Abstract
Hürthle cell carcinoma (HCC) is a variant of a follicular carcinoma with a tendency to higher frequency of metastases and a lower survival rate. However, intracavitary cardiac metastases from thyroid HCC are extremely rare. We describe the case of a 57-year-old female with thyroid HCC, 5 years after total thyroidectomy, who presented with dyspnea associated with hypoxia and hypotension. The computed tomography angiogram showed extensive pulmonary embolism and a 6-cm right atrial mass while the lower-extremity deep vein thrombosis studies were negative. This patient received a cardiac thrombectomy using cardiopulmonary bypass support. However, intraoperatively, we found out that the mass was from the mediastinum, directly extending into the heart and clearly unresectable since it effaced at least 1/3 of the right atrial wall. The core biopsy of the mass confirmed that it was metastatic poorly differentiated HCC of thyroidal origin. The patient eventually died of respiratory failure due to a massive pulmonary embolism. For cancer patients with unexplained dyspnea, cardiac metastases should be considered regardless of anticoagulation prophylaxis, especially when there is no deep vein thrombosis in the lower limbs. Early recognition of intracavitary cardiac metastases may help in providing prompt treatment and improving the prognosis.
Key Words: Hürthle cell carcinoma, Thyroid cancer, Intracardiac metastasis, Pulmonary embolism
Introduction
Hürthle cell carcinoma (HCC), a variant of a follicular carcinoma, is referred to as follicular carcinoma (oxyphilic type). It only accounts for 3–10% of the differentiated thyroid, although the average age-adjusted annual incidence for thyroid cancer in the USA is less than 40 cases per 1 million people [1]. It behaves in a more aggressive fashion than other well-differentiated thyroid cancers, with a tendency to higher frequency of metastases and a lower survival rate. The intracardiac metastases, especially right atrial metastases, are very rare. We present a case of intracardiac metastases in the right atrium which caused a massive pulmonary embolism (PE).
Case Presentation
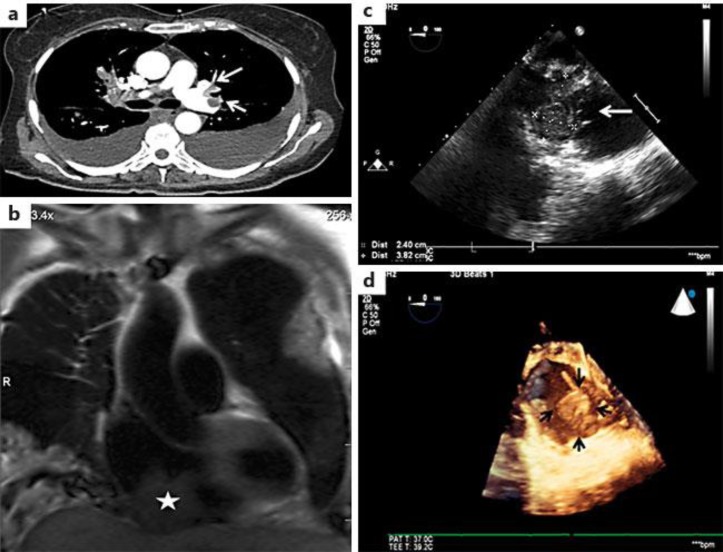
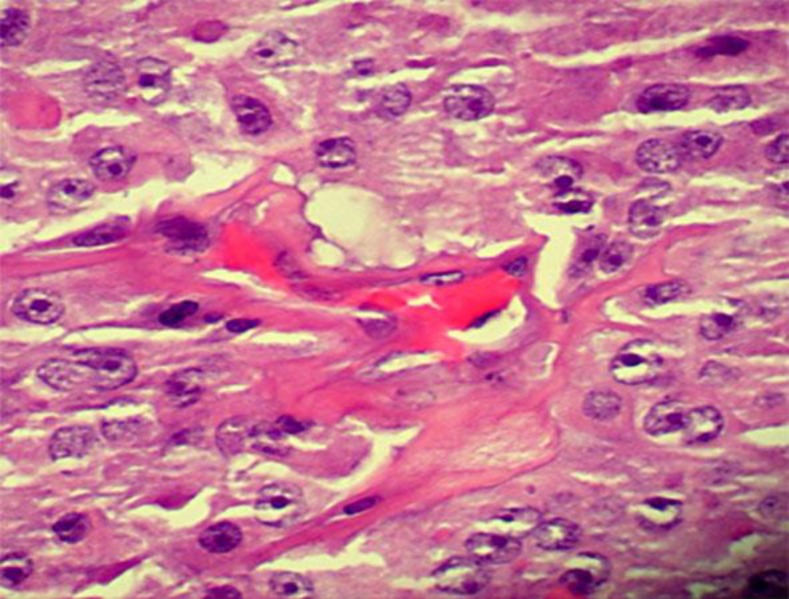
A 57-year-old female with a 5-year history of HCC presented to the emergency room with a sudden onset of dyspnea. Three years after a total thyroidectomy, she developed a right femoral pathological fracture due to bone metastasis of HCC. She started experimental treatment with lenvatinib 3 months prior to admission. At present, she had tachycardia, hypoxia and hypotension. Her computed tomography angiogram (CTA) showed