Abstract
Purpose.
To compare a 44-mer pigment epithelial–derived factor (PEDF) peptide with neurotrophic activity, and a 34-mer PEDF with antiangiogenic properties in association with docosahexaenoic acid (DHA) in corneal nerve regeneration after experimental surgery.
Methods.
A corneal stromal dissection was performed in rabbits. Treatment groups received topical 44-mer, 34-mer, or full PEDF plus DHA. Corneal sensitivity and Schirmer's test were performed weekly. Rabbits were euthanized at 2 and 4 days and 8 weeks. Two- and 4-day samples were stained for neutrophils and CD11b+ cells. Corneal nerves were stained with βIII tubulin and calcitonin gene-related peptide (CGRP) antibodies in specimens collected at 8 weeks. Subepithelial nerve plexus density was calculated. A PEDF-receptor (PEDF-R) was analyzed in rabbit corneal epithelial cells (RCEC) by Western blot and immunofluorescence.
Results.
Infiltration of CD11b+cells and neutrophils was reduced by treatment with 44-mer PEDF+DHA. A 3-fold increase in subepithelial corneal nerves and CGRP-positive nerves was found in the 44-mer PEDF+DHA-treated group compared with the 34-mer PEDF+DHA- and vehicle-treated groups. There was a 75% recovery of corneal sensitivity by week 7, and Schirmer's test reached control values in the 44-mer PEDF+DHA-treated corneas at 7 weeks. A PEDF-R protein with homology to calcium-independent phospholipase A2ς was expressed in RCEC.
Conclusions.
The 44-mer PEDF+DHA, but not the 34-mer PEDF+DHA, promotes functional regeneration of damaged corneal nerves. Forty four–mer PEDF, by activating a corneal epithelial receptor, in conjunction with DHA could be a novel therapeutic agent for the treatment of neurotrophic keratitis and dry eye that develops as a result of corneal nerve damage.
Keywords: corneal nerves, nerve regeneration, pigment epithelial-derived factor, 44-mer peptide, docosahexaenoic acid, lamellar keratectomy
A 44-mer pigment epithelial–derived factor (PEDF) peptide with neurotrophic activity plus docosahexaenoic acid (DHA), but not a 34-mer PEDF+DHA, stimulates corneal nerve regeneration, and increases sensitivity and tear secretion after experimental surgery. This may be a novel therapy for neurotrophic keratitis and dry eye that develops after corneal damage.
The cornea is densely innervated, and several studies have shown that the health of corneal nerves is vital to maintain homeostasis of the ocular surface and tissue clarity.1–4 Many diseases that affect the cornea can compromise corneal innervation, leading to a decrease in tear production and blink reflex as well as impaired epithelial wound healing.4–8 As a result, neurotrophic keratitis and corneal opacification may ensue. It is estimated that 1.57 million people suffer from corneal blindness worldwide.9 In turn, some of surgical interventions such as refractive surgery and corneal transplantation sever corneal nerves, and studies have shown that corneal sensation can take years to recover, and in some cases it is permanently reduced.10,11 Unfortunately, there are few therapeutic interventions available today that can successfully promote the recovery of corneal sensation. Acknowledging our current limitations, the National Eye Institute (NEI) has identified the need for development of therapeutic agents that can stimulate corneal nerve regeneration as one of the highest priorities in vision research.12
Several molecules have been studied that may have a positive effect on corneal nerve regeneration, including nerve growth factor (NGF), VEGF, semaphorins, neurotrophins 3 and 4 (NT-3; NT-4), growth associated protein-43 (GAP-43), and others, all with variable success.13–17 Neuropeptides, such as substance P (SP) and insulin-like growth factor-1 (IGF-1), have been used to treat delayed wound healing due to corneal nerve damage when traditional treatments have failed.18
Pigment epithelial-derived factor (PEDF) is a glucoprotein discovered in the culture media of retinal pigment epithelial (RPE) cells and is widely expressed in different tissues, including the cornea.19,20 Pigment epithelial-derived factor has broad neurotrophic, neuroprotective, and antiangiogenic activity.21 Work in our laboratory has previously shown that treatment with PEDF in association with the ω-3 fatty acid, docosahexaenoic acid (DHA), after lamellar keratectomy increases regeneration of rabbit corneal nerves.22 We have also shown that corneal sensation returns to normal levels in treated animals only 8 weeks after surgical injury.23 The mechanisms of the neuroregenerative action of PEDF and DHA is not completely understood, but our studies suggest that it involves the synthesis of neuroprotectin D1 (NPD1), a docosanoid synthetized from DHA with strong anti-inflammatory and neuroprotective activity.24–26
Pigment epithelial-derived factor is a 50-kDa protein, and synthetic peptides derived from the sequence of human PEDF, which maintain part of its bioactivity, have been tried in models of choroid and corneal neovascularization, and in diabetic retinopathy.27–29 A 44-amino-acid fragment of PEDF, corresponding to positions Val78-Thr121 of the 418 amino acids of PEDF, has neuroprotective activity and has been shown to stimulate survival and differentiation of spinal motor neurons.30 However, the adjacent 34-amino-acid peptide, corresponding to positions Asp44–Asn77, has been shown to be antiangiogenic.31
Some therapeutic advantages of using small peptides include better tissue penetration, a narrower spectrum of action with reduced side effects, and ease of synthesis in reproducibly large-scale quantities, while a possible disadvantage could be faster biodegradation and lower efficacy of the drug. These are all important considerations in the development of therapeutic agents. In this study we compare the effect of the synthetic 44-mer PEDF and 34-mer PEDF peptides and the whole PEDF molecule in association with DHA on the regeneration of corneal nerves.
