Abstract
CAPSULE
Clinical outcomes using INVOcell device with ICSI.
OBJECTIVE
Intravaginal culture of oocytes (INVO) procedure is an intravaginal culture system that utilizes the INVOcell device in which the fertilization and embryo culture occur. In this procedure, the vaginal cavity serves as an incubator for oocyte fertilization and early embryonic development. The objective of this study was to evaluate the clinical outcomes of this intravaginal culture system in intracytoplasmic sperm injection (ICSI).
METHODS
A total of 24 cycles INVO-ICSI (study group) and 74 cycles of ICSI (control group) were included in the study. The cleaved oocytes at day 3/total injected oocytes, embryo quality, pregnancy rate (PR), implantation rate (IR), and miscarriage rate (MR) were compared between both groups.
RESULTS
At day 3, there was no difference in the cleaved oocyte rate (78.7 and 76.1%) and embryo quality (77 and 86.8%) for the study and control groups, respectively. In the study group, more embryos were significantly transferred compared to the control group (2.63 ± 0.58 versus 1.93 ± 0.25; P < 0.05). PRs, IRs, and MRs were similar for the study group compared with the control group (PR: 54.2% versus 58.1%; IR: 31.7% versus 33.6%; MR: 7.7% versus 20.9%).
CONCLUSIONS
Good PR and IR can be obtained using the INVOcell device, and the INVO-ICSI procedure can be considered as an alternative option to infertile patients.
Keywords: INVO, INVOcell, ICSI, pregnancy rate, implantation rate
Introduction
Since the birth of Louise Brown by in vitro fertilization (IVF) in 1978, scientific advances, such as ovarian stimulation, embryo cryopreservation, and intracytoplasmic sperm injection (ICSI), have been accomplished in assisted reproduction technologies (ART); thus, between 1% and 4% of children are born through ART.1 Procedures such as IVF and ICSI imply that the fertilization of the oocyte by the sperm occurs in the laboratory, simulating the physiological conditions where embryos are developed in vivo. Embryos are cultured in vitro until transferred into the uterus.
Ranoux et al2 developed an intravaginal culture system named INVO (intravaginal culture of oocytes). They proposed a simplified alternative option to conventional IVF.2 The INVO procedure is simple, requires minimal laboratory equipment, and has a low cost, utilizing the vaginal cavity environment for oocyte fertilization and incubation of early embryo development. The vaginal cavity provides the pCO2, pO2, and temperature for the culture of the gametes and embryos.3 The INVOcell device,4 specially designed for the INVO procedure, is permeable to gas, allowing the equilibrium between the pCO2 of the vagina and the pCO2 of the culture medium to maintain a pH between 7.2 and 7.4 during the entire period of vaginal incubation. Moreover, INVO procedures have been performed in several countries such as Austria, Bolivia, Brazil, Ecuador, India, Mexico, Nicaragua, Pakistan, Panama, Spain, Turkey, and Venezuela with pregnancy rates (PRs) ranging from 19.6% to 31%.2,5–8
In the conventional IVF procedure, the oocytes are inseminated with a total of 50,000–100,000 spermatozoa with fertilization and cleavage rates of 80% and 95% respectively. In the INVO procedure, the inner chamber of the INVOcell has a volume of 1.08 mL, and there the oocytes are inseminated with a maximum of 30,000 spermatozoa to avoid the risk of polyspermic embryos; however, this reduced spermatozoa concentration in high culture media volume results in low percentages of cleaved embryos at day 3 (30%–60%).8 The INVO procedure cannot be examined within the first 16–18 hours following insemination to validate 2PN fertilization. As a result, abnormal fertilization rates are reduced by ICSI, which shows similar results as IVF in fertilization and cleavage rates.9 All in all, injecting each oocyte with a single sperm greatly increases the available number of zygotes and cleavage embryos and, thus, the efficiency of the procedure.
It is of utmost importance to point out that IVF requires expensive and complex electromechanical devices, along with respective monitoring and calibration procedures. The cost is also increased when considering that temperature and CO2 and O2 concentrations must be maintained at constant levels. In contrast, the INVOcell takes into consideration all the aforementioned factors and makes use of the vaginal cavity, efficiently providing the required homeostatic conditions in vivo, disregarding any undesired effects characteristic of the in vitro procedure. It is in this manner that the process of fertilization and the prospective embryonic development can take place in a natural microenvironment and at a reduced cost for the patients.