extensive PE (fig. 1a), and an MRI showed multiple defects in the right atrium (fig. 1b). Her transesophageal echocardiogram demonstrated a large mobile mass in the right atrium (fig. 1c, d). She had been on enoxaparin for thrombosis prophylaxis for 3 months, and the lower-extremity deep vein thrombosis (DVT) studies were negative. The patient subsequently underwent a cardiac thrombectomy using a cardiopulmonary bypass support. Intraoperatively, the cardiac mass was found to be a direct extension from the mediastinum into the heart. It was unresectable since it affected at least 1/3 of the right atrial wall. A core biopsy demonstrated poorly differentiated HCC of thyroidal origin (fig. 2). The patient died of respiratory failure 2 weeks later due to a massive PE.
Fig. 1.
The patient had intracardiac metastases of HCC with a massive PE. a The CTA showed a bilateral effusion and extensive pulmonary embolisms involving the major branches of the left upper and lower lobes. b An MRI showed a large mass contiguous with the right ventricular and atrial septum. c The intraoperative transesophagal echocardiogram. d 3-dimensional echocardiography showed a large mass (about 6 cm) attached to the back wall of the posterior right atrium.
Fig. 2.
The patient's core biopsy demonstrated a poorly differentiated HCC of thyroidal origin.
Discussion
Thyroid cancer can be divided into 3 general subtypes based on pathology, i.e. differentiated (papillary, follicular, and Hürthle cell), medullary, and anaplastic thyroid cancers. Of the differentiated cancers, only 3% are Hürthle cell or oxyphil tumors while 85% are papillary cancers and 10% have a follicular histology [1]. HCC is more likely to metastasize into the soft tissue of the neck and other distant sites than into the cervical lymph nodes. Patients then have a higher mortality than in other differentiated thyroid cancers [2]. Intracardiac metastases are very rare. Left atrial metastasis mimicking myxoma was once reported [3]. Only a few cases of right atrial metastases have been reported by literature research. A dangerous complication of right-side intracardial metastases is PE, refractory to anticoagulation treatment.
The incidence rate for cardiac metastasis is approximately 1.23% of all metastatic diseases of HCC, and only less than 20% of them occur in the right chambers [4]. Cardiac metastases to the myocardium are much less frequented than those to the pericardium/epicardium. Intracavitary, endocardial or valvular metastatic deposits occur in less than 6% of cases [5]. Surprisingly, our patient has right atrial intracavity metastases involving at least 1/3 of the atrial wall.