Methods
Surgery and Treatment
Animals were treated according to the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic Research and the Institutional Animal Care and Use Committee (IACUC) at the Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Male New Zealand albino rabbits weighing 2 to 3 kg were used. Anesthesia was achieved by intramuscular injection of xylazine (10 mg/kg) and ketamine hydrochloride (50 mg/kg). Topical anesthesia in the form of proparacaine drops was also used. The surgery was performed as previously described22 and consisted of a lamellar keratectomy in the right eye of each rabbit performed by manual dissection at the level of the midcorneal stroma using a 3-mm incision. The area to be dissected was previously marked with an 8-mm disposable trephine. No sutures were used. All surgeries were performed on the same day and by the same surgeon (MSC). Topical moxifloxacin was used for prophylaxis postoperatively. A temporary tarsorrhaphy was performed at the end of surgery and then was removed on the postoperative day 3.
Treatment was initiated immediately after surgery via a 72-hour collagen shield (Oasis, Glendora, CA, USA) soaked in the drug (PEDF+DHA; 34-mer PEDF+DHA or 44-mer PEDF+DHA) or vehicle for the treatment and control groups, respectively. Treatments were not tested in corneas without surgery. Pigment epithelial-derived factor was obtained from BioProducts MD (Middletown, MD, USA) with greater than 90% purity by SDS-PAGE. The 34-mer and the 44-mer PEDF peptides were prepared by Gen Way Biotech, Inc. (San Diego, CA, USA) with purity greater than 95%. Fifty nanograms of PEDF, 34-mer PEDF, or 44-mer PEDF were dissolved in PBS and 10 μg of DHA (Cayman Chemical, Ann Arbor, MI, USA) complexed to 2.5% albumin was used per shield. Previous studies in our laboratory had shown that the collagen shield absorbs approximately 25% of DHA.10 Collagen shields were changed twice a week for 6 weeks. Animals were euthanized in subgroups at 2, 4 days, and 8 weeks after surgery with an overdose of sodium pentobarbital via ear vein injection.
Corneal Sensitivity
Corneal sensitivity measurements were performed weekly inside the surgical area (central cornea) using the Cochet-Bonnet aesthesiometer (Luneau Ophtalmologie, Chartres Cedex, France) following a previously described technique.30 Briefly, the length of the monofilament was varied from 6.0 to 0.5 cm in 0.5-cm fractions until the corneal touch threshold was found. The cornea was tested four times at each filament length. The response was considered negative when no blink was elicited by monofilament touch. A positive response was considered when the animal blinked in equal or more than 50% of the times tested. When filament length approached the threshold, the number of times tested was increased from 4 to 10 to improve accuracy and consistency. If no blink response could be elicited at a monofilament length of 0.5 cm, corneal sensitivity was recorded as 0. The left eye was also measured and used as a control. The same examiner (JH), who was blinded to the treatment, performed all measurements.
Tear Secretion
Schirmer's test (Zone-Quick; Menicon America, Inc., San Mateo, CA, USA) was used to assess tear production after surgery, according to manufacturer's instructions. The test was performed without anesthesia. Measurements were obtained weekly in the injured as well as the uninjured eyes for comparison in every group. The same examiner (JH), who was blinded to the different treatment groups, performed all the measurements.
Immunohistochemistry and Imaging of Rabbit Corneas
After rabbits were euthanized, whole corneas were excised and fixed with 2% fresh paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 hours at room temperature. Corneas were then washed with PBS three times for 5 minutes each and incubated with mouse monoclonal anti–βIII-tubulin antibody (1:1000; Convance Antibody Services, Inc., Berkeley, CA, USA) or chicken anti-CGRP (1:500; Chemicon International, Temecula, CA, USA) in 1% goat normal serum plus 0.15% Tritron X-100 in 0.1 M PBS for 24 hours at room temperature. After washing (4 × 15 minutes), the tissues were incubated with the corresponding secondary antibodies Alexa Fluor-594 goat anti-mouse IgG or Alexa Fluor 488 donkey anti-chicken IgG (Invitrogen, Eugene, OR, USA).
The injured area was viewed and photographed with a fluorescence microscope (Nikon Eclipse TE200; Nikon, Inc., Melville, NY, USA) equipped with a photometric camera (Cool Snap HQ, Tucson, AZ, USA) using MetaVue imaging software (Molecular Devices, Inc., Sunnyvale, CA, USA). The βIII-tubulin and the CGRP-positive tissue areas at the subepithelial nerve plexus were calculated and compared with the total area using an image analysis program (Adobe Photoshop, Adobe Systems, Inc., San Jose, CA, USA). Only the nerve fibers in sharp focus for each image were traced to calculate the nerve area. The investigators obtaining the images and calculating the nerve area were blinded to the condition of each specimen. Ten different images of different areas within the injured zone were analyzed per rabbit.
To evaluate inflammatory cell responses, corneas from rabbits killed on days 2 and 4 after surgery were removed along the limbus and immediately fixed in 4% paraformaldehyde at room temperature for 2 hours, cut in half, and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc., Torrance, CA, USA). Serial cryostat sections (6 μm) were cut, air dried, and stored at −20°C until use. For immunofluorescence staining, the sections were washed in PBS, blocked with 10% goat serum, 0.1% Tritron X-100 in PBS for 30 minutes at room temperature, and then incubated overnight at 4°C with a rat monoclonal anti-neutrophil antibody (1:500; Abcam, Cambridge, MA, USA), or a rat monoclonal [M1/70] anti-CD11b antibody (1:300; Abcam). Afterward, the sections were incubated with Alexa Fluor 488 goat anti-rat IG (H+L) antibody (Invitrogen) for 1 hour at room temperature. To localize the nuclei, 4′-6′-diamino-2-phenylindole (DAPI; Sigma-Aldrich Corp., St. Louis, MO, USA) staining was performed. The sections were examined with a Nikon fluorescent microscope under ×200 magnification. Positive cells were counted in a blind fashion from five randomly selected fields per cornea and averaged.
