Abstract
Purpose.
To establish the regulatory roles that pericytes have in coordinating retinal endothelial cell (EC) growth and angiogenic potential.
Methods.
Pericytes were derived from donor diabetic (DHuRP) or normal (NHuRP) human retinae, and characterized using vascular markers, coculture, contraction, morphogenesis, and proliferation assays. To investigate capillary “cross-talk,” pericyte-endothelial coculture growth, and connexin-43 (Cx43) expression assays were performed. Paracrine effects were examined via treating EC with pericyte-derived conditioned media (CM) in proliferation, angiogenesis, and angiocrine assays. The effects of sphingosine 1-phosphate (S1P) were assessed using receptor antagonists.
Results.
The DHuRP exhibit unique proliferative and morphologic properties, reflecting distinctive cytoskeletal and isoactin expression patterns. Unlike NHuRP, DHuRP are unable to sustain EC growth arrest in coculture and display reduced Cx43 expression. Further, CM from DHuRP (DPCM) markedly stimulates EC proliferation and tube formation. Treatment with S1P receptor antagonists mitigates DPCM growth-promotion in EC and S1P-mediated pericyte contraction. Angiocrine assays on normal and diabetic pericyte secretomes reveal factors involved in angiogenic control, inflammation, and metabolism.
Conclusions.
Effects from the diabetic microenvironment appear sustainable in cell culture: pericytes derived from diabetic donor eyes seemingly possess a “metabolic memory” in vitro, which may be linked to original donor health status. Diabetes- and pericyte-dependent effects on EC growth and angiogenesis may reflect alterations in bioactive lipid, angiocrine, and chemomechanical signaling. Altogether, our results suggest that diabetes alters pericyte contractile phenotype and cytoskeletal signaling, which ultimately may serve as a key, initiating event required for retinal endothelial reproliferation, angiogenic activation, and the pathological neovascularization accompanying proliferative diabetic retinopathy.
Keywords: S1P, connexin 43, neovascularization, cytoskeleton, contractility, microcirculation, endothelial cell, retina
Pericytes derived from diabetic and normal human retinae were characterized using cytoskeletal effector, coculture, force-generation, endothelial morphogenesis, and growth-based assays. These data point to the pivotal role that pericytes may have in enabling pathologic angiogenesis during diabetes.
Diabetic retinopathy (DR) remains one of the leading causes of adult blindness in the United States.1 As the incidence of diabetes is projected to rise to 33% of the United States population by 2050,2 there is an urgent need for developing therapeutics that could successfully target diabetes-associated ocular complications. Currently, treatments are focused on inhibiting pathologic angiogenesis through growth factor targeting and antibody-based growth factor neutralization.3 However, decades of global growth factor ablative therapies may produce unintended consequences on macrovascular physiology, blood pressure regulation, and/or neural retinal physiology, as the long-term effects of chronic growth factor inhibition remain unknown.4 Thus, we anticipate that a detailed understanding of the pathophysiologic changes and mechanisms controlling microvascular cell dysfunction could inform strategies aimed at creating advanced molecular or cellular therapies able to abrogate the pathogenesis of proliferative DR.
Foundational work revealed that pericytes have a pivotal role in modulating microvascular physiology and pathological angiogenesis.5–9 Situated within the basement membrane of capillary and postcapillary venules, which they help to organize and synthesize, pericytes are notably absent in histologic preparations of late-stage diabetic retinae, where acellular capillaries and areas of central nonperfusion can be observed.10 Conferring increased vascular stability through varying degrees of capillary coverage,11 pericytes have been shown to repress endothelial proliferation, and modulate capillary remodeling.12,13 In contrast, pericyte-deficient microvessels or pericyte-dysfunctional capillaries may be prone to becoming angiogenically-active or permissive for angiogenic sprouting.14 Indeed, it has been suggested that pericyte drop-out is a prerequisite for pathological angiogenic induction.15,16 However, recent work from our group17,18 and others19 suggests that pericyte dysfunction, rather than their frank loss from the microvasculature, could be sufficient to initiate an “angiogenic switch,” therein enabling the ensuing pathological angiogenesis associated with DR. Thus, it is plausible that the loss of physical associations between endothelial cells (ECs) and pericytes alone is insufficient in explaining the regulatory roles that pericytes have in governing endothelial growth dynamics and pathological angiogenic activation during diabetes.
Microvascular angiogenic potential involves contact-dependent and -independent signaling between pericytes and ECs. Transforming growth factor-β (TGF-β), pericyte force generation, and gap junctions have been identified as a subset of key cell- and molecular-based signaling pathways controlling pericyte-endothelial “cross-talk.” Capillary stabilization mediated by activation of TGF-β is achieved by close investment of the endothelium by pericytes,20 which not only confers endothelial growth arrest, but also acts as a mechanochemical signal21 to stimulate α-smooth muscle actin (αSMA) expression and contractile phenotype in pericytes. Moreover, pericyte contractility, regulated by cytoskeletal organization and Rho signaling, has been shown to mediate endothelial proliferation and capillary tone.17,22,23 Pericytes have been shown to form functional heterotypic gap junctions that electrically couple with the endothelium,24,25 and it is hypothesized that signaling through gap junctions could influence angiogenic potential.
Contact-independent signals, such as soluble growth factors, including angiopoietin-1 (Ang-1), platelet-derived growth factor (PDGF), FGF, and VEGF can promote either vessel maturation or proangiogenic signals.26–29 While these factors are highly studied, bioactive lipids, in particular sphingosine 1-phosphate (S1P), are generating considerable interest as mediators of vascular physiology.30,31 S1P has a wide range of vascular effects, including proliferation, migration, and permeability.32,33 Vessel-derived S1P can be produced by ECs34 and pericytes,34–36 and primarily signals through S1P receptors 1 to 3 (S1P1–3) due to vascular-restricted expression.37 Recently, pericyte-derived S1P was shown to mediate mural cell–lymphocyte interactions36 and endothelial barrier integrity.35 Furthermore, S1P signaling appears to be a driving force in pathologic ocular angiogenesis as anti-S1P antibodies attenuate retinal neovascularization,38 and S1P2-null mice have reduced angiogenesis in ischemia-driven models of retinopathy.39 Therefore, S1P may represent a vital therapeutic target in DR.
In this study, we isolated microvascular pericytes from diabetic and nondiabetic human retinae (DHuRP and NHuRP, respectively), and then explored their phenotypic differences while interrogating the regulatory roles pericytes have in orchestrating retinal capillary endothelial growth dynamics. While normal and diabetic pericytes express bona fide pericyte markers, including 3G5 and NG2, and do not bind or internalize acyl-LDL (as do ECs), DHuRP are phenotypically and functionally different from NHuRP. First, DHuRP possess an altered cytoskeleton compared to NHuRP, since the steady state levels of αSMA and the resultant F-actin–containing cytoskeleton are uniquely different from NHuRP. Indeed, isoactin-containing stress fibers found in DHuRP are diminutive as are the cells' morphology reflective of this diabetes-induced state; for example, unable to undergo cytoskeletal rearrangements, which are seen in NHuRP following growth factor stimulation. Furthermore, DHuRP actomyosin-mediated force-transducing abilities are diminished when compared to NHuRP silicone substrate-deforming capacity. Moreover, the expression and localization of the gap junction protein connexin 43 (Cx43) in pericyte mono- and cocultures are reduced in DHuRP. In vitro coculture assays reveal a disruption in DHuRP-mediated contact-dependent and -independent control of endothelial growth. Furthermore, conditioned media (CM) from diabetic retinal pericyte populations stimulate EC growth and tube formation assays, an effect partially mediated by S1P. We showed that S1P not only influences the endothelium, but also markedly alters pericyte contractility. Further investigation of the pericyte secretome using angiocrine profiling reveals that the balance of pro- and anti-angiogenic factors is disrupted, immune cell modulation may be affected, and effector molecules linked to S1P signaling are upregulated in DHuRP. Altogether, DHuRPs display aberrant S1P signaling, cytoskeletal organization, and contractility, culminating in dysfunctional microvascular mechanochemical signals.
