Abstract
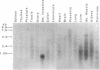
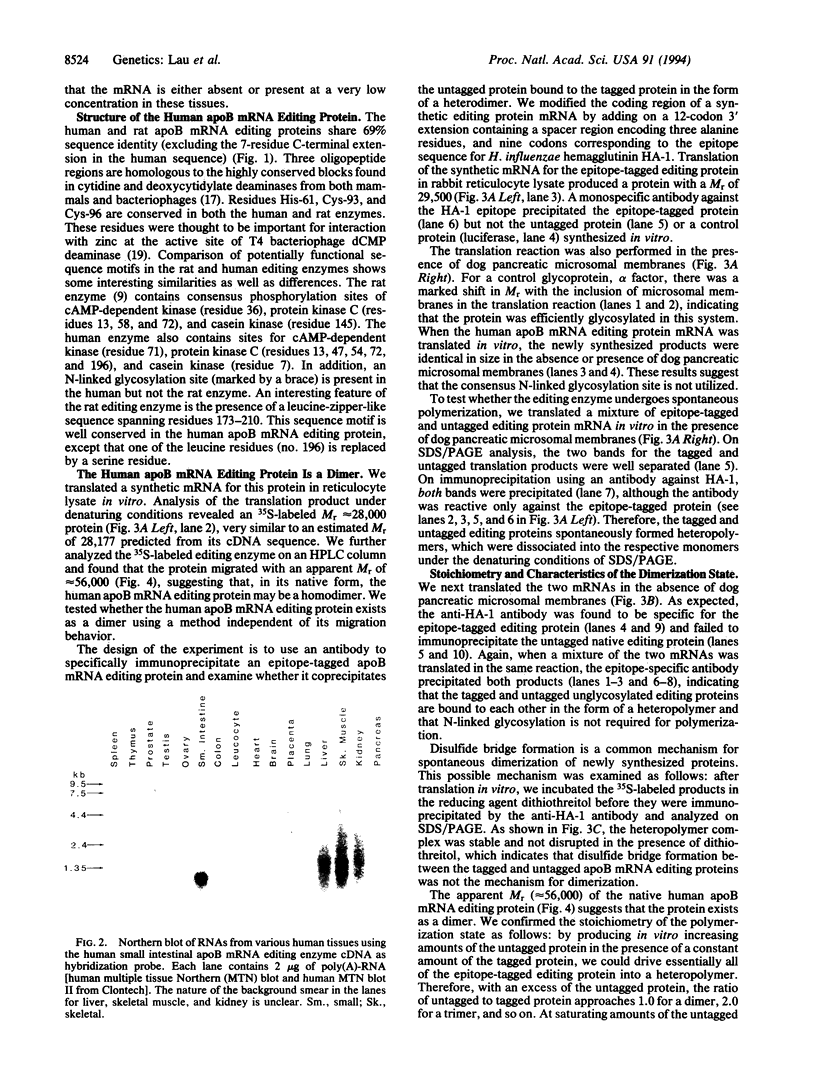
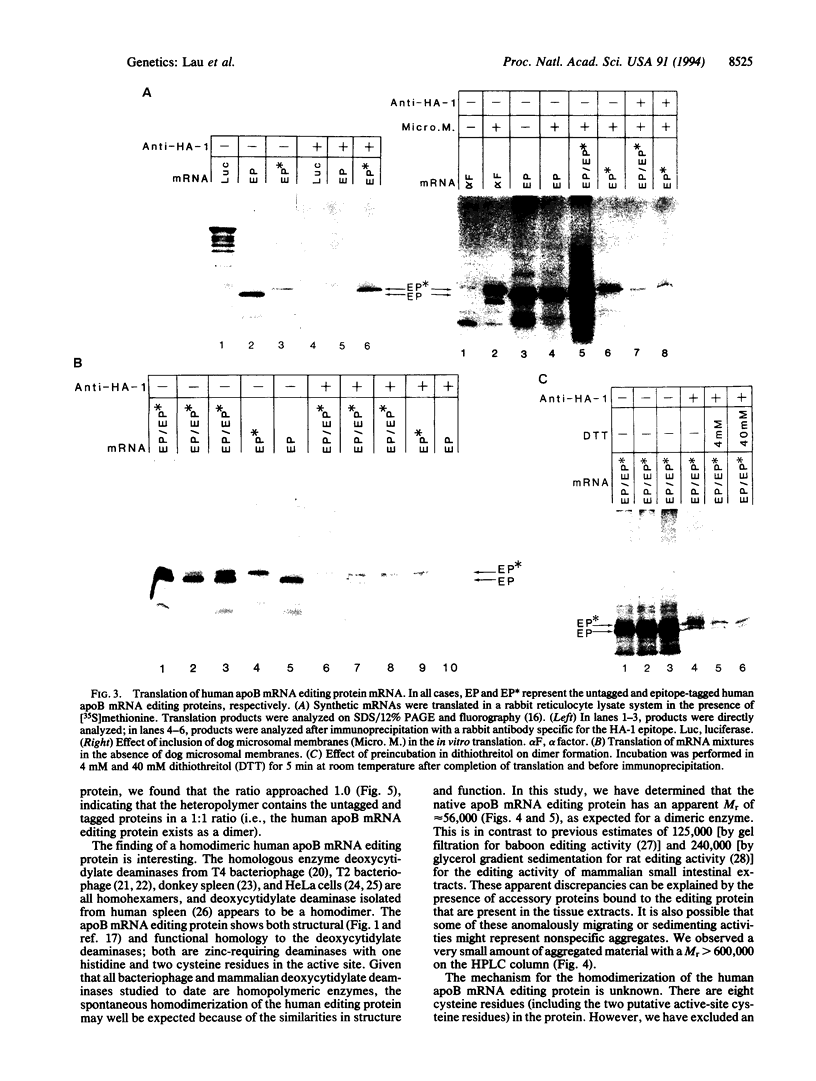
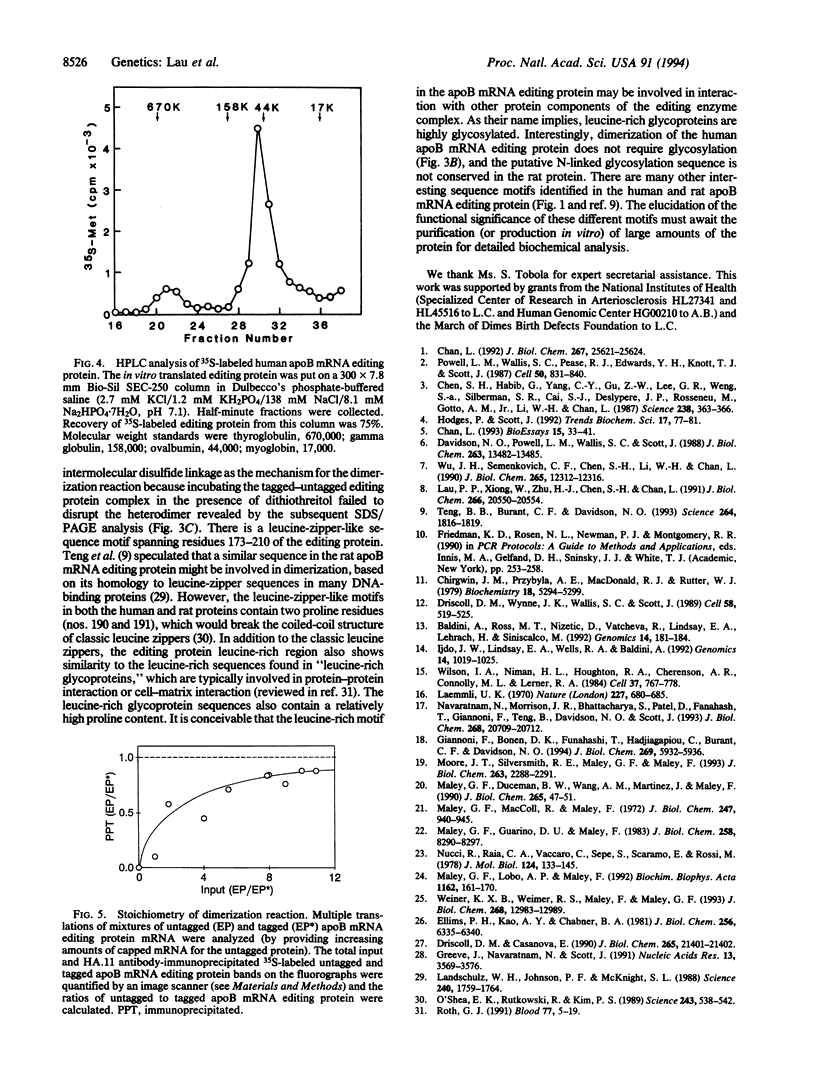
Apolipoprotein B (apoB) mRNA editing consists of a posttranscriptional C-->U conversion involving the first base of the codon CAA encoding glutamine-2153 to UAA, a stop codon, in apoB mRNA. Using a cloned rat cDNA as a probe, we cloned the cDNA and genomic sequences of the gene for a human apoB mRNA editing protein. Expression of the cDNA in HepG2 cells results in editing of the intracellular apoB mRNA. By fluorescence in situ hybridization, we localized the gene for the editing protein to chromosome band 12p13.1-p13.2. By Northern blot analysis, it was shown that the human editing protein mRNA is expressed exclusively in the small intestine. The cDNA sequence predicts a translation product of 236-aa residues. By attaching an epitope tag sequence to the C terminus of the editing protein, we examined the polymerization state of the editing protein synthesized in vitro. We found that the editing protein undergoes spontaneous polymerization. The migration of the human apoB mRNA editing protein on an HPLC column and the stoichiometry of polymeric epitope-tagged to untagged protein indicate that the protein exists as a dimer. Dimerization does not require glycosylation of a consensus N-linked glycosylation sequence present in the protein and is not mediated by disulfide bridge formation. The human apoB mRNA editing protein is a cytidine deaminase showing structural homology to some known mammalian and bacteriophage deoxycytidylate deaminases. The latter enzymes exist as homopolymers. The fact that the apoB mRNA editing protein also exists as a homodimer has important