Metastatic disease to the heart is rare. The incidence quoted in the current literature, based on autopsy, is approximately 1.23% [4]. They are 20–40 times more common than the primary cardiac tumors, accounting for approximately 96.5% of all cardiac tumors [6]. Cardiac metastases involving the myocardium are much less frequent than those involving the pericardium/epicardium, which is the most common site. Intracavitary, endocardial or valvular metastatic deposits (presented in our case) occur in less than 6% of known cases [5]. Approximately 80% of this type of metastases occur in the right chambers and only rarely in the left chambers. The cardiac metastases can be located by multiple spreading methods, including lymphatic, direct or hematogenous spread dependent on the location and histopathology of the primary malignancy. In our case, it was found that the tumor directly extended from the mediastinum into the right atrium.
Patients with cancer have a high risk of developing venous thromboembolism (VTE), which incorporates DVT and PE, with 20% of VTE events occurring in cancer patients [7]. The VTE incidences vary by cancer site. For thyroid cancer, the hazard rates are only 1.8 (CI 0.8–3.7), not significantly higher than in controls [7]. A recent study suggested that the hazard ratio of PE in HCC patients treated with lenvatinib was only 2.7%, which is not significantly higher than that in placebo groups (1.5%) [8]. Therefore, we did not speculate that our patient's PE was caused by the lenvatinib treatment. She developed a massive PE without any evidence of DVT despite thrombosis prophylaxis with enoxaparin. This suggested that her PE might have been caused by mechanical changes of the right atrial metastases rather than by common risk factors such as immobility and hypercoagulation.
The diagnosis of cardiac metastases is clinically difficult because the symptoms are nonspecific. Left-sided intracardial metastases mimicking myxoma could induce obstructive heart failure, syncope or stroke; right-sided intracardial metastases can cause recurrent PE, refractory to anticoagulation treatment. It may be unrecognized until positive image finding. Therefore, image study is essential in the diagnosis of cardiac metastases. Currently, the echocardiography remains the most efficient method for an initial diagnosis. The transesophageal echo may better visualize the atrial and the great vessels, while CT/MRI is more useful for tumor location, morphological features and associated mediastinal and/or pulmonary involvement. The real-time 3-dimensional echocardiography is used in many intraoperative assessments such as ventricular endomyocardial biopsies and the placement of catheter delivered valvular devices.
Conclusions
Intracavitary cardiac metastases from thyroid HCC are extremely rare. For cancer patients with unexplained dyspnea, cardiac metastases should be considered regardless of anticoagulation prophylaxis, especially when there is no DVT in the lower limbs. Early recognition of intracavitary cardiac metastases may help in providing prompt treatment and improving the prognosis.
Statement of Ethics
The authors declare that all examinations and interventions have been examined and approved by the appropriate ethics committee of Raritan Bay Medical Center and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Trigo JM, Capdevila J, Grande E, et al. Thyroid cancer: SEOM clinical guidelines. Clin Transl Oncol. 2014;16:1035–1042. doi: 10.1007/s12094-014-1224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chindris AM, Casler JD, Bernet VJ, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015;100:55–62. doi: 10.1210/jc.2014-1634. [DOI] [PubMed] [Google Scholar]
- 3.Giovanella L, Treglia G, Ceriani L, et al. Left atrial metastasis of Hürthle-cell thyroid carcinoma mimicking myxoma. J Nucl Cardiol. 2014;21:406–407. doi: 10.1007/s12350-013-9826-8. [DOI] [PubMed] [Google Scholar]
- 4.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 5.Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol. 1988;18:195–201. [PubMed] [Google Scholar]
- 6.Borsaru AD, Lau KK, Solin P. Cardiac metastasis: a cause of recurrent pulmonary emboli. Br J Radiol. 2007;80:e50–e53. doi: 10.1259/bjr/94870835. [DOI] [PubMed] [Google Scholar]
- 7.Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]