Materials and Methods
Isolation of Normal and Diabetic Pericytes
Microvascular pericytes were isolated from retinae, which were derived from either normal or diabetic human donors with limited medical histories beyond age of donor, sex, and disease state. Microvascular retinal cells and pericytes were isolated from donor eyes between 36 and 48 hours post mortem, and were provided by National Disease Research Interchange (NDRI; Philadelphia, PA, USA); globes were shipped via courier on wet ice within specially crafted containers able to securely hold each globes. This work reports on postmortem ocular tissues. Nevertheless, the procurement of the postmortem tissue performed by NDRI is in compliance with the World Medical Association Declaration of Helsinki. Posterior poles were separated and globes enucleated using well-established methods that have been developed in the laboratory.40 Cells from individual retinae were pooled to generate a “diabetic” and “normal” pericyte pool possessing heterotypic population and genetic diversity.
Cell Culture
All cells were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Grand Island, NY, USA) supplemented with bovine calf serum (BCS) or fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA), 25 mM HEPES (Sigma-Aldrich Corp., St. Louis, MO, USA), 1% L-glutamine (Life Technologies), and 1% penicillin-streptomycin-fungizone (PSF; Life Technologies). The NHuRP were cultured in fully supplemented 10% BCS-containing 1.0 g/L D-glucose DMEM, and DHuRP were cultured in fully supplemented 20% FBS-containing 4.5 g/L D-glucose DMEM. To aid in culture expansion, DHuRP growth media was further supplemented with 10 ng/mL recombinant BFGF and PDGF-BB (Austral Biologicals, San Ramon, CA, USA). The NHuRP and DHuRP were used from passages 2 to 7. To test whether growth factor treatment would affect pericyte phenotype, normal or diabetic cells were plated separately onto glass coverslips and allowed to attach in their basal growth media. Then, cells were allowed to grow in either their basal media, 10 ng/mL FGF and PDGF (in 2% BCS DMEM 1.0 g/L glucose), or 1 ng/mL TGF-β1 (in 2% BCS DMEM 1.0 g/L glucose) for 48 hours before fixing and staining. Retinal ECs were isolated and cultured as characterized previously,41 used at passages 5 to 12, and grown in fully supplemented 5% BCS-containing 1.0 g/L D-glucose DMEM.
CM Collection
The NHuRP and DHuRP were cultured to approximately 75% to 90% confluence in growth media as described above. Then, pericytes were placed in 2% BCS-containing 1.0 g/L D-glucose DMEM supplemented with 25 mM HEPES, 1% L-glutamine, and 1% PSF for 48 hours. The CM was collected under sterile conditions and immediately placed on ice, while complete media then was replaced on the living cultures. Then, CM was centrifuged to remove cellular debris before sterile filtering through 0.22-μm filters (Millipore, Billerica, MA, USA) before use in experiments.
Immunofluorescence
The DHuRP and NHuRP were seeded on glass coverslips, coated with 3 μg/mL rat tail collagen I (BD Biosciences, San Jose, CA, USA) diluted in PBS for 30 minutes at 37°C, and incubated for 24 hours in their respective growth media. Subsequently, cultures were fixed stained, and imaged as reported previously.42 Staining was performed with the Alexa-Fluor 488-acetylated LDL (Life Technologies), and antibodies toward NG2 (1:100; Millipore), 3G5 (18 mg/mL),40 SMA (1:100; BioGenex, Fremont, CA, USA), Alexa Fluor 488-conjugated secondary (1:200; Life Technologies), and Alexa Fluor 546-conjugated phalloidin (1:25–1:50; Life Technologies). Images were captured with a Zeiss Axiovert 200 M equipped with a Hamamatsu (Orca ER) camera and a xenon fluorescence light source using MetaMorph Imaging software as described previously.42
In Vitro Contractility Assay
The DHuRP and NHuRP were seeded onto silicone-coated glass coverslips prepared as described.43,44 Cultures were incubated for 24 hours, followed by imaging using bright field optics as published previously.17,43
Pericyte-Endothelial Cocultures: 5-Ethynyl-2′-Deoxyuridine (EdU) Assays
Coculture experiments, where ECs and NHuRP or DHuRP are plated together in the same well, were performed as described here and previously.17 After 20 hours of coculture, cells were incubated with 10 μM EdU (Life Technologies) for 4 hours before coculture fixation and permeabilization. The Click-iT EdU Alexa Fluor 594 imaging kit (Life Technologies) was employed using the manufacturer's instructions to assess EC S-phase entry. All cells were colabeled with Hoechst33342 (1:1000; Life Technologies) to identify nuclei. Experiments were performed in triplicate, and repeated three times. Statistical significance was assessed via unpaired Student's t-tests. At the same time, cocultures also were labeled with pericyte- and cytoskeletal-specific antibodies (below).
Pericyte-Endothelial Coculture: Cx43 Localization
The NHuRP and DHuRP were plated on collagen I-coated glass coverslips, and allowed to attach for 24 hours. Subsequently, ECs were seeded on the same coverslips, to achieve a 1:1 ratio, pericyte:EC. After 24 hours of coculture, cells were fixed and permeabilized as described above. Cocultures were stained with Anti-Cx43 antibody (1:1000; gift of David Paul, Harvard Medical School, Boston, MA, USA) and Alexa Fluor 488-conjugated secondary antibody (1:200), before imaging at ×40 magnification. Then, Cx43 puncta were quantified at every pericyte-EC junction. Experiments were performed in triplicate, and repeated three times. Statistical significance was assessed via unpaired Student's t-tests.
Pericyte-Derived CM: Endothelial Proliferation Assay
The ECs were seeded in 24-well plates at an initial cell density of 10,000 cells/well, and cultured in fully supplemented 5% BCS-containing 1.0 g/L D-glucose DMEM. After cells attached overnight, control and treatment media were added to EC cultures at 24 and 96 hours after plating. Control media included 1.0 g/L D-glucose DMEM supplemented with 1% BCS, 25 mM HEPES, 1% L-glutamine, and 1% PSF (referred to as “serum control”), and 10 ng/mL FGF in 1% BCS-containing 1.0 g/L D-glucose DMEM (positive control). Treatments included pericyte-derived CM, in the presence or absence of 1 μM JTE013 (Tocris Biosciences, Bristol, UK) or 1 μM VPC23019 (Avanti Polar Lipids, Alabaster, AL, USA). After 5 days of culture, EC counts were obtained using a Z1 Beckman Coulter Counter (Indianapolis, IN, USA). Experiments were performed in triplicate, n = 6. An ANOVA followed by Student-Newman-Keuls posttests were used to assess statistical significance.
Retinal EC Morphogenesis and Tube Formation Assays
Growth factor reduced Matrigel (BD Biosciences) was polymerized for 1 hour at 37°C in 8-well chamber slides as described previously (USA Scientific, Ocala, FL, USA).42 The ECs were plated atop the gel at a density of 50,000 cells/well with the following treatments: serum control, 10 ng/mL FGF, normal pericyte-derived CM (NP CM), diabetic pericyte-derived CM (DP CM), DP CM + 1 μM VPC23019, and DP CM + 1 μM JTE013. The ECs were incubated for 16.5 hours at 37°C, and imaged. Tube length was assessed using Image J software. Experiments were performed in triplicate, n = 3. An ANOVA followed by Student-Newman-Keuls posttests were used to assess statistical significance.
Western Blotting
Total cell lysates from NHuRP and DHuRP were separated by SDS-PAGE before Western blotting as previously described. In brief, cells were lysed with ×1 sample buffer (125 mM Tris pH 7.0, 2% SDS, 10% Glycerol, 10% β-mercaptoethanol) after 48 hours of culture in 2% BCS-containing 1.0 g/L D-glucose DMEM supplemented with 25 mM HEPES, 1% L-glutamine, and 1% PSF for 48 hours. Parallel cultures were trypsinized and counted (Coulter Electronic, Z1, Hialeah, FL, USA) to insure that lysates from the control and experimental groups represented equal cell population densities. Proteins were transferred overnight onto nitrocellulose membranes. Primary antibodies and dilutions were as follows: phosphorylated Sphingosine Kinase 1 (pSphK1, 1:1000; ECM Biosciences, Versailles, KY, USA), Sphingosine Kinase 1 (SphK1, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), SMA (1:1000; Sigma-Aldrich Corp.), Cx43 (1:10,000; gift of David Paul), lamin A/C (1:10,000; gift of Larry Gerace, The Scripps Research Institute, LaJolla, CA, USA), and horseradish peroxidase (HRP)-conjugated secondary antibodies (1:1000; Santa Cruz Biotechnology). Western blots from an equivalent number of experiments were performed three times, and analyzed using band densitometry (ImageJ; provided in the public domain by the National Institutes of Health [NIH], Bethesda, MD, USA). Statistical significance was determined by unpaired Student's t-test.