Western Blot and Immunostaining of Rabbit Corneal Epithelial Cells
Rabbit corneal epithelial cells (RCEC) were cultured as described previously,32 and Western blot analysis was performed as denoted previously.33 Briefly, proteins from cell extracts were resolved by SDS-PAGE using precast gels from Invitrogen (4%–12%) and were transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen). Biotinylated protein molecular weight standards were applied in one line of each gel. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS; 20 mM Tris-Cl, 150 mM NaCl, pH 7.6) plus 0.1% Tween-20 for 1 hour and then probed with polyclonal anti–PEDF receptor (R) antibody (also known as antiadipose triglyceride lipase or calcium-independent phospholipase A2ς, iPLA2Aς; Cayman Chemical), overnight at 4°C. When the blocking peptide was used, blocking peptide and anti–PEDF-R were incubated at room temperature for 1 hour, then diluted to optimal concentration and processed as described above. Membranes were washed with the same buffer and were further incubated with appropriate HRP-conjugated secondary antibodies. Protein bands were visualized using chemiluminescence detection reagents (ECL-Plus; Amersham, Piscataway, NJ, USA).
To determine the location of the PEDF-R, RCEC were cultured in a 48-well plate and, upon reaching 50% to 60% confluence, the cells were fixed with methanol for 30 minutes, blocked with 10% normal goat serum and 1% BSA in PBS for 30 minutes at room temperature, and incubated for 24 hours at 4°C with rabbit polyclonal anti–PEDF-R antibody and mouse monoclonal protein disulfide isomerase (PDI) antibody, an endoplasmic reticulum (ER) marker (Abcam). After washing, the cells were incubated with the appropriate secondary antibodies, IgG Alexa-Fluor 488 goat anti-rabbit and IgG Alexa-Fluor 594 goat anti-mouse. After each step, the slides were washed three times with PBS. No staining was observed when the primary antibody was omitted. DAPI staining was performed to localize the nuclei. Cells were examined with an Olympus 1X71 fluorescence microscope (Center Valley, PA, USA), and the images were captured with an Olympus XM10 camera.
Statistical Analysis
All data are expressed as mean ± SD. The number of rabbits analyzed in each experiment is described in the figure legends. Results represent the average of two independent experiments. Comparison between the different groups was performed by ANOVA and Student's t-test. P values less than 0.05 were considered significant.
Results
44-Mer PEDF+DHA Stimulates Corneal Nerve Regeneration
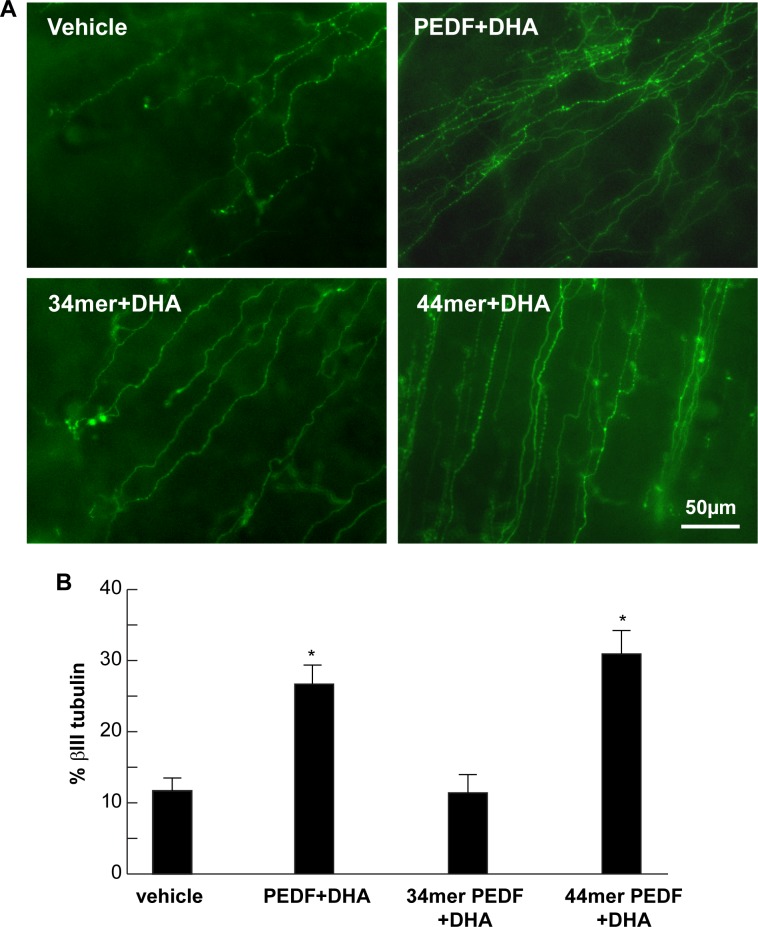
Six weeks after treatment and 8 weeks after surgery, the percentage of nerve area in whole mounts of rabbit corneas stained with βIII tubulin was 26.7 ± 2.6% in the PEDF+DHA-treated group, 30.9 ± 3.3% in the 44-mer PEDF+DHA-treated group, 11.4 ± 2.5% in the 34-mer PEDF+DHA-treated group and 11.7 ± 1.7% in the vehicle-treated group (Fig. 1). Both the PEDF+DHA and the 44-mer PEDF+DHA groups showed a statistically significant increase in corneal nerve area when compared with rabbits receiving vehicle alone (P < 0.01 and P < 0.001, respectively). The βIII tubulin-positive corneal nerve area in the group treated with 34-mer PEDF+DHA was not different from the vehicle group (P = 0.97). The highest corneal nerve area was found in the group receiving treatment with 44-mer PEDF+DHA, although this difference was not statistically significant when compared with the PEDF+DHA-treated group.
Figure 1.
Eight weeks post surgery, 44-mer PEDF+DHA increases corneal nerve area. (A) Immunocytochemistry of subbasal corneal nerves stained with βIII tubulin in rabbit corneal wholemounts 8 weeks after lamellar keratectomy. Images within the surgical area are shown for all study groups. (B) Quantification of subbasal nerve area. *Statistically significant higher nerve area in the PEDF+DHA (n = 6 rabbits) and 44-mer PEDF+DHA (n = 9 rabbits) groups compared with 34-mer PEDF+DHA (n = 9 rabbits) and vehicle groups (n = 6 rabbits).