implications for the mechanism of apoB mRNA editing in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini A., Ross M., Nizetic D., Vatcheva R., Lindsay E. A., Lehrach H., Siniscalco M. Chromosomal assignment of human YAC clones by fluorescence in situ hybridization: use of single-yeast-colony PCR and multiple labeling. Genomics. 1992 Sep;14(1):181–184. doi: 10.1016/s0888-7543(05)80303-9. [DOI] [PubMed] [Google Scholar]
- Chan L. Apolipoprotein B, the major protein component of triglyceride-rich and low density lipoproteins. J Biol Chem. 1992 Dec 25;267(36):25621–25624. [PubMed] [Google Scholar]
- Chan L. RNA editing: exploring one mode with apolipoprotein B mRNA. Bioessays. 1993 Jan;15(1):33–41. doi: 10.1002/bies.950150106. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Powell L. M., Wallis S. C., Scott J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J Biol Chem. 1988 Sep 25;263(27):13482–13485. [PubMed] [Google Scholar]
- Driscoll D. M., Casanova E. Characterization of the apolipoprotein B mRNA editing activity in enterocyte extracts. J Biol Chem. 1990 Dec 15;265(35):21401–21403. [PubMed] [Google Scholar]
- Driscoll D. M., Wynne J. K., Wallis S. C., Scott J. An in vitro system for the editing of apolipoprotein B mRNA. Cell. 1989 Aug 11;58(3):519–525. doi: 10.1016/0092-8674(89)90432-7. [DOI] [PubMed] [Google Scholar]
- Ellims P. H., Kao A. Y., Chabner B. A. Deoxycytidylate deaminase. Purification and some properties of the enzyme isolated from human spleen. J Biol Chem. 1981 Jun 25;256(12):6335–6340. [PubMed] [Google Scholar]
- Giannoni F., Bonen D. K., Funahashi T., Hadjiagapiou C., Burant C. F., Davidson N. O. Complementation of apolipoprotein B mRNA editing by human liver accompanied by secretion of apolipoprotein B48. J Biol Chem. 1994 Feb 25;269(8):5932–5936. [PubMed] [Google Scholar]