Angiocrine Assays
The NHuRP/DHuRP were plated at 20K to 40K/well in a 12-well plate; cells were allowed to attach in normal growth media overnight. Medias were conditioned as above: 1 mL of 2% BCS containing DMEM 1.0 g/L glucose with 1% PSF and L-glut was added to cells for 48 hours. Medias were collected on ice, centrifuged, and then subjected to the Proteome Profiler: human angiogenesis array (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's suggestions. Membranes were visualized for 10 minutes using x-ray film. Densitometry of spots was performed using ImageJ, and this assay was repeated in triplicate. Statistical significance was determined by unpaired Student's t-test.
Results
Characterization of Cells Derived From Normal and Diabetic Retinae
Donor eyes from individuals with proliferative diabetic retinopathy or normal donors were obtained from NDRI on wet ice and via courier mail within 24 hours of expiration (see Materials and Methods). Posterior poles and retinae were dissected from the globes, and retinal microvascular isolates and pericyte cultures were derived using well-established protocols.40 As shown in Figure 1, pericytes derived from pooled donor eyes, “normal” or “diabetic;” that is, NHuRP or DHuRP, are morphologically distinct when cultured in vitro. The NHuRP are heterogeneous cells, possessing ruffled edges indicative of a migratory phenotype, together with phase contrast-dense stress fibers (Fig. 1A). Cells are typically mononucleate and appear, under bright-field microscopy, reminiscent of other primary pericyte cultures derived from mammalian and human tissues.40,45–47 On the other hand, the pericytes isolated from diabetic retina have a greater spread of cell size (Fig. 1B) and are on average 5.7-fold larger than NHuRP (67702 ± 34959–11929 ± 6723 A.U., respectively). Leading edges are largely absent from DHuRP, as are the straight, axially aligned phase dense stress fibers typically present under bright-field phase microscopy (Fig. 1B). Instead, DHuRP display curved bundles of stress fibers reflecting the circular morphology of these extensive cells; moreover, many DHuRP appear multinucleate, which may indicate potential cytokinetic defects culminating in aneuploidy. In normal growth conditions, DHuRP proliferate at reduced rates compared to NHuRP, as the normal cells reach confluence in 2 to 3 days, while DHuRP require approximately 2 weeks to reach 50% to 60% confluence (data not shown).
Figure 1.
Normal and diabetic pericyte culture from human donor retinae. The NHuRP and DHuRP were isolated from human donor retinae and are sustainable in culture for approximately 10 and 6 passages, respectively. Note the dramatically different morphology and spread of cellular sizes between (A) NHuRP and (B) DHuRP. Scale bar: 250 μm. Coculture of pericytes with retinal EC demonstrate an uptake of Alexa-Fluor 488 acetylated LDL specifically by EC and not by (C) NHuRP or (D) DHuRP. Scale bar: 100 μm.
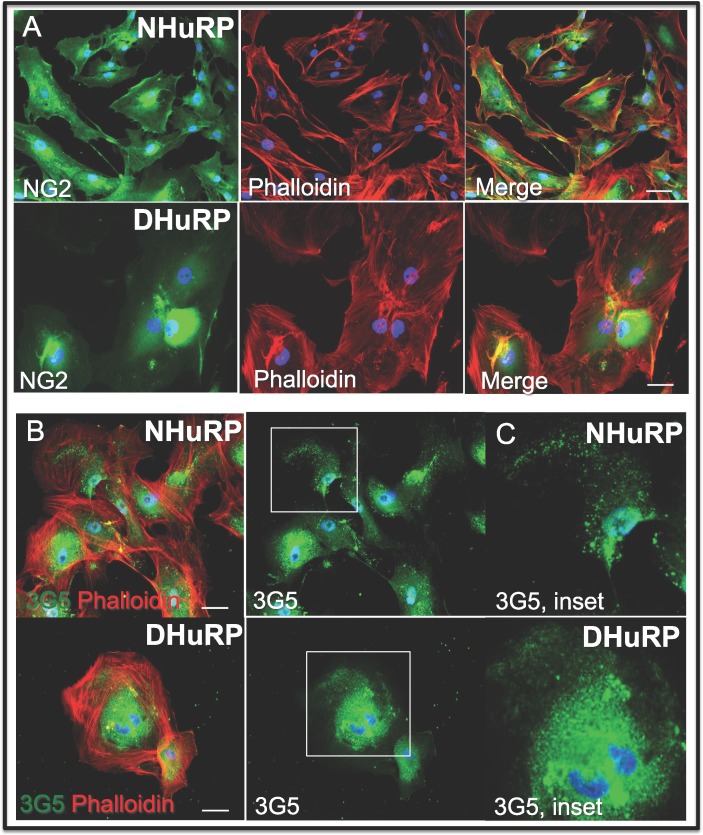
To establish the identity of the human donor-derived retinal microvascular cell populations and to confirm whether or to what extent these cell populations possess cell surface, extracellular matrix and cytoskeletal markers previously identified by our laboratory and others to be representative of bona fide pericytes, we performed several antibody- and vital dye-based assays. Firstly, we cocultured EC with NHuRP or DHuRP to test the cells' ability to bind and internalize acetylated-LDL. While the vascular ECs, which possess the LDL scavenger receptor, internalized the Alexa Fluor 488-labeled acyl-LDL, pericytes derived from diabetic or normal human donor eyes neither bind nor internalize this fluorescent lipid derivative (Figs. 1C, 1D). Further, NHuRP and DHuRP express the pericyte markers NG2 and 3G5 (Figs. 2A–C) with normal diffuse/cytoplasmic and punctuated, cell surface-staining patterns, respectively.
Figure 2.
NHuRP and DHuRP express pericyte markers. Normal and diabetic retinal pericytes were costained with Alexa Fluor-546 phalloidin (red), and the pericyte markers (A) NG2 and (B) 3G5. Note the normal punctuated staining of 3G5 in NHuRP and DHuRP (C). Scale bar: 50 μm.