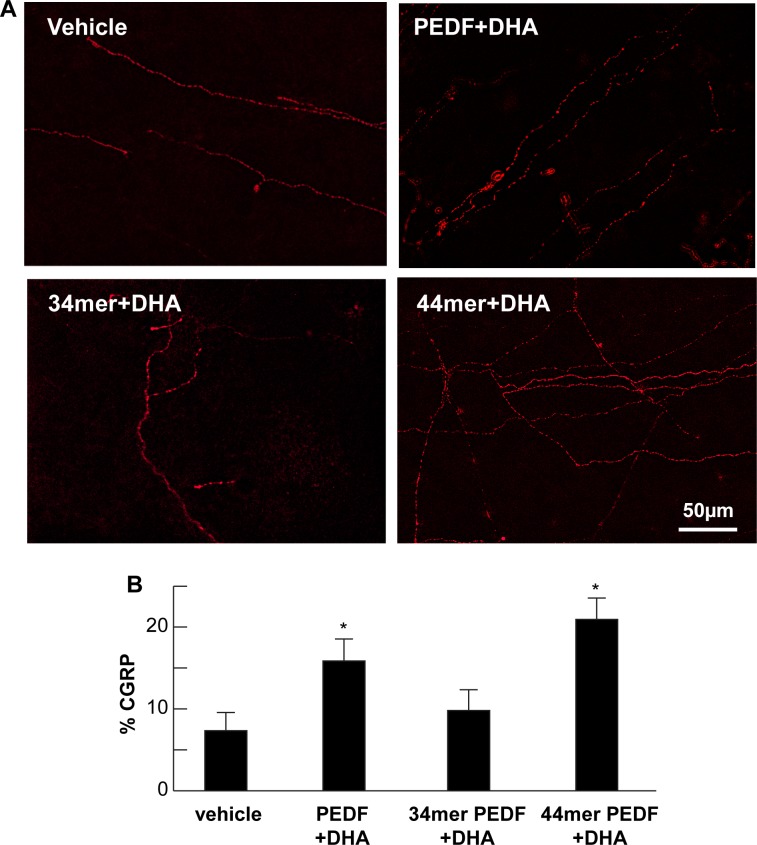
Previous studies from our laboratory had shown that rabbit corneal nerve fibers express the sensory neuropeptide CGRP and that combination of DHA and PEDF treatment enhances recovery of nerves expressing CGRP.23 In this study, the nerve area showing positive stain for CGRP was highest in the 44-mer PEDF+DHA-treated group (20.9 ± 2.6%) followed by the PEDF+DHA-treated group (15.9 ± 2.6%). The CGRP-positive nerve area in the 34-mer PEDF+DHA group was 9.8 ± 2.5%, and it was 7.3 ± 2.2% in the vehicle group (Fig. 2). The group treated with 44-mer PEDF+DHA showed that a greater effect occurred on regeneration of corneal nerves that can express CGRP above all groups, even when compared with the group receiving the whole PEDF molecule (P = < 0.001).
Figure 2.
Regenerated corneal nerves express CGRP. (A) Immunofluorescence of subbasal corneal nerves stained with anti-CGRP antibody in rabbit corneal wholemounts 8 weeks after lamellar keratectomy. Images within the surgical area are shown for each study group. (B) Quantification of CGRP positive subbasal nerves within the surgical area for each group. (PEDF+DHA, n = 6 rabbits; 44-mer PEDF+DHA, n = 9 rabbits; 34-mer PEDF+DHA, n = 9 rabbits; vehicle, n = 6 rabbits). *Significant differences compare with vehicle and 34-mer PEDF+DHA treated rabbits. **Significant increase between rabbits treated with PEDF+DHA and 44-mer PEDF+DHA.
44-Mer PEDF + DHA Treatment Accelerates Recovery of Corneal Sensation and Improves Tear Secretion
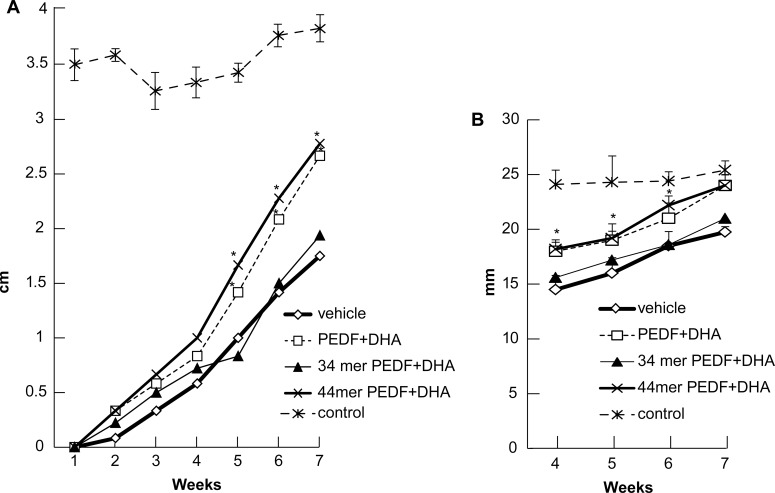
Figure 3A shows the corneal sensitivity measurements obtained from the different treatment groups. We observed a complete absence of corneal sensation at postoperative week one in all groups. Some sensation was recovered by week 2 through 4, but no differences were found among the groups. By postoperative week 5, the 44-mer PEDF+DHA- and the PEDF+DHA-treated groups showed a statistically significant increase in corneal sensation when compared with the 34-mer PEDF+DHA and vehicle groups. This difference was maintained throughout the remaining weeks. Average normal rabbit corneal sensitivity, as measured in the nonsurgical control eyes, was 3.6 cm. At 7 weeks, the 44-mer PEDF+DHA and the PEDF+DHA groups had recovered approximately 75% corneal sensation (2.8 cm and 2.7 cm, respectively).
Figure 3.