- Greeve J., Navaratnam N., Scott J. Characterization of the apolipoprotein B mRNA editing enzyme: no similarity to the proposed mechanism of RNA editing in kinetoplastid protozoa. Nucleic Acids Res. 1991 Jul 11;19(13):3569–3576. doi: 10.1093/nar/19.13.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges P., Scott J. Apolipoprotein B mRNA editing: a new tier for the control of gene expression. Trends Biochem Sci. 1992 Feb;17(2):77–81. doi: 10.1016/0968-0004(92)90506-5. [DOI] [PubMed] [Google Scholar]
- Ijdo J. W., Lindsay E. A., Wells R. A., Baldini A. Multiple variants in subtelomeric regions of normal karyotypes. Genomics. 1992 Dec;14(4):1019–1025. doi: 10.1016/s0888-7543(05)80125-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lau P. P., Xiong W. J., Zhu H. J., Chen S. H., Chan L. Apolipoprotein B mRNA editing is an intranuclear event that occurs posttranscriptionally coincident with splicing and polyadenylation. J Biol Chem. 1991 Oct 25;266(30):20550–20554. [PubMed] [Google Scholar]
- Maley G. F., Duceman B. W., Wang A. M., Martinez J., Maley F. Cloning, sequence analysis, and expression of the bacteriophage T4 cd gene. J Biol Chem. 1990 Jan 5;265(1):47–51. [PubMed] [Google Scholar]
- Maley G. F., Guarino D. U., Maley F. Complete amino acid sequence of an allosteric enzyme, T2 bacteriophage deoxycytidylate deaminase. J Biol Chem. 1983 Jul 10;258(13):8290–8297. [PubMed] [Google Scholar]
- Maley G. F., Lobo A. P., Maley F. Properties of an affinity-column-purified human deoxycytidylate deaminase. Biochim Biophys Acta. 1993 Mar 5;1162(1-2):161–170. doi: 10.1016/0167-4838(93)90143-f. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Maccoll R., Maley F. T2r + bacteriophage-induced enzymes. II. The subunit structure of deoxycytidylate deaminase. J Biol Chem. 1972 Feb 10;247(3):940–945. [PubMed] [Google Scholar]