The aim of the present study was to evaluate the development capacity, embryo quality, and clinical outcomes using the INVOcell device. The procedure itself revealed to be efficient and safer as an alternative treatment for infertile patients as part of ICSI.
Materials and Methods
Patients
This is a retrospective nonrandomized study obtained from 24 INVO-ICSI cycles (study group; 23 patients) done at FERTILAB Laboratory of Assisted Reproduction (Lima, Peru) between February 2012 and December 2014. Seventy-four ICSI cycles performed in the same period were used as the control group (70 patients). Patients gave their written, informed consent to be included in the study.
Difference in ages among males and females within both groups was insignificant (Table 1). The clinical characteristics from patients in the study and control group were female factor (29.2% and 18.9%), male factor (12.5% and 18.9%), and multiple factor (58.3% and 62.2%), respectively. Cases of severe endometriosis, egg donation, severe male factor, and azoospermia were not included. The Institutional Review Board and the corresponding Ethics Committee from Clínica Oncogyn (Lima, Peru) approved the study.
Table 1.
Comparison of patient’s age and hormonal stimulation in both evaluated groups.
| STUDY GROUP | CONTROL GROUP | |
|---|---|---|
| No. of cycles | 24 | 74 |
| Female age (years) (Mean ± SD) |
35.29 ± 4.65 | 33.66 ± 4.32 |
| Male age (years) (Mean ± SD) |
40.78 ± 9.83 | 37.86 ± 6.65 |
| No. of days of stimulation (Mean ± SD) |
7.96 ± 1.23 | 8.03 ± 1.26 |
| No. of rFSH/patient (IU) (Mean ± SD) |
1212.67 ± 418.43 | 1327.08 ± 307.35 |
| No. of HMG/patient (IU) (Mean ± SD) |
1654.19 ± 411.55 | 1425.02 ± 646.92 |
Note: P = not significant.
Ovarian stimulation and oocyte collection
The menstrual cycles of patients were stimulated using recombinant follicle stimulating hormone (FSH) (Gonal®, Merck Serono Laboratories, Peru) and HMG (Menopur®, Ferring Farmaceutical, Peru) according to previously established stimulation protocols.10 There were no differences in hormonal stimulations in both evaluated groups (Table 1). Medication was started on day 2 of the menstrual cycle until at least three follicles reached ~18 mm in diameter. The oocyte recovery was performed by vaginal ultrasound 36 h after the intramuscular administration of Human Chorionic Gonadotropin (hCG; Pregnyl®, Ferring Farmaceutical). Patients underwent oocyte recovery under general anesthesia with 200 mg of Propofol intravenous (Diprivan® 1% P/V; AstraZeneca Laboratories, UK).
During the follicular aspiration procedure, oocytes were recovered in Global®-HEPES–buffered medium (IVFonline, Canada) supplemented with 10% vol/vol Serum Substitute Supplement (SSS; Irvine Scientific, USA). After retrieval, cumulus–oocyte complexes were manually denuded from cumulus cells using sterile needles and cultured in ~200 μL drops of Global®-Fertilization medium (IVFonline) plus 10% SSS under oil at 37°C and an atmosphere containing 6% CO2, 5% O2, and 89% N2 for 5 hours before the ICSI procedure.
Semen samples
Semen samples were collected by masturbation after 3–5 days of abstinence and on the day of oocyte retrieval for ICSI. Semen analysis was performed according to World Health Organization criteria.11 After liquefaction, motile spermatozoa were separated from the seminal plasma by centrifugation at 300 × g for 10 minutes through 1.0-mL 95% and 45% isolate gradients (Irvine Scientific). The pellet was washed once by centrifugation for 5 minutes and was resuspended in 0.1 mL of Global Fertilization medium +10% SSS for ICSI.
INVOcell device preparation
The INVOcell device (INVO Bioscience, USA) is composed of an inner chamber with a rotating valve and a protective outer rigid shell (Fig. 1). On the morning of the INVO procedure, the inner chamber was filled with Global® medium (IVFonline) supplemented with 10% vol/vol SSS, closed and maintained inside the incubator at 37°C and an atmosphere of 6% CO2, 5% O2, and 89% N2 until the oocytes were loaded inside.
Figure 1.

Inner chamber and outer rigid shell of the INVOcell device.