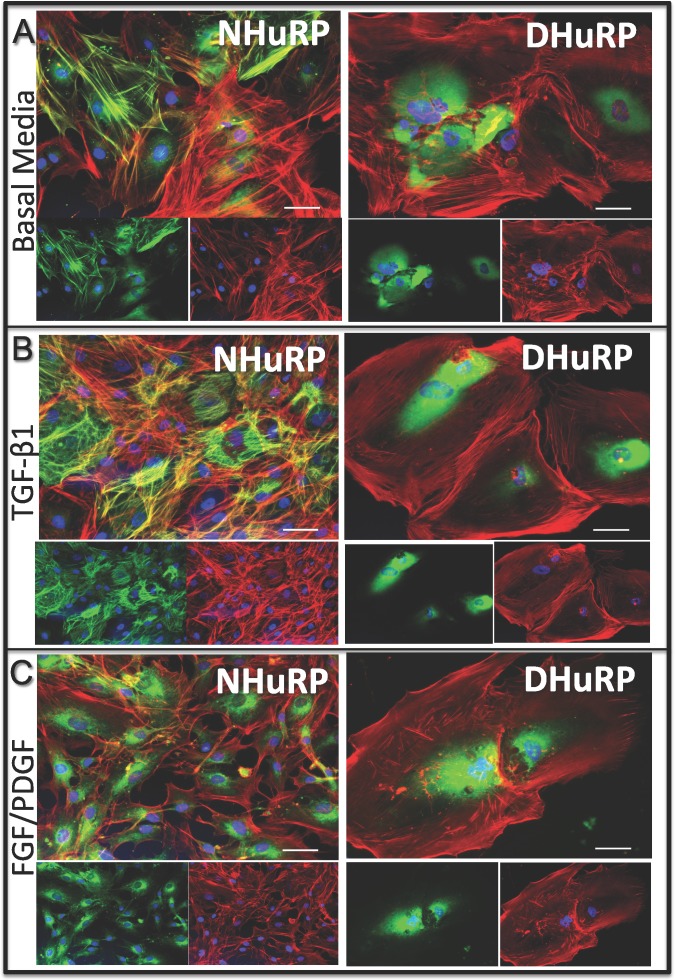
Pericytes in culture have been shown to assume a more migratory and proliferative phenotype in response to treatment with the growth factors FGF and PDGF-BB, while exposure to TGF-β shifts these cells into a more hypertrophic/contractile state,21,48–50 responses which are recapitulated by NHuRP (Figs. 3A–C). Regardless of exposure of DHuRP to TGF-β or normal serum, incorporation of SMA into filamentous structures is strikingly absent (Figs. 3A, 3B); moreover, the ratio of SMA to phalloidin intensities are many fold higher in NHuRP (data not shown). While we do observe qualitative changes in the phalloidin-stained cytoskeletal structures in NHuRP and DHuRP, cytokine application is unable to mobilize αSMA into stress fibers. This is in contrast to NHuRP, which incorporate SMA into filamentous structures to a greater extent after application of TGF-β, and are diminished in stress fiber associated αSMA post treatment with FGF/PDGF. Thus, while cells have been isolated from their individual normal or diabetic microenvironments do retain standard pericyte markers, we find that cytoskeletal differences persist upon culturing these pericytes in vitro.
Figure 3.
Growth factor stimulation alters cytoskeletal organization in NHuRP and DHuRP. Normal and diabetic retinal pericytes were costained phalloidin (red) and SMA (green) after culture in (A) basal growth media, or low serum media containing either (B) TGF-β1, or (C) a combination of FGF and PDGF-BB. Scale bar: 50 μm.
DHuRPs Exhibit Altered Cytoskeletal Organization
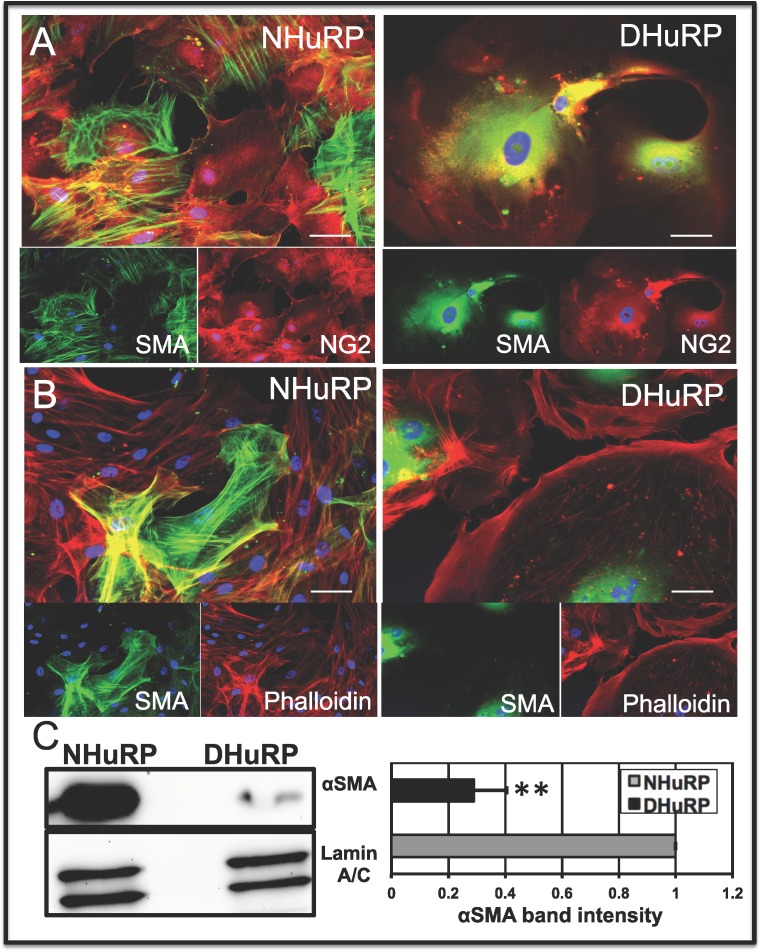
Pericyte isoactin network formation and organization underlies pericyte shape and contractile phenotype, and perturbations to the cytoskeleton result in altered cell shape and physiology.48,51 As we found that treatment with FGF/PDGF and TGF-β was unable to mobilize SMA to DHuRP stress fibers, we further sought to characterize DHuRP cytoskeletal dynamics. We demonstrated that all NHuRP in normal growth express the pericyte marker NG2, while SMA expression and organization varies among cells (Fig. 4A, left). While DHuRP express NG2, anti-αSMA localization is diffuse and perinuclear, and is not stress fiber-associated (Fig. 4A, right). Additionally, staining with phalloidin (total actin) and anti-αSMA reveals marked cytoskeletal alterations between DHuRP and NHuRP. Previous work characterized isoactin arrays in normal pericytes,46 and in alignment with these results, NHuRP were shown to possess robust F-actin and αSMA-enriched stress fibers that traverse the entirety of the cell and colocalize (Fig. 4B, bottom left). In contrast, DHuRP were largely devoid of cell-spanning stress fibers, with F-actin localizing to the edge of the cell membrane and/or bundled into circular arrays; furthermore DHuRP display a qualitative diminution in αSMA expression, and repositioned from stress fibers to a more diffuse, perinuclear and peripheral cytoplasmic position (Fig. 4B, right).
Figure 4.
Alterations in DHuRP isoactin, and cytoskeletal architecture and expression. The DHuRP and NHuRP were costained with either (A) NG2 and αSMA or (B) phalloidin and αSMA. Note the variable degree of SMA staining and cytoskeletal incorporation into NG2+ NHuRP, while NG2+ DHuRP display diffuse perinuclear SMA staining in (A). Colocalization of SMA with phalloidin staining reveals a stress fiber localization of SMA in NHuRP, but not in DHuRP (B), merged panels. Scale bar: 50 μm. (C) Pericyte lysates were immunoblotted with anti-αSMA and anti-lamin A/C. Quantification of αSMA band intensities normalized to lamin A/C, and represented as an average fold of NHuRP ± SE. **P < 0.01.
Western blotting was used to confirm the immunofluorescence-based analyses of diminished αSMA expression in DHuRP. Compared to NHuRP, αSMA expression was reduced by 3.45-fold in DHuRP (Figs. 4C, 4D). Taken together, these results suggested that diabetes influences cytoskeletal dynamics in pericytes by affecting muscle and nonmuscle actin organization, as well as reducing αSMA expression.
Diabetic Microenvironment Alters Pericyte Contractile Phenotype
It has been well established that cellular force generation is acto-myosin–dependent.23 Since DHuRP cytoskeletal organization is extensively altered compared to NHuRP, and the smooth muscle-like cytoskeletal elements are seemingly and significantly diminished as a result of diabetic insult, we hypothesized that contractile function would be similarly altered. To test this directly, we studied whether or to what extent normal or diabetic pericytes are able to generate force, therein “wrinkling” or deforming their underlying substrates upon which they are anchored. Using live cell imaging coupled to our in vitro substrate deformation assay, we observed marked differences in morphology and contractile potential when comparing pericyte populations derived from normal or diabetic donor eyes. The NHuRP deform their underlying anchoring substrates, generating parallel arrays of straight “wrinkles,” where overlying deforming forces dictate the underlying patterns produced (Fig. 5, left). Conversely, DHuRP create two notable types of deformation patterns: small wrinkles localized to the cellular cortex and curvilinear wrinkles reflective of the cellular shape, which lacks a bona fide leading or trailing edge characteristic of migrating cells (Fig. 5, right). Quantitatively, NHuRP display a 2.3-fold increase in the percent of contracting cells compared to DHuRP (52.7% ± 4.9–22.7% ± 6.0, respectively; Fig. 5). These experiments suggest that the cytoskeletal disorganization observed in DHuRP culminates in altered contractile status and reduced force production.