- Moore J. T., Silversmith R. E., Maley G. F., Maley F. T4-phage deoxycytidylate deaminase is a metalloprotein containing two zinc atoms per subunit. J Biol Chem. 1993 Feb 5;268(4):2288–2291. [PubMed] [Google Scholar]
- Navaratnam N., Morrison J. R., Bhattacharya S., Patel D., Funahashi T., Giannoni F., Teng B. B., Davidson N. O., Scott J. The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase. J Biol Chem. 1993 Oct 5;268(28):20709–20712. [PubMed] [Google Scholar]
- Nucci R., Rala C. A., Vaccaro C., Sepe S., Scarano E., Rossi M. Freezing of dCMP aminohydrolase in the activated conformation by glutaraldehyde. J Mol Biol. 1978 Sep 5;124(1):133–145. doi: 10.1016/0022-2836(78)90152-3. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Roth G. J. Developing relationships: arterial platelet adhesion, glycoprotein Ib, and leucine-rich glycoproteins. Blood. 1991 Jan 1;77(1):5–19. [PubMed] [Google Scholar]
- Teng B., Burant C. F., Davidson N. O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993 Jun 18;260(5115):1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- Weiner K. X., Weiner R. S., Maley F., Maley G. F. Primary structure of human deoxycytidylate deaminase and overexpression of its functional protein in Escherichia coli. J Biol Chem. 1993 Jun 15;268(17):12983–12989. [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wu J. H., Semenkovich C. F., Chen S. H., Li W. H., Chan L. Apolipoprotein B mRNA editing. Validation of a sensitive assay and developmental biology of RNA editing in the rat. J Biol Chem. 1990 Jul 25;265(21):12312–12316. [PubMed] [Google Scholar]