ICSI and embryo culture
Five hours after aspiration, the cumulus–oocyte complexes were denuded with 80 UI/mL of hyaluronidase (IVFonline) and the oocytes in metaphase II were injected according to methods previously described.12
In the study group, 5.29 injected oocytes were transferred to the inner chamber and close to the rotating valve on average. The inner chamber was placed into the outer rigid shell and immediately inserted into the vaginal cavity altogether with a diaphragm as a retention system. During the incubation time, the patients had no intercourse, bath, swimming, or vaginal douche.
After 72 hours of the culture period, the INVOcell device was removed manually from the vaginal cavity. The outer rigid shell was opened and discarded; embryos were retrieved from the inner chamber, placed under mineral oil in 10-μL droplets of Global® medium (IVFonline) supplemented with 10% vol/vol SSS, and their quality was evaluated under the microscope. Additionally, how the INVO are not examined after 16–18 hours to validate 2PN fertilization, therefore we compared the percentages of cleaved oocyte at day 3/total injected oocytes between evaluated groups. In the control group, after the ICSI procedure (day 0), all injected oocytes were cultured at 37°C in an atmosphere of 6% CO2, 5% O2, and 89% N2. The fertilization was evaluated 16–18 hours post injection by the presence of two pronuclei (day 1). The zygotes were individually cultured under mineral oil, in 10-μL droplets of Global® medium (IVFonline) supplemented with 10% vol/vol SSS from day 1 to day 3. On day 3, the embryos were moved to fresh 10-μL droplets of Global® medium +10% SSS and cultured 2 days more up to the transfer day in blastocyst stage.
Good quality day 3 embryos were defined as those with 6–8 cells, no multinucleation, and ≤10% of fragmentation. Good quality blastocysts were defined as having an inner cell mass (ICM) and trophoectoderm type A or B.13 The ICM score was evaluated as follows: type A = compact area, many cells present; type B = cells are loosely grouped. The trophoectoderm was scored as follows: type A = many cells forming a tight epithelial network of cells; type B = few cells forming a loose network of cells.
Embryo transfer
Embryos were transferred on day 3 or 5 using an Emtrac embryo transfer catheter (Gynétics Medical Products, Lommel, Belgium) that had been previously washed with culture medium. The catheter was completely filled with the culture medium having the embryos in the last 10 μL of the catheter. All transfers were performed according to the methods previously described by Mansour.14 The embryos that were not transferred were cryopreserved or discarded according to their morphology.
Pregnancy determinations
The biochemical pregnancy was assessed 14 days after the embryo transfer by measuring the hCG-beta subunit in blood. The clinical pregnancy was determined by transvaginal ultrasonography to detect gestational sacs and fetal heartbeats at approximately 21 and 28 days after transfer, respectively.
Statistical analysis
Statistical analysis was carried out using the statistic package Stata 10 (StataCorp, College Station, TX, USA). Data are represented as mean ± SD. Group comparisons were made using the χ2 test and Student’s t-test. It was considered a statistically significant difference when P < 0.05. The clinic PR was calculated from the number of patients with at least one gestational sac divided by the total number of embryo transfers by 100. The implantation rate (IR) was calculated by dividing the number of gestational sacs observed by ultrasound at the 21st day post transfer by the total number of embryos transferred by 100. The miscarriage rate (MR) was defined as the number of pregnancies with total loss of gestational sacs before 20 weeks of gestation between the numbers of pregnancies by 100.
Results
Laboratory and clinical outcomes obtained from the study group (INVO-ICSI cycles) and the control group (ICSI cycles) are shown in Table 2. A total of 140 and 627 oocytes were collected from both study and control groups, respectively. One hundred and twenty-seven from the study group and 544 oocytes from the control groups were injected. There were a total of 78.7% and 76.1% of cleaved oocytes at day 3/total injected oocytes, in the study and control groups, respectively. There was no difference in the number of media cells and the quality of embryo at day 3 between groups. The patients of the study group significantly received more embryos compared to those patients from the control group (2.63 ± 0.58 versus 1.93 ± 0.25; P < 0.05).
Table 2.