Figure 5.
Diabetes-induced alterations in pericyte contraction-based force transduction. The HuRP were plated on collagen-I coated deformable silicone substrates, and live imaging 24 hours after plating reveals divergent morphologic and deformation patterns in NHuRP (top left) and DHuRP (top right). Scale bar: 50 μm. Pericyte contractility was quantified as previously described and depicted as a mean percentage ± SE. **P < 0.01.
DHuRPs Promote EC Cycle Entry
Pericytes have been shown to govern endothelial quiescence through cell contact- and soluble-mediated mechanisms.17,18,20 Further, alterations in pericyte contractile phenotype have been shown to relieve contact-dependent EC growth arrest and promote angiogenic induction.17,18 As pericytes isolated from diabetic retinae exhibit unique contractile protein expression, motility, and force transduction profiles compared to those from normal donors, we tested for differential ability to regulate endothelial growth by assaying S-phase entry in EC cocultures with DHuRP and NHuRP. When ECs are cocultured and in contact with NHuRP, approximately one-third (31.2% ± 3.5%) of ECs are observed in S-phase. However, under identical cell-matched conditions, ECs in direct contact with DHuRP experience a doubling (69.7% ± 2.7%) in S-phase entry (Fig. 6). While ECs in coculture with NHuRP are in large part growth arrested, the soluble milieu of DHuRP-EC cocultures appears to stimulate endothelial growth, as isolated ECs were shown to enter S-phase at a 1.7-fold higher rate than isolated ECs cultured together with NHuRP (61.3% ± 4.5%–36.6% ± 4.8%, respectively; Fig. 6). These results implicate a diabetic-induced disruption of pericyte-mediated endothelial growth arrest, by contact-dependent and soluble mechanisms.
Figure 6.
Diabetic pericytes are unable to sustain retinal capillary endothelial growth arrest in vitro. The NHuRP and DHuRP were cocultured with EC for 24 hours, and pulsed with 10 μM EdU for the last 4 hours of coculture. Blue and pink nuclei indicate EdU-negative and -positive cells, respectively. (A) The NHuRP (asterisk) maintain contacted (arrowheads) and isolated (diamonds) EC quiescence, while (B) DHuRP (asterisk) are unable to maintain contacted and isolated endothelial S-phase entry. Scale bar: 50 μm. (C) Quantification of EdU-positive nuclei in pericyte contacted and isolated EC as an average percentage ± SE. **P < 0.01.
Modulation of Connexin 43 Expression in DHuRPs Impacts Heterotypic Gap Junction Formation
Gap junction communication between vascular cells relies on the expression of the connexin subunits, Cx37, Cx40, Cx43, and Cx45.52 Furthermore, in vitro and in vivo diabetic models have demonstrated an attenuation of Cx43 expression and localization.53,54 With these observations in mind, together with our previous data demonstrating loss of contact-dependent cell cycle arrest in DHuRP-EC cocultures, we studied Cx43 expression and localization in retinal pericytes isolated from diabetic and nondiabetic donors. To ascertain whether heterotypic gap junction formation was affected by diabetic conditions, NHuRP or DHuRP were cocultured with ECs for 24 hours, fixed, and stained for Cx43. The DHuRP-EC junctions demonstrate diminished Cx43 puncta (Fig. 7B), whereas NHuRP-EC contact points have numerous brightly stained plaques (Fig. 7A). Quantitatively, there were approximately twice as many Cx43 puncta per pericyte-contacted EC in NHuRP cocultures (9.7 ± 1.7 vs. 4.7 ± 0.8; Fig. 7C). Derived from confluent monolayers of pericytes alone, NHuRP and DHuRP lysates were compared using Western blot, and quantitative densitometry reveals significant 6-fold reductions in the levels of Cx43 in DHuRP when compared to NHuRP and normalized to lamin A/C (Fig. 7D). Thus, pericytes derived from diabetic human retinae demonstrated decreased Cx43 expression as well as a reduction in heterotypic gap junction formation.
Figure 7.
Diabetes-induced perturbations in pericyte connexin 43 expression. (A, B) The NHuRP-EC and DHuRP-EC cocultures were visualized with DIC (left) and Cx43 (right). Selected pericyte-EC contact points are highlighted (asterisks); right indicate insets of selected HuRP-REC contact points demarcated in the DIC image with dotted black boxes. Note the diminution in Cx43 punctae and in fluorescence intensity in DHuRP-EC cocultures as compared to control NHuRP-EC panels. Scale bar: 50 μm. (C) Quantification of the number of puncta per pericyte contacted REC as an average ± SE. *P < 0.05. (D) Cx43 immunoblot of pericyte lysates normalized to lamin A/C with accompanying band densitometry as an average fold of NHuRP ± SE. **P < 0.01.
DHuRP-Derived Conditioned Media Alters Endothelial Growth and Angiogenic Potential
Retinal neovascularization is an essential step in the pathogenesis of diabetic retinopathy.55 Furthermore, soluble factors derived from pericytes normally act to maintain endothelial quiescence.56 However, here we observed an increase in contact-independent S-phase entry of EC in coculture with DHuRP. Therefore, we sought to determine whether CM collected from DP CM alters EC angiogenic potential compared to media conditioned by NHuRP (NP CM). To this end, we used the well-characterized, in vitro Matrigel model of angiogenesis. In agreement with past studies,57 treating EC with NP CM inhibits network formation, as tubes appear retracted and multiple cellular aggregates are present, and quantitatively, morphogenesis is significantly decreased when compared to positive (10 ng/mL FGF) and negative (low serum) controls (Figs. 8A, 8B). Interestingly, when ECs are exposed to DP CM, the endothelium forms a highly interconnected microvascular network displaying a highly branched cellular array possessing connectivity and resembling an in vivo capillary EC-derived angiogenic array (Fig. 8A); this represents a 2-fold increase in networks formed when compared to identical EC cultures, which were treated with cell number-matched equivalents of NP CM (Fig. 8B).
Figure 8.
Pericyte CM modulate retinal capillary endothelial growth and morphogenesis. (A) EC were plated on Matrigel and exposed to the various conditions: FGF in low serum (1% BCS), low serum only, NPCM or DPCM. 16.5 hours post plating tube formation on Matrigel was visualized. Note the decrease in tube formation in EC treated with NPCM, an effect that is reversed with DPCM. Scale bar: 100 μm. (B) Quantification of EC tube formation as an average of arbitrary units ± SE. ANOVA P < 0.0001; Student Newman-Keuls posttest *P < 0.05, **P < 0.01 relative to serum control; ##P < 0.01 relative to DP CM. (C) As above, EC were treated with pericyte-derived conditioned media, FGF or serum control for 120 hours. Compared to serum control, NP CM significantly inhibited REC proliferation, whereas DP CM enhanced growth. The REC proliferation measured as an average cell number per well ± SE. ANOVA, P < 0.001; Student-Newman-Keuls posttest, *P < 0.05, **P < 0.01 compared to serum control; #P < 0.05, ##P < 0.01 compared to DP CM.
As proliferation is a key step in angiogenesis, and as DP CM has a positive effect on endothelial tube formation, we sought to determine if DP CM would affect EC proliferation. Indeed, DP CM possesses twice the mitogenic activity when compared to serum control and NP CM–treated cells, showing a 1.7- and 2.7-fold increase 5 days after treatment, respectively (Fig. 8C). Taken together, in comparison with NHuRP, which normally antagonize the angiogenic process, DHuRP produce soluble factors, which are capable of inducing a robust angiogenic response in EC.
