targeting of survivin gene expression results in inhibition of cellular proliferation [28], whereas survivin over-expression promotes cell cycle entry with an accelerated S phase shift and resistance to G1 arrest [28,29]. The relationship between induction of apoptosis by GA and inhibition of the cell cycle in K562 cells were not studied. Further studies will include this point to validate the findings of the study.
Conclusions
In summary, our present findings demonstrated that GA could induce K562 cells death selectively with less toxicity to normal cells. GA might trigger K562 cells death via the mitochondrial apoptotic pathway. Besides, GA induced cell death through down-regulation of NF-κB, c-myc, PI3K and p-AKT. Our results provide a basis for developing GA into a potential therapeutic agent for the treatment of human leukemia. Yet it remains to be continued to study the mechanisms of in detail and confirmed in vivo.
Footnotes
Source of support: This study was supported by Medical Science and Technology Key Project of Nanjing, NJGL-2011196, Jiangsu Provincial Special Program of Medical Science BL2012005 and Medical Science and Technology Project of Nanjing ZKX10012
References
- 1.Wu Z-Q, Guo Q-L, You Q-D, et al. Gambogic acid inhibits proliferation of human lung carcinoma spc-a1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mrna expression in the cells. Biol Pharm Bull. 2004;27:1769–74. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- 2.Pandey MK, Sung B, Ahn KS, et al. Gambogic acid, a novel ligand for transferrin receptor, potentiates tnf-induced apoptosis through modulation of the nuclear factor-κb signaling pathway. Blood. 2007;110:3517–25. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Guo Q-L, You Q-D, et al. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line bgc-823. World J Gastroenterol. 2005;11:3655–59. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H-Z, Kasibhatla S, Wang Y, et al. Discovery, characterization and sar of gambogic acid as a potent apoptosis inducer by a hts assay. Bioorg Med Chem. 2004;12:309–17. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Guo Q-L, You Q-D, et al. Gambogic acid-induced g2/m phase cell-cycle arrest via disturbing cdk7-mediated phosphorylation of cdc2/p34 in human gastric carcinoma bgc-823 cells. Carcinogenesis. 2006;28:632–38. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Ichikawa H, Garodia P, et al. From traditional ayurvedic medicine to modern medicine: Identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets. 2006;10(1):87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κb and nuclear factor-κb–regulated gene products in ifn-α-sensitive and ifn-α-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–30. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 9.Xiong CS, Drewe J, Kasibhatla S. A chemical genetics approach for the discovery of apoptosis inducers: From phenotypic cell based hts assay and structure-activity relationship studies, to identification of potential anticancer agents and molecular targets. Curr Med Chem. 2006;13:2627–44. doi: 10.2174/092986706778201521. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Hu Y, Gu HY, et al. Oroxylin a induces g2/m phase cell-cycle arrest via inhibiting cdk7-mediated expression of cdc2/p34 in human gastric carcinoma bgc-823 cells. J Pharm Pharmacol. 2008;60:1459–63. doi: 10.1211/jpp/60.11.0006. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Fu H, Tie Y, et al. Mir-34a inhibits migration and invasion by down-regulation of c-met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Park JH, Lee M, et al. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–19. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S, Pandey MK, Yadav VR, Aggarwal BB. Gambogic acid inhibits stat3 phosphorylation through activation of protein tyrosine phosphatase shp-1: Potential role in proliferation and apoptosis. Cancer Prev Res. 2011;4:1084–94. doi: 10.1158/1940-6207.CAPR-10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chen K, Kovaříková A. Pharmacology and toxicology of toad venom. J Pharm Sci. 1967;56:1535–41. doi: 10.1002/jps.2600561202. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Chen D, Li S, et al. Gambogic acid enhances proteasome inhibitor-induced anticancer activity. Cancer Lett. 2011;301:221–28. doi: 10.1016/j.canlet.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Q-L, You Q-D, Wu Z-Q, et al. General gambogic acids inhibited growth of human hepatoma smmc-7721 cells in vitro and in nude mice. Acta Pharmacologica Sinica. 2004;25:769–74. [PubMed] [Google Scholar]
- 17.Wang T, Wei J, Qian X, et al. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262:214–22. doi: 10.1016/j.canlet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Chen X, Li X, et al. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent bcr-abl downregulation. Clin Cancer Res. 2014;20:151–63. doi: 10.1158/1078-0432.CCR-13-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Peng H, Xia G, et al. Anticancer effect of two diterpenoid compounds isolated from annona glabra linn. Acta Pharmacol Sin. 2004;25:937–42. [PubMed] [Google Scholar]
- 20.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa b and its significance in prostate cancer. Oncogene. 2001;20:7342–51. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 21.Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korz C, Pscherer A, Benner A, et al. Evidence for distinct pathomechanisms in b-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood. 2002;99:4554–61. doi: 10.1182/blood.v99.12.4554. [DOI] [PubMed] [Google Scholar]
- 23.Calin GA, Ferracin M, Cimmino A, et al. A microrna signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Li H, Yuan H, et al. Humanin delays apoptosis in k562 cells by downregulation of p38 map kinase. Apoptosis. 2005;10:963–71. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 25.Duangmano S, Dakeng S, Jiratchariyakul W, et al. Antiproliferative effects of cucurbitacin b in breast cancer cells: Down-regulation of the c-myc/htert/telomerase pathway and obstruction of the cell cycle. Int J Mol Sci. 2010;11:5323–38. doi: 10.3390/ijms11125323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Q-L, Lin S-S, You Q-D, et al. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma smmc-7721 cells. Life Sci. 2006;78:1238–45. doi: 10.1016/j.lfs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Guo Q-L, You Q-D, et al. Gambogic acid induces apoptosis and regulates expressions of bax and bcl-2 protein in human gastric carcinoma mgc-803 cells. Biol Pharm Bull. 2004;27:998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation bysurvivin gene targeting. J Biol Chem. 1998;273:11177–82. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 29.Sarela A, Verbeke C, Ramsdale J, et al. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886–92. doi: 10.1038/sj.bjc.6600133. [DOI] [PMC free article] [PubMed] [Google Scholar]