Corneal nerve sensation and tear secretion is restored after surgical injury with 44-mer PEDF+DHA. (A) Corneal sensitivity measured by Cochet Bonnet esthesiometer for each study group up to 7 weeks post injury. Higher sensitivity measurements is shown in PEDF+DHA (n = 6 rabbits) and 44-mer PEDF+DHA (n = 9 rabbits) groups than in 34-mer PEDF+ DHA (n = 9 rabbits) and vehicle (n = 6 rabbits) groups beginning at week 5. (B) Tear secretion measured by Schirmer's test showing results from week 4 to week 7 after injury for all treatment groups and control (nonsurgical) group. Higher Schirmer's scores and normal control levels are reached by week 7 with PEDF+DHA and 44-mer PEDF+DHA. *Statistically significant difference with respect to 34-mer PEDF+DHA- and vehicle-treated groups.
Tear secretion, as measured by the Schirmer's test, was also reduced after corneal nerve damage in all groups compared with normal controls (Fig. 3B). A higher Schirmer's test score was had in the 44-mer PEDF+DHA- and PEDF+DHA-treated groups had than the 34-mer PEDF+DHA- and vehicle-treated groups at all-time points measured, reaching normal control scores at postoperative week 7 (Fig. 3).
44-Mer PEDF+DHA Treatment Reduces the Inflammatory Response Triggered by Corneal Injury
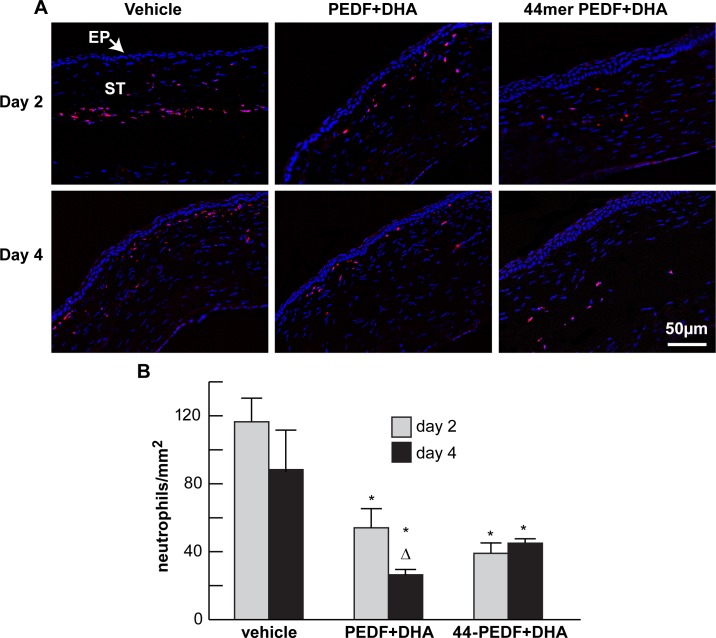
Our previous studies had shown that PEDF+DHA treatment decreases the inflammatory response in corneas after injury produced by lamellar keratectomy.24 To determine if the 44-mer PEDF+DHA had similar actions, neutrophil infiltration within the surgical area was examined on postoperative days 2 and 4 (Fig. 4) in rabbits treated with the 44-mer PEDF+DHA, the full length PEDF+DHA or vehicle. For this, an antibody that recognizes a 40-kDa antigen expressed by polymorphonuclear cells, but absent in macrophages, was used. The vehicle group showed an increase in neutrophil infiltration at day 2 that persisted through day 4 (average of 116 ± 13 and 87 ± 23 cells/mm2, respectively). We found that both treatments (PEDF+DHA and 44-mer PEDF+DHA) decreased neutrophil infiltration into the stroma. The difference was statistically significant for days 2 and 4 in the 44-mer PEDF+DHA group and the PEDF+DHA group. Also, when comparing days 2 and 4, the PEDF+DHA group was the only one to show a significant decrease in the number of cells by day 4 (average 54 ± 11 vs. 26 ± 4 cells/mm2, P = 0.007).
Figure 4.
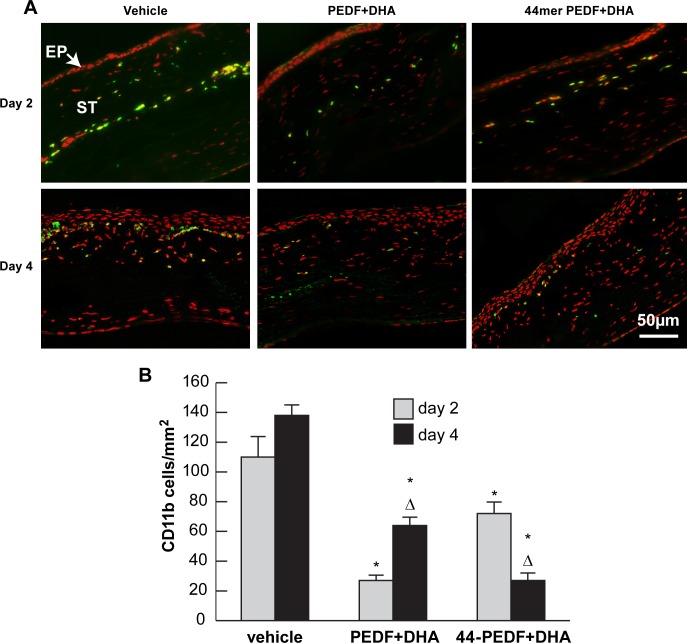
Treatment with PEDF+DHA and the 44-mer PEDF+DHA decreases neutrophil infiltration after surgery. (A) Images of frozen sections within the surgical area obtained at postoperative days 2 and 4 and stained with antineutrophil antibody and DAPI. (B) Quantification of the number of neutrophil-positive cells (red) at days 2 and 4 for each group. *Statistically significant difference with respect to vehicle group. ΔStatistically significant difference with respect to day 2 in the same group. EP, epithelium; ST, stroma.
The number of CD11b+ cells infiltrating the surgical area was also reduced in both treated groups compared with the vehicle group at postoperative days 2 and 4 (Fig. 5). The vehicle group showed a nonsignificant increase in CD11b+ cells from day 2 to 4. However the group treated with 44-mer PEDF+DHA not only displayed a significantly lower number of cells at both time points examined, but also showed a reduction in CD11b+ cells from postoperative day 2 to 4 (mean, 72 ± 4 vs. 27 ± 6 cells/mm2 respectively, P = 0.0001). Interestingly, the group treated with PEDF+DHA presented the reverse phenomenon, with very few CD11b+ cells on day 2 that increase by day 4 (mean, 27 ± 8 vs 64 ± 5 cells/mm2, P = 0.0007).