DP CM Promotes Angiogenesis Through an S1P-SphK1-Dependent Mechanism
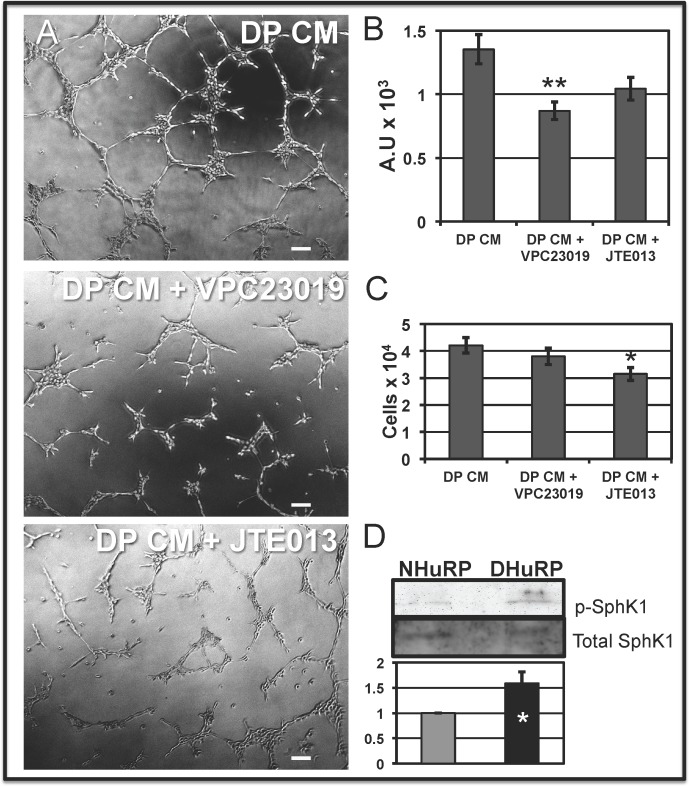
As DP CM, alone, stimulates EC-driven angiogenesis in vitro, we investigated whether soluble secreted factor(s) were responsible for the angiogenic- and growth-promoting activities. S1P, a potential candidate, has been shown recently to enhance EC mitogenesis58 and tubulogenesis,59 yet the role and/or effects of pericyte-produced S1P on EC cycle dynamics or angiogenic potential, as well as whether diabetes could induce perturbations in pericyte-derived S1P signaling have not been studied to our knowledge. To explore whether S1P signaling is involved in the DP CM-mediated angiogenic response, we used the pharmacologic inhibitors VPC23019 and JTE013. Inhibitor VPC23019 is a competitive antagonist of S1P1 and S1P3 with no activity toward S1P2, whereas JTE013 acts as a selective antagonist of S1P2 only, and optimal doses for selective inhibition of each S1P receptor isoform have been optimized and defined previously.60 When DP CM was combined with 1 μM VPC23019, EC tube formation was significantly reduced by 36% compared to DP CM alone (Figs. 9A, 9B). While a slight decrease in tube formation occurred in conditions containing DP CM and 1 μM JTE013, this effect was not statistically different from DP CM alone (Figs. 8A, 8B). Interestingly, addition of S1P to pericytes plated on deformable substrates increases normal pericyte contractility, an effect that can be mitigated by addition of the SIP receptor antagonists, especially by the S1P2 inhibitor, JTE013 (Supplementary Fig. S1). Together, we concluded that the S1P signaling pathway has a critical role in the microvasculature physiology, and that the S1P1/S1P3 signaling pathway is largely responsible for the increase in EC tube formation upon exposure to DP CM.
Figure 9.
DHuRP S1P-SphK1 signaling axis influences microvascular interactions. (A) The EC were plated on Matrigel, to analyze tube formation in the presence of DP CM alone or with DP CM with the S1PR antagonists, JTE013 and VPC23019, for 16.5 hours. Scale bar: 100 μm. (B) Quantification of EC tube formation as an average of arbitrary units ± SE. ANOVA, P < 0.0001; Student Newman-Keuls posttest **P < 0.01. (C) Treatment of DP CM with JTE013 significantly abrogated DP CM mediated EC mitogenesis. The REC proliferation was measured as an average cell number per well ± SE. ANOVA, P < 0.001; Student-Newman-Keuls posttest, *P < 0.05. (D) Populations of NHuRP and DHuRP were cultured in identical serum conditions for 48 hours before lysis. Pericyte lysates were separated via SDS-PAGE, and immunoblotted with anti-phosphorylated serine 225 SphK1 and anti-total SphK1. Quantification of pSphK1 and total SphK1 band intensities normalized to lamin A/C, and represented as an average fold of pSphK1 to total SphK1 in NHuRP lysates ± SE. *P < 0.05.
We examined whether the increase in EC proliferation upon stimulation with DP CM was, in part, due to signaling through the S1P receptors. In vitro proliferation assays revealed that selective inhibition of S1P2 (JTE013), but not S1P1,3 (VPC23019), abrogates the stimulating activity of the DP CM (Fig. 9C). Thus, DP CM exerts its progrowth effect via S1P2 while endothelial tube morphogenesis is mediated by the S1P1/S1P3.
Because DP CM was shown to drive EC growth and tube formation, in part, by S1P/S1PR signaling, we posited that changes in the SphK1 phosphorylation status might result from diabetes-induced perturbations in posttranslational modification of the rate limiting enzymes responsible for S1P production itself. Activation of SphK1 relies on phosphorylation of the serine 225 residue.61 To these ends, we took advantage of phospho-specific SphK1 antibodies and Western blotting to ascertain whether the diabetic microenvironment influences the phosphorylation status of SphK1. Indeed, while total kinase expression remains unchanged, there is a significant 1.58 ± 0.22-fold increase in the ratio of phosphorylated SphK1 to total SphK1 in DHuRP compared to NHuRP (Fig. 9D). This increase in SphK1 activation in DHuRP may be responsible for increases in S1P secretion, which subsequently acts through S1P2 or S1P1/S1P3 to stimulate endothelial proliferation and morphogenesis, respectively.
Diabetes-Induced Alterations in the Pericyte Secretome
To further understand how diabetes might alter retinal microvascular phenotype, and to learn whether the diabetic state impacts retinal pericytes' release and/or secretion of angiogenic modulators, including proteases and their inhibitors, cytokines and their cognate receptors, growth and survival agents, and accompanying receptors, and angiogenesis inhibitors/activators that are differentially regulated in NHuRP and DHuRP, we performed “secretome” analyses of CM produced by each cell type in vitro. Comparable population densities of retinal pericytes derived from normal or diabetic donor eyes were plated as described (see Materials and Methods). Interestingly, several factors involved in angiogenic control, inflammation, and immunity, and protein metabolism are either significantly different (c.f. Fig. 10), distinctly altered, or are secreted but unchanged in medias conditioned by pericytes, which were derived from normal versus diabetic donors (Fig. 10; Table). That activators and inhibitors of angiogenesis, for example, endostatin and TSP-1, as well as other key cytokines, including GM-CSF, are modulated in DP CM (Fig. 10) is of interest, especially linked to the regulatory role that immune surveillance cells have in shaping microvascular cell responses to injury, including angiogenic activation. Also, linked to diabetes, per se, it is extremely interesting to point out that the insulin-like growth factor binding proteins 2 and 3, which have growth, bioactive-lipid, and pathological angiogenesis-altering activities,62–64 are significantly upregulated in DP CM (Fig. 9). Indeed, IGFBP3 has been shown to induce angiogenesis through an SphK1-dependent mechanism,65,66 further solidifying the link between DP CM and S1P-signaling. Other factors secreted by pericyte populations, but not significantly altered between groups. Thus, diabetic insult causes a shift in the secretome of DHuRP that is sustainable in culture.
Figure 10.
Pericyte secretome profiles are altered downstream of diabetes. The NHuRP and DHuRP were plated at identical cell numbers per well: 40K cells in a 24 well plate (top). Scale bar: 250 μm. After attachment in their growth media, normal and diabetic pericytes were treated with identical serum media for 48 hours, and this conditioned media was collected and analyzed with an angiocrine assay (see Materials and Methods). Factors that were differentially expressed are depicted in the graph (*P < 0.01, **P < 0.001 in t-test analyses).
Table.