Comparison of laboratory and clinical results between the study and control groups.
| STUDY GROUP | CONTROL GROUP | |
|---|---|---|
| No. of total oocytes | 140 | 627 |
| No. of total injected oocytes | 127 | 544 |
| No. of total fertilized oocytes (2PN) (%) | – | 423 (77.8) |
| Cleaved oocyte at day 3/total injected oocytes (%) | 78.7 (100/127) | 76.1 (414/544) |
| No. of cell/embryo at day 3 (Mean ± SD) | 6.79 ± 1.28 | 7.47 ± 0.72 |
| Good quality embryos at day 3 (%) | 77 (77/100) | 86.8 (367/423) |
| Blastocyst formation rate (%) | – | 50.6 (214/423) |
| No. of embryo transferred/patient (Mean ± SD) | 63 (2.63 ± 0.58)* | 143 (1.93 ± 0.25) |
| Pregnancy rate (%) | 54.2 (13/24) | 58.1 (43/74) |
| Implantation rate (%) | 31.7 (20/63) | 33.6 (48/143) |
| Miscarriages (%) | 7.7 (1/13) | 20.9 (9/43) |
| Biochemical pregnancy rate (%) | 0– | 4.4 (2/45) |
| Single pregnancy (%) | 61.5 (8/13) | 88.4 (38/43) |
| Twin pregnancy (%) | 23.1 (3/13) | 11.6 (5/43) |
| Triple pregnancy (%) | 15.4 (2/13) | 0– |
Note:
P < 0.05 compared to the control group.
PR, IR, and MR were similar in the study and the control group (PR: 54.2% and 58.1%; IR: 31.7% and 33.6%; MR: 7.7% and 20.9%; P = not significant) (Fig. 2). For the study group, one and two gestational sacs were observed in 8 (61.5%) and 3 (23.1%) patients, respectively. For the control group, one and two gestational sacs were observed in 38 (88.4%) and 5 (11.6%), respectively. These percentages were similar in both evaluated groups. Additionally, three gestational sacs were observed in two patients from the study group (15.4%) (Table 2).
Figure 2.
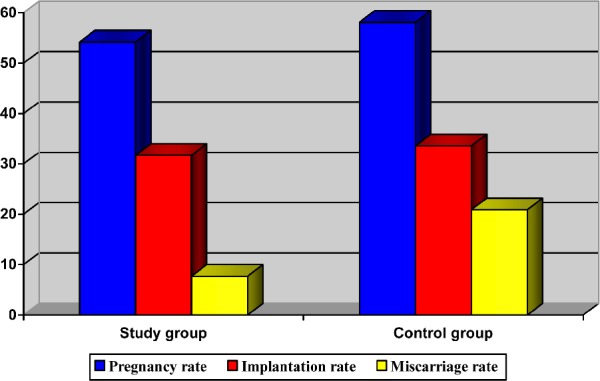
PRs, IRs, and MRs.
Discussion
The INVO procedure consists of utilizing the vaginal cavity environment for the oocyte fertilization and embryo development.2 We assessed this procedure using the INVOcell device and evaluated the PR, IR, and MRs. Our data showed that the formation of normal zygotes and good quality embryos after microinjection is possible, as well as good clinical outcomes. The INVOcell device can be inserted transiently in the vagina for up to 3 or 5 days without bleeding or any clinical impact or endometrial receptivity. An important result of this study was to find that the INVO-ICSI procedure results in PR and IRs similar to those achieved by classical ICSI procedures (PR: 54.2% versus 58.1%; IR: 31.7% versus 33.6%), exacerbating that the INVOcell device effectively minimizes any external factors that could negatively affect both gametes and embryos as those observed during in vitro procedures. In INVO-ICSI group, more embryos were transferred to patients and this may explain the similar pregnancy and implantation results; however, Abdelmassih et al15 and Karaki et al16 reported lower IRs (18.5% versus 45.3% and 13% versus 26%, respectively) with more transferred embryos on day 3 compared with day 5 (4.0 versus 5.0 and 3.5 versus 2.0, respectively). In the present study, we modified the classical INVO procedure, injecting the metaphase II oocytes to ensure a higher zygote number and cleavage embryos. These changes have allowed us to achieve high cleavage and PRs compared to those prelaunched results reported by Ranoux8 (88.3% versus 49.9% and 43.3% versus 17.1%, respectively).