Figure 5.
Treatment with PEDF+DHA and the 44-mer PEDF+DHA significantly decreases CD11b+ cells at 2 and 4 days after surgery. (A) Frozen sections of rabbit corneas obtained from the central surgical area at 2 and 4 days after lamellar keratectomy. Representative images of each group at both time points showing staining with anti-CD11b antibody. (B) Quantification of the number of CD11b+ cells (green) for each study group. *Statistically significant difference with respect to vehicle group. ΔStatistically significant difference with respect to day 2 in the same group.
Inflammatory cells appear in the midstroma at day 2, but their distribution change toward a more subepithelial distribution by day 4 post surgery (Figs. 4, 5). This could be due to the fact that the corneal lamella incision was close to the midstroma and that, at an early postoperative time (2 days), cell infiltration took place at the incision site. When the injury healed, the inflammatory response moved to the anterior stroma and subepithelial layer, the site in which the flap had been created. Keratocyte apoptosis and/or activation of the surrounding stromal cells could be a cause of the inflammatory response at the site.
Rabbit Corneal Epithelial Cells Express a PEDF-R
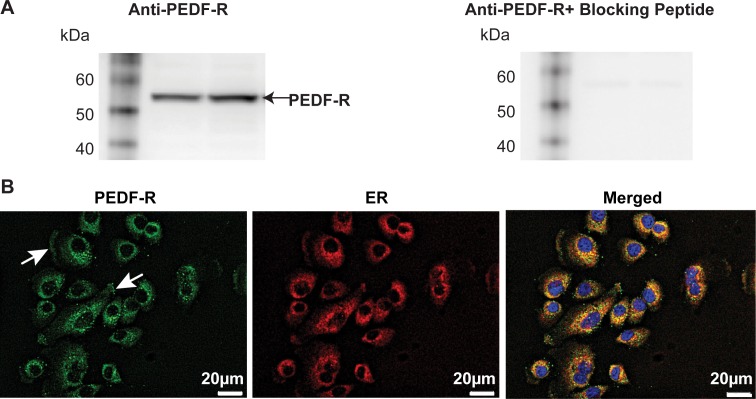
The PEDF-R was found to be present in corneal rabbit epithelial cells by Western blot analysis (Fig. 6A). The blocking peptide specifically abolished the PEDF-R band. The subcellular localization of PEDF-R was investigated by double immunofluorescence staining labeling with PEDF-R and the PDI antibodies (Fig. 6B). There was positive staining around the nuclei, in a punctuated fashion and in the plasma membrane (arrows). The merged images show the colocalization of PEDF-R and endoplasmic reticulum (ER) marker.
Figure 6.
Rabbit corneal epithelial cells express a PEDF-R that shares strong homology with members of the Ca2+ independent PLA2 (iPLA2). (A) Thirty micrograms of protein from RCEC were resolved by SDS-PAGE and analyzed by Western blot. The PEDF-R is shown as a 56-kDa band. When the blocking peptide was added to the primary antibody, no bands were observed. (B) Immunocytochemistry of rabbit corneal epithelial cells in culture showing the presence of PEDF-R (green) and ER (red) and the merged image of both staining patterns, nuclei stained with DAPI.
Discussion
Corneal integrity relies upon a competent corneal nerve supply. A wide variety of conditions can be responsible for corneal nerve alterations and neurotrophic keratopathy, such as infectious agents, congenital anomalies, multiple ocular and systemic diseases, trauma, corneal dystrophies, and surgical interventions.4–8,10 It is well established that corneal nerve damage can result in dry eye and impaired epithelial wound healing because of the disruption of neuronal circuits involved in tear production and secretion as well as the lack of neuropeptides, which provide a supportive effect to the corneal epithelium.34,35 As a consequence of corneal nerve damage, ocular surface complications can ensue, including persistent epithelial defects, corneal ulcers, melts, perforations, and limbal stem cell dysfunction.36,37 In addition, pain and discomfort related to dry eye might constitute a significant burden to patients.
Studies have shown that, approximately, only a 60% recovery of corneal innervation occurs more than 3 years after laser in situ keratomileusis.10,38 Likewise, after complete transection of stromal nerves in penetrating keratoplasty, little recovery of corneal innervation is observed 30 to 40 years later.11 In this study, we show that in a model of surgically induced nerve damage at the level of the stroma, the 44-mer PEDF peptide in combination with DHA can selectively accelerate the regeneration of corneal nerves. We had previously shown that PEDF requires the addition of DHA to stimulate corneal nerve regeneration in the same animal model.22 The recovery of corneal sensation as soon as 7 weeks after surgery, as well as improved Schirmer's scores and the presence of CGRP within the regenerated neurites, indicate not just anatomical recovery but also improved nerve function in this group.23 The effect of the more selective 44-mer PEDF+DHA was significantly higher than that of the complete PEDF molecule. A clear difference was also found when compared to the 34-mer PEDF+DHA, which showed no effect in corneal nerve regeneration, corneal sensitivity, or tear secretion. In light of these results, there might be a possible advantage to the use of 44-mer PEDF in lieu of the whole molecule to increase selectivity and specificity of treatment.