Pericyte Secretome Analysis of Factors Upregulated, Downregulated, or Unchanged in Medias Conditioned by Normal and Diabetic Human Retinal Pericytes

Discussion
We provided evidence that challenges the notion that reactivation of the microvasculature in DR requires pericyte dropout using NG2+/3G5+/LDLR− diabetic and age-matched control human retinal pericytes. It is well known that diabetes alters the microvascular environment, and currently accepted models of DR describe the histopathologic features of late-stage disease as a step-wise progression, beginning with hyperglycemia and inflammation, followed by pericyte loss, and ending with neovascularization15,16,67–69 (Fig. 11, top). However, our results allowed us to speculate that the diabetic microenvironment acts as a modifier of retinal pericyte physiology, producing a switch from anti- to pro-angiogenic signaling (Fig. 11, bottom). We determined that diabetic cells are markedly different from control cells in cytoskeletal protein expression, force generation, gap junction regulation, and sphingolipid signaling. Ultimately, in coculture and CM experiments, we demonstrate that DHuRP are unable to sustain normal control of endothelial growth dynamics. For the first time, we reveal that DHuRP mechanochemical signaling alters endothelial growth potential, which may be the functional consequence of altered cellular tension, gap junction formation, and S1P secretion.
Figure 11.
Angiogenic induction of the retinal microcirculation is a pericyte-driven process. Current model of proliferative diabetic retinopathy: inflammatory mediators and hyperglycemia activate proapoptotic pathways and/or migratory events in pericytes. Loss of retinal pericytes creates an environment permissive to endothelial activation, and subsequently, neovascularization (top). The bottom depicts our proposed model of DR as our study implicates that pathologic retinal angiogenesis may be affected in early stages of this disease. Hyperglycemia and inflammatory responses may reprogram retinal pericytes and ECs to a proangiogenic phenotype. Reduction of pericyte contractility downstream of smooth muscle and non-muscle actin reorganization in addition to pathologic S1P signaling release the endothelium from its growth inhibited state. Attenuated gap junction formation between pericytes and ECs appears to be another feature of altered pericyte physiology in diabetes. In agreement with past studies, the ultimate histopathologic picture of diabetic retinopathy is retinal microvessels devoid of pericytes.
Our results suggested that diabetes regulates pericyte actin network reorganization, which subsequently alters pericyte contractile status. We observed that muscle and nonmuscle actin networks are markedly deformed in DHuRP, and this degeneration in cytoskeletal architecture leads to decreased force generation by these cells. Furthermore, DHuRP not only are markedly altered in cytoskeletal architecture and are unable to mobilize the αSMA isoform into stress fibers, reminiscent of pericytes inhibited in their Rho signaling cascade which selectively disassembly αSMA-containing stress fibers, with no influence on the gamma stress fiber network,51 but also display reduced pericyte contractility, similar to pericytes treated with a ROCK inhibitor.17 As Rho is a central regulator of actin dynamics,70 future studies aimed at elucidating the Rho GTPase status of DHuRP should be undertaken.
Our results provide in vitro evidence of altered Cx43 dynamics in human diabetic retinal disease. In agreement with mouse models53 and bovine retinal pericyte mono- and cocultures,54 we found that not only are DHuRP monolayers deficient in Cx43 compared to pericytes derived from healthy human retinae, but also, the number of Cx43 puncta are reduced at DHuRP-EC membrane contact points, which may be a mechanism of pericyte regulation of EC growth, as our data indicate a loss in EC quiescence in a DHuRP contact-dependent fashion. Interestingly, cancer cell proliferation appears sensitive to Cx43 subunit expression levels, suggesting a role for Cx43-mediated growth regulation.71 Therefore, determining the extent to which pericyte Cx43 functions to regulate proliferative capacity, by homo- and heterotypic junctional communication, is a priority that will shed insight into the pathologic significance of decreased pericyte Cx43 expression in diabetes.
Pericytes have been identified recently as a microvascular source of S1P, which may act to reinforce endothelial tight junctions.35 To our knowledge, our in vitro studies are the first to suggest that pericyte-derived S1P may function to initiate endothelial proliferation and angiogenesis during diabetes. The DHuRP stimulate EC mitogenesis and morphogenesis through distinct S1PR subtypes: in part, proliferation is S1P2 mediated, while tube formation is S1P1/S1P3 mediated. In contrast with our proliferation results, studies using exogenous S1P identify S1P2 as a negative regulator of EC migration, angiogenesis and growth in vitro.72,73 Our experiments were performed with CM, and, therefore, the incongruence of our results may reflect coupling of S1P signaling with an undetermined growth factor (or factors) contained in DP CM. Indeed, there is considerable crosstalk between S1P and other growth factor signaling pathways, including VEGF74 and connective tissue growth factor (CTGF),75 and preliminary data suggest that other factors are elevated in the DHuRP secretome, including VEGF, PDGF-AA, and Ang-1 and -2 (data not shown). Moreover, in vivo ischemic retinal neovascularization experiments may further support this notion, as S1P2 signaling appears to be principally involved in abnormal angiogenesis not proliferation.39 Clearly, it is critical to further elucidate the pathologic effects of DP CM, and how S1P and other proangiogenic signaling pathways cooperate in DR.
We provided evidence of enhanced proliferation among isolated ECs in our DHuRP-EC cocultures, suggesting an imbalance in anti-/proproliferative factor secretion. There are coculture studies that show TGF-β–dependent and independent mechanisms foster capillary quiescence.17,20 While previous studies have ruled out a TGF-β effect downstream of alterations in Rho signaling,17 and preliminary data from angiocrine assays do not reveal a change in TGF-β levels between NHuRP and DHuRP (data not shown), we have found that sphingolipid signaling is altered in diabetic pericytes, which influences endothelial growth and morphogenesis. S1P induces an increase in normal pericyte contractile phenotype, DHuRP are diminished in force generation, yet display increased pSphK1, and levels of SphK1 have been shown to influence arteriolar tonus in vitro76; thus, these elevated levels of active SphK1 may reflect a feedback mechanism. Physiologically, pericytes likely produce S1P in an autocrine fashion to maintain pericyte tone, which, by an unknown mechanism, becomes ineffective to regulate tone in diabetic pericytes. Thus, to overcome this resistance and regain normal tonus, DHuRP may secrete increased levels of S1P, which may be explained by the increase in active SphK we observed. As perivascular S1P levels are dramatically elevated, activation of adjacent ECs may occur in DR. Future experiments will determine whether and to what extent Rho signaling downstream of S1P govern microvascular cell interactions in diabetes.
Interestingly, we demonstrated that diabetes induces changes in pericyte secretome profiles, possibly providing insight into the mechanism by which angiogenic activation is initiated during DR. Importantly, diabetic upregulation of the IGF-like binding proteins, specifically IGFBP-3, not only has relevance to the diabetic microenvironment, but also strengthens our newly described involvement of DHuRP in production/activation of S1P and SphK. As we demonstrated that IGFBP-2 and -3 are upregulated in DHuRP, ECs derived from human diabetic retinae also exhibit increases in IGF-1, and the stimulatory IGFBPs -1, -2, -3, and -5.77 Additionally, IGFBP3 alone directly activates SphK and increases SphK message levels.66 Furthermore, IGFBP3 positively influences angiogenic induction in cell culture and animal models via IGF-1/IGF1R signaling, and subsequent S1P production and SphK activation.65 While data suggest that IGFBP has growth promoting and growth inhibiting properties,62,78 these effects are likely to be tissue and context-specific. For example, IGFBP3 may be a good candidate for cancer therapy as this molecule is specifically expressed on ECs isolated from tumor vessels and not normal ECs,79 and suppresses tumor growth and angiogenesis.78 On the other hand, IGFBP3 mediates endothelial precursor cell differentiation, migration, and capillary formation; further, expression of IGFBP3 protects against hyperoxia-induced vaso-obliteration and promotes proper vascular repair after ischemic insult to the retina.80 More work in this area will shed insight into the mechanism by which IGFBP3 acts in a vascular cell- or tissue- and disease-specific context to influence angiogenesis or pathogenesis. For example our data lend credence to the notion that the elevations in DP CM-derived IGFBP3 signals to promote angiogenic induction via stimulating DHuRP S1P production, SphK phosphorylation, RhoGTP-, and downstream cytoskeletal effector activation/signaling.