Although human embryos can develop successfully in atmospheric concentrations of oxygen (20%), some authors have suggested that low oxygen concentrations (5%) resemble the physiological conditions of the uterus effectively, and thereby improve the quality, viability, and embryo morphology.17,18 Bavister19 and Karagenc et al20 showed that embryo culturing in 20% of O2 produces damage and morphological disorganization with few vacuolated cells in the embryonic ICM. Moreover, the INVOcell device is permeable to gas, allowing adequate equilibrium of pO2 that resembles the uterine cavity atmosphere of around 5% of oxygen; and pCO2 that maintains the pH of the culture medium between 7.2 and 7.4 during the entire period of vaginal incubation. These gas concentrations ensure an adequate energetic metabolism necessary for the gametes’ viability, fertilization, and embryonic development, which were demonstrated with similar clinical outcomes between INVO and ICSI cycles in the present study.
There are many commercial media formulations for stage-specific use. Recently, a single medium was formulated that presents all embryo components during all stages of post fertilization in in vitro development.21 Classically, the movement of embryos to fresh medium on day 3 has been suggested as a technique to avoid exposure of embryos to the potential buildup of ammonium from the breakdown of amino acids or volatile atmospheric compounds. The Global® single-culture medium designed to limit the buildup of ammonium by replacement of glutamine with a more stable form, could be used for continuous uninterrupted culture of human embryos.22,23 A preliminary study of 12 cycles with extended culture to 5 days without medium renewal resulted in similar PRs and IRs to those observed in patients who were transferred at day 3 (data unpublished). Our preliminary data showed that the same medium could be used throughout the 5 days of culture without medium renewal on day 3. However, a larger sample size and well-designed prospective studies should be carried out with zygotes and embryos cultured in IVF labs with all variables controlled, in order to reveal more advantages regarding the application of extended culture without medium renewal.
Additionally, reports suggest that the INVO procedure entails psychological benefits for the patients, allowing them to be directly involved in the fertilization and embryonic development procedures by carrying the INVOcell device in the vaginal cavity, thus rendering the technique as reliable and reducing the anxiety levels experienced.24 This has led to increased acceptance levels of the procedure and a decrease in stress levels characteristic of assisted reproduction patients. Nevertheless, we suggest that further studies be carried out in order to evaluate the psychological evolution of the patients who undergo the INVO procedure, thereby revealing its potential advantages.
Finally, our results show that the INVO procedure combined with ICSI allows the procurement of high-quality embryos with similar IRs as those observed during IVF and ICSI. This in turn validates its relevance as an alternative treatment for infertile couples at a lower cost. Therefore, we suggest that further research and well-designed studies be carried out, in which all variables are controlled including chorionic villus sampling or amniocentesis test, in order to reveal more advantages regarding the utilization of the INVOcell device as part of standard ART.
Conclusion
The presented results allow us to suggest the INVOcell device as an intravaginal culture procedure with acceptable clinical outcomes, hence rendering it as an additional alternative to the existing assisted reproduction procedures.
Footnotes
ACADEMIC EDITOR: Zeev Blumenfeld, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JGF, RH. Analyzed the data: JGF, DL, LV. Wrote the first draft of the manuscript: JGF, RH, DL. Contributed to the writing of the manuscript: JGF, RH, DL. Agree with manuscript results and conclusions: JGF, RH, DL, LV, RR, PZ, JDC. Jointly developed the structure and arguments for the paper: JGF. Made critical revisions and approved final version: JGF, RH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Hansen M, Boer C, Mine E, de Klerk N, Kurinczuck JJ. Assisted reproductive techonologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20:328–338. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]
- 2.Ranoux C, Aubriot FX, Dubuisson JB, et al. A new in vitro fertilization technique: intravaginal culture. Fertil Steril. 1988;49:654–657. doi: 10.1016/s0015-0282(16)59835-5. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M, Fukuda K, Ranoux C. Unexpected low oxygen tension of intravaginal culture. Hum Reprod. 1996;11:1293–1295. doi: 10.1093/oxfordjournals.humrep.a019374. [DOI] [PubMed] [Google Scholar]