The role of the immune system in nerve regeneration is yet not well understood but mounting evidence suggests that both nervous and immune systems actively interact under healthy and pathological conditions.4,6,39 Biochemical communication between the two systems is accomplished by means of cytokines and neuropeptides. Studies have shown a direct physical association between resident macrophage populations and nerves in the normal murine cornea40 and that CD11b+GR+ myeloid cells can secrete NGF and promote trigeminal ganglion neurite growth in mice.39 On the other hand, a strong correlation has been shown between the increase in dendritic cell numbers and the decrease in subbasal corneal nerves during infectious keratitis,6 and there is evidence that immunomodulation with cyclosporine may retard nerve sprouting from transected trunks.41 As such, it is likely that just the right amount of immune activity is needed to provide the optimal environment for healthy corneal nerves and/or to allow for their regeneration after injury. In our study, neutrophil and CD11b+ (marker of myeloid linage cells) cellular infiltration was investigated. As expected, the number of inflammatory cells within the corneal stroma increased early on after surgery. This immune response was significantly reduced by treatment with 44-mer PEDF+DHA at 2 days after treatment. Interestingly, neutrophil infiltration tended to remain stable from day 2 to day 4 in the vehicle or 44-mer PEDF+DHA groups. However, animals treated with PEDF+DHA showed a statistically significant decrease in neutrophils from day 2 to day 4. Additionally, when the effect of treatment on CD11b+ cells was analyzed, we observed that, overall, the number was reduced in both treatment groups but that there was a higher numbers of cells on day 4 than day 2, except for the 44-mer PEDF+DHA group. These different effects in the modulation of inflammation may be in part explained by additional effects carried out by other active parts of the whole PEDF molecule that differ from the 44-mer PEDF peptide activity. Studies in mice had shown that denervation by trigeminal nerve ablation induces angiogenesis and increases CD11b+ cells, while PEDF cornea expression decreases.42 This suggests a role for PEDF to maintain the avascular state of the tissue. In our experiments, the injury damage the nerves, but the inflammation is quickly resolved. The cornea wound heals in 1 week, and we did not observe formation of new vessels when mice were treated with 44-mer–PEDF+DHA. In addition to its neuroprotective and neurotrophic activity, an anti-inflammatory effect of PEDF has previously been shown in Müller and endothelial cells.43
Due to the multifunctional activities of PEDF, more than one cell surface receptor for PEDF has been described.44,45 Our data shows that rabbit corneal epithelial cells express the PEDF-R that shares strong homology with members of the iPLA2; in addition, the neuroprotective activity of PEDF has been attributed to the N-terminal 44-mer peptide, which contains the receptor binding region of this receptor.46,47 Another previously described PEDF-R, the laminin receptor, interacts with a region of PEDF containing the amino acid sequence of the 34-mer PEDF and is involved in the antiangiogenic activity45; In our model, the 34-mer PEDF +DHA does not stimulate corneal nerve regeneration. The PEDF-R expressed in the RCEC with phospholipase activity has four transmembrane domains, and previous in vitro studies have shown that liberates fatty acids from phospholipids.46 Pigment epithelial–derived factor can strongly induce the synthesis of NPD1 from its precursor DHA in retinal pigment epithelial cells,48 and mass spectrometry analysis has confirmed NPD1 synthesis in rabbit corneas treated with PEDF+DHA.22 In this context, we can speculate that some of the anti-inflammatory and neuroprotective activity of the 44-mer PEDF+DHA after corneal injury may be carried out through NPD1 synthesis.48 It is important to point out that the addition of DHA is needed since rabbit corneas contain very low amounts of DHA49 and do not synthesize significant amounts of NPD1 in the absence of DHA.22 It has recently been demonstrated that PEDF enhances retinal ganglion cell axon regeneration when injected intravitreally in rats after an optic nerve crush.50 Unlike corneal epithelial cells, neurons are enriched in DHA, so it is possible that in these cells, PEDF alone could increase the synthesis of NPD1.
In summary, our study demonstrates that the peptide 44-mer PEDF in combination with DHA is an effective and selective treatment for corneal nerve injury that can modulate the inflammatory response, promote the recovery of corneal sensation, and increase tear production. In the absence of an effective treatment available to the clinician for treating disorders that affect corneal nerves, 44-mer PEDF in combination with DHA may represent a potentially valuable therapeutic strategy and deserves further investigation.
Acknowledgments
The authors thank Charles A. Cefalu, a medical student who participated in the Summer Undergraduate Neurosciences (SUN) Program at the Neuroscience Center of Excellence, Louisiana State University Health Sciences Center, New Orleans, for his excellent technical assistance.
Supported by National Institutes of Health (Bethesda, MD, USA) Grant R01 EY019465.
Disclosure: J. He, None; M.S. Cortina, None; A. Kakazu, None; H.E.P. Bazan, None
References
- 1. Muller, LJ,, Marfurt CE,, Kruse F,, Tervo TMT. Corneal nerves: structure contents and function. Exp Eye Res. 2003; 76: 521–542. [DOI] [PubMed] [Google Scholar]
- 2. He J,, Bazan NG,, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010; 91: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel DV,, McGhee CN. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005; 46: 4485–4488. [DOI] [PubMed] [Google Scholar]
- 4. Shaheen BS,, Bakir M,, Jain S. Corneal nerves in health and disease. J Surv Ophthalmol. 2014; 59: 263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamrah P,, Curzat A,, Dastjerdi MH,, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: in vivo confocal microscopy after herpes keratitis. Ophthalmology. 2010; 117: 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruzat A,, Witkin D,, Bnisadi N,, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011; 52: 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He J,, Bazan HEP. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2012; 119: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He J,, Bazan HEP. Corneal nerve architecture in a donor with unilateral epithelial basement membrane dystrophy. Ophthalmic Res. 2013; 49: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pascolini D,, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 10. Lee BH,, McLaren JW,, Erie JC,, et al. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002; 43: 3660–3664. [PubMed] [Google Scholar]
- 11. Patel SV,, Wrie JC,, McLaren JW,, Bourne WM. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and in late endothelial failure. Arch Ophthalmol. 2007; 125: 1693–1698. [DOI] [PubMed] [Google Scholar]
- 12. Vision Research: Needs Gaps and Opportunities. National Institutes of Health: National Eye Institute. Available at: http://www.nei.nih.gov/strategicplanning/pdf/VisionResearch2012 Accessed January 10, 2014.