- 4.Frydman R, Ranoux C. INVO: a simple, low cost effective assisted reproductive technology. ESHRE monographs, special task force on “developing countries and infertility”. Hum Reprod. 2008;163:85–89. [Google Scholar]
- 5.Ranoux C, Seibel MM. New techniques in fertilization: intravaginal culture and microvolume straw. J In Vitro Fert Embryo Transf. 1990;7:6–8. doi: 10.1007/BF01133876. [DOI] [PubMed] [Google Scholar]
- 6.Bonaventura L, Ahlering P, Morris R, Mouchel J, Scheiber M, Batzofin J. The INVOcell, a new medical device for intra vaginal fertilization and culture. Fertil Steril. 2006;86(3):S164. [Google Scholar]
- 7.Lucena E, Saa AM, Navarro DE, Pulido C, Lombana O, Moran A. INVO procedure: minimally invasive IVF as an alternative treatment option for infertile couples. Sci World J. 2012;2:1–6. doi: 10.1100/2012/571596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranoux C. In vivo embryo culture device. In: Nagy ZP, Varghese A, Agarwal A, editors. Practical Manual of In Vitro Fertilization. Berlin: Springer; 2012. pp. 161–169. [Google Scholar]
- 9.Palermo GD, Neri QV, Monahan D, Kocent J, Rosenwaks Z. Development and current applications of assisted fertilization. Fertil Steril. 2012;97:248–259. doi: 10.1016/j.fertnstert.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Tavmergen E, Goker E, Sendag F, Levi R. Comparison of short and long ovulation induction protocols used in ART applications according to the ovarian response and outcome of pregnancy. Arch Gynecol Obstet. 2002;266:5–11. doi: 10.1007/pl00007494. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Laboratory Manual for Examination and Processing. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 12.García J, Noriega Hoces L, Gonzales GF. Sperm chromatin stability and its relationship with fertilization rate after intracytoplasmic sperm injection (ICSI) in an assisted reproduction program. J Assist Reprod Genet. 2007;24:587–593. doi: 10.1007/s10815-007-9174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainly: Infertility and Genetics Beyond. UK: Parthenon press; 1999. p. 378. [Google Scholar]
- 14.Mansour R. Minimizing embryo expulsion after embryo transfer: a randomized controlled study. Hum Reprod. 2005;20:170–174. doi: 10.1093/humrep/deh573. [DOI] [PubMed] [Google Scholar]
- 15.Abdelmassih V, Balmaceda JP, Nagy ZP, Abdelmassih S, Abdelmassih R. ICSI and day 5 embryo transfers: higher implantation rates and lower rate of multiple pregnancy with prolonged culture. Reprod Biomed Online. 2001;3:216–220. doi: 10.1016/s1472-6483(10)62039-1. [DOI] [PubMed] [Google Scholar]
- 16.Karaki RZ, Samarraie SS, Younis NA, Lahloub TM, Ibrahim MH. Blastocsyt culture and transfer: a step toward improved in vitro fertilization outcome. Fertil Steril. 2002;77:114–118. doi: 10.1016/s0015-0282(01)02939-9. [DOI] [PubMed] [Google Scholar]
- 17.Gardner D, Lane M. Embryo culture in textbook of assisted reproductive techniques: laboratory and clinical perspective. In: Gardner D, Weissman A, Howles C, Shoham Z, editors. Laboratory and Clinical Perspectives. London: Martin Dunitz Press; 2001. pp. 203–222. [Google Scholar]
- 18.Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2000;6:84–96. doi: 10.1016/s1472-6483(10)62060-3. [DOI] [PubMed] [Google Scholar]
- 19.Bavister BD. The role of animal studies in supporting human ART. Reprod Fert Dev. 2004;16:719–728. doi: 10.1071/rd04087. [DOI] [PubMed] [Google Scholar]
- 20.Karagenc L, Sertkaya Z, Ciray N. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod Biomed Online. 2004;9:409–417. doi: 10.1016/s1472-6483(10)61276-x. [DOI] [PubMed] [Google Scholar]
- 21.Sepúlveda S, García J, Arriaga E, Díaz J, Noriega-Portella L, Noriega-Hoces L. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2008;91:1765–1770. doi: 10.1016/j.fertnstert.2008.02.169. [DOI] [PubMed] [Google Scholar]
- 22.Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling embryo study. Fertil Steril. 2009;92:1783–1786. doi: 10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Borges N, Belles M, Herreros J, et al. Single medium culture in a time-lapse incubator until the blastocyst stage with or without medium renewal on Day-3: a prospective randomized study with donor oocytes. Hum Reprod. 2013;28(suppl 1):167. [Google Scholar]
- 24.Vieira GH, Colucci FA. Comparative preliminary study between the conventional IVF/ICSI and the INVO intra-vaginal device: stress-related psychological impact. JBRA Assist Reprod. 2013;17:300–303. [Google Scholar]