- 13. Esquenazi S,, Bazan HE,, Bui V,, et al. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005; 46: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 14. Boninni S,, Lambiase A,, Rama P,, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000; 107: 1347–1351. [DOI] [PubMed] [Google Scholar]
- 15. Omoto M,, Yoshida S,, Miyashita H,, et al. The semaphoring 3A inhibitor SM-345431 accelerates peripheral nerve regeneration and sensitivity in a murine corneal transplantation model. PLoS One. 2012; 7: e47716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu CQ,, Zhang M,, Matis KI,, Kim C,, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Invest Ophthalmol Vis Sci. 2008; 49: 3870–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaudhary S,, Namavari A,, Yco L,, et al. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea. 2012; 31: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishida T. Translational research in corneal epithelial wound healing. Eye Contact Lens. 2010; 5: 300–304. [DOI] [PubMed] [Google Scholar]
- 19. Tombran-Tink J,, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev. Neurosci. 2003; 4: 628–636. [DOI] [PubMed] [Google Scholar]
- 20. Becerra SP. Focus on molecules: pigment epithelium-derived factor (PEDF). Exp Eye Res. 2006; 82: 739–740. [DOI] [PubMed] [Google Scholar]
- 21. Barstable CJ,, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004; 23: 561–577. [DOI] [PubMed] [Google Scholar]
- 22. Cortina MS,, He J,, Li N,, Bazan NG,, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010; 51: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortina MS,, He J,, Li N,, Bazan NG,, Bazan HE. Recovery of corneal sensitivity calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012; 130: 76–83. [DOI] [PubMed] [Google Scholar]
- 24. Cortina MS,, He J,, Russ T,, Bazan NG,, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013; 54: 4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calandria JM,, Marcheselli VL,, Mukherjee PK,, et al. Selective survival rescue in 15-lipoxygenase-1-deficeint retinal pigment epithelial cells by the novel dosahexaenoic acid-derived mediator, neurprotective D1. J Biol Chem. 2009; 248: 17877–17882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bazan NG. Cell survival matters: docosahexanoic acid signaling, neuroprotection and photorecpetors. Trends Nuerosci. 2006; 29: 263–271. [DOI] [PubMed] [Google Scholar]
- 27. Amaral J,, Becerra SP. Effects of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010; 51: 1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsui T,, Nishino Y,, Maeda S,, Yamagishi S. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc Res. 2012; 84: 105–108. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y,, Leo LF,, McGregor C,, Grivitishsili A,, Barnstable CJ,, Tombran-Tink J. Pigment epithelial-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2 (Akita) mice. Mol Med. 2012; 18: 1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Houenou LJ,, D'Costa AP,, Li L,, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. Comp Neurol. 1999; 412: 506–514. [PubMed] [Google Scholar]
- 31. Mamral J,, Becerra SP. Effect of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization. Invest Ophahalmol Vis Sci. 2010; 51: 1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hurst J,, Ma X,, Bazan HEP. PAF binding to a single receptor in corneal epithelium plasma membrane. Invest Ophthalmol Vis Sci. 1999; 40: 790–795. [PubMed] [Google Scholar]
- 33. Kakazu A,, Chandrasekher G,, Bazan HEP. HGF protects corneal epithelial cells from apoptosis by the PI_3K/Akt-1/Bad-but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci. 2004; 45: 3485–3492. [DOI] [PubMed] [Google Scholar]
- 34. Belmonte C,, Acosta MC,, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004; 78: 513–525. [DOI] [PubMed] [Google Scholar]
- 35. Kubilus JK,, Linsenmayer TF. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonini S,, Rama P,, Olzi D,, et al. Neurotrophic keratitis. Eye (Lond). 2003; 17: 989–995. [DOI] [PubMed] [Google Scholar]
- 37. Ueno H,, Ferrari G,, Hattori T,, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest ophthalmol Vis Sci. 2012; 53: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aclvillo MP,, McLaren JW,, Hodge DO,, et al. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004; 45: 3991–3996. [DOI] [PubMed] [Google Scholar]
- 39. Sarkar J,, Chaudhary S,, Jassim SH,, et al. CD11+GR+myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration. Invest Ophthalmol Vis Sci. 2013; 54: 5920–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seyed-Tazavi Y,, Chinnery HR,, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Invest Ophthalmol Vis Sci. 2014; 55: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 41. Namavari A,, Chaudhary S,, Chang JH,, et al. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Invest Ophthalmol Vis Sci. 2012; 53: 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrari G,, Hajrasouliha AR,, Sadrai Z,, Ueno H,, Chauhan SK,, Nerves Dana R. and neovessels inhibit each other in the cornea. Invest Ophthalmol Vis Sci. 2013; 54: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang SX,, Wang JJ,, Gao G,, Shao C,, Mott R,, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006; 20: 323–325. [DOI] [PubMed] [Google Scholar]
- 44. Notari L,, Baladron V,, Aroca-Aguilar JD,, et al. Identification of a lipase-linked cell membrane receptor for pigment epitheium-derived factor. J Biol Chem. 2006; 284: 10480–10490. [DOI] [PubMed] [Google Scholar]
- 45. Bernard A,, Gao-Li J,, Franco CA,, Bouceba T,, Huet A,, Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelial-derived factor. J Biol Chem. 2009; 284: 10480–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bilak MM,, Becerra SP,, Vincent AM,, Moss BH,, Aymerich MS,, Kuncl RW. Identification of the neuroprotective molecular region of pigment epithelium-derived factor and its binding sites on motor neurons. J Neurosci. 2002; 22: 9378–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aymerich MS,, Alberdi EM,, Martinez A,, Becerra SP. Evidence for pigment epithelium-derived factor receptors in the neural retina. Invest Ophthalmol Vis Sci. 2001; 42: 3287–3293. [PubMed] [Google Scholar]
- 48. Mukherjee PK,, Marcheselli VL,, Barreiro S,, Hu J,, Bok D,, Bazan NG. Neurotrophins enhance retinal pigment cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007; 104: 13152–13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bazan HEP,, Bazan NG. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea: comparison of epithelium, stroma and endothelium. Curr Eye Res. 1984; 3: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 50. Vigneswara V,, Bery M,, Logan A,, Ahmed Z. Pigment epithelium-derived factor is ratinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Invest Ophthalmol Vis Sci. 2013; 54: 2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]