Our study provided evidence that pericyte loss may not be required to induce angiogenesis in DR; rather, the diabetic microenvironment may alter pericyte physiology, thereby acting as the angiogenic switch that tips the retinal microcirculation from stable and quiescent to proliferative and sprouting. While interpretations regarding our results may be limited because of insufficient patient and donor medical histories, ongoing and planned studies are aimed to better understand the causal links that are likely to exist between the diabetic retinal microenvironment and the resultant regulatory roles that pericyte chemo-mechanics have in controlling endothelial growth dynamics and pathological angiogenic induction. Importantly, we show for the first time to our knowledge a novel role for S1P in the microvasculature: pericyte contractility is promoted by S1P, and pericyte-derived S1P may act to signal though endothelial S1PRs to induce EC proliferation and morphogenesis. Our findings point to a paradigm shift in pericyte biology, carrying potential clinical implications in that microvascular lesions may occur earlier than previously thought. If so, therapeutic approaches should not only be limited to inhibiting pathologic neovascularization, but also aimed at stabilizing the preexisting, diseased retinal pericyte population in attempts to prevent initial angiogenic induction. Second, bioactive lipid signaling may prove to be a viable target in the treatment of proliferative DR. With emerging mesenchymal and adipose-derived stem cell technologies,81 creation of pro-/antiangiogenic pericytes may be used in tumor angiogenesis, DR and wound healing through manipulation of mechanochemical signaling pathways. Our work highlights the complex interplay between mechanical and soluble regulation of angiogenic potential, and highlights the need to elucidate the molecular players in pericytes as a means to combat microvascular disease.
Acknowledgments
Supported by Grants NIH T32DK07542, NIH HL069770 (JTD), NIH EY 15125, EY 19533, and EY 022063 (IMH).
Disclosure: J.T. Durham, None; B.M. Dulmovits, None; S.M. Cronk, None; A.R. Sheets, None; I.M. Herman, None
References
- 1. Crawford TN,, Alfaro DV, III, Kerrison JB,, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009; 5: 8–13. [DOI] [PubMed] [Google Scholar]
- 2. Boyle JP,, Thompson TJ,, Gregg EW,, Barker LE,, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence mortality, and prediabetes prevalence. Popul Health Metr. 2010; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durham JT,, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011; 11: 253–264. [DOI] [PubMed] [Google Scholar]
- 4. Saint-Geniez M,, Kurihara T,, Sekiyama E,, Maldonado AE,, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009; 106: 18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crocker DJ,, Murad TM,, Geer JC. Role of the pericyte in wound healing. an ultrastructural study. Exp Mol Pathol. 1970; 13: 51–65. [DOI] [PubMed] [Google Scholar]
- 6. Cogan DG,, Kuwabara T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br J Ophthalmol. 1984; 68: 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willard AL,, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol. 2012; 2012: 209538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armulik A,, Genove G,, Betsholtz C. Pericytes: developmental physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 9. Hirschi KK,, D'Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997; 79: 419–428. [DOI] [PubMed] [Google Scholar]
- 10. Garner A. Histopathology of diabetic retinopathy in man. Eye (Lond). 1993; 7: 250–253. [DOI] [PubMed] [Google Scholar]
- 11. Shepro D,, Morel NM. Pericyte physiology. FASEB J. 1993; 7: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 12. Hellstrom M,, Gerhardt H,, Kalen M,, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001; 153: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benjamin LE,, Hemo I,, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998; 125: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 14. Murakami T,, Suzuma K,, Takagi H,, et al. Time-lapse imaging of vitreoretinal angiogenesis originating from both quiescent and mature vessels in a novel ex vivo system. Invest Ophthalmol Vis Sci. 2006; 47: 5529–5536. [DOI] [PubMed] [Google Scholar]
- 15. Geraldes P,, Hiraoka-Yamamoto J,, Matsumoto M,, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009; 15: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfister F,, Feng Y,, vom Hagen F,, et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008; 57: 2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kutcher ME,, Kolyada AY,, Surks HK,, Herman IM. Pericyte rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol. 2007; 171: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durham JT,, Surks HK,, Dulmovits BM,, Herman IM. Pericyte contractility controls endothelial cell cycle progression and sprouting: Insights into angiogenic switch mechanics. Am J Physiol Cell Physiol. 2014; 307: C878–C892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozerdem U,, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004; 7: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antonelli-Orlidge A,, Saunders KB,, Smith SR,, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989; 86: 4544–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sieczkiewicz GJ,, Herman IM. TGF-beta 1 signaling controls retinal pericyte contractile protein expression. Microvasc Res. 2003; 66: 190–196. [DOI] [PubMed] [Google Scholar]
- 22. Kutcher ME,, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009; 77: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S,, Zeiger A,, Maloney J,, Maciej K,, Van Vliet K,, Herman IM. Pericyte actomyosin-mediated contraction at the cell–material interface can modulate the microvascular niche. J Phys Condens Matter. 2010; 22: 1–11. [DOI] [PubMed] [Google Scholar]
- 24. Larson DM,, Carson MP,, Haudenschild CC. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc Res. 1987; 34: 184–199. [DOI] [PubMed] [Google Scholar]
- 25. Wu DM,, Minami M,, Kawamura H,, Puro DG. Electrotonic transmission within pericyte-containing retinal microvessels. Microcirculation. 2006; 13: 353–363. [DOI] [PubMed] [Google Scholar]
- 26. Thurston G,, Suri C,, Smith K,, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286: 2511–2514. [DOI] [PubMed] [Google Scholar]
- 27. Lindahl P,, Johansson BR,, Leveen P,, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997; 277: 242–245. [DOI] [PubMed] [Google Scholar]
- 28. Connolly DT,, Heuvelman DM,, Nelson R,, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989; 84: 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lobb RR,, Alderman EM,, Fett JW. Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochemistry. 1985; 24: 4969–4973. [DOI] [PubMed] [Google Scholar]
- 30. Hemmings DG. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2006; 373: 18–29. [DOI] [PubMed] [Google Scholar]
- 31. Allende ML,, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002; 1582: 222–227. [DOI] [PubMed] [Google Scholar]
- 32. Lee H,, Goetzl EJ,, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol. 2000; 278: C612–C618. [DOI] [PubMed] [Google Scholar]
- 33. Lee JF,, Gordon S,, Estrada R,, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009; 296: H33–H42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ancellin N,, Colmont C,, Su J,, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002; 277: 6667–6675. [DOI] [PubMed] [Google Scholar]
- 35. McGuire PG,, Rangasamy S,, Maestas J,, Das A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol. 2011; 31: e107–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zachariah MA,, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010; 328: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takuwa Y,, Okamoto Y,, Yoshioka K,, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta. 2008; 1781: 483–488. [DOI] [PubMed] [Google Scholar]
- 38. Caballero S,, Swaney J,, Moreno K,, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res. 2009; 88: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skoura A,, Sanchez T,, Claffey K,, Mandala SM,, Proia RL,, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007; 117: 2506–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nayak RC,, Herman IM. Bovine retinal microvascular pericytes: isolation, propagation, and identification. Methods Mol Med. 2001; 46: 247–263. [DOI] [PubMed] [Google Scholar]
- 41. Gitlin JD,, D'Amore PA. Culture of retinal capillary cells using selective growth media. Microvasc Res. 1983; 26: 74–80. [DOI] [PubMed] [Google Scholar]
- 42. Durham JT,, Herman IM. Inhibition of angiogenesis in vitro: a central role for beta-actin dependent cytoskeletal remodeling. Microvasc Res. 2009; 77: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kotecki M,, Zeiger AS,, Van Vliet KJ,, Herman IM. Calpain- and talin-dependent control of microvascular pericyte contractility and cellular stiffness. Microvasc Res. 2010; 80: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aebi U,, Pollard TD. A glow discharge unit to render electron microscope grids and other surfaces hydrophilic. J Electron Microsc Tech. 1987; 7: 29–33. [DOI] [PubMed] [Google Scholar]
- 45. Boroujerdi A,, Tigges U,, Welser-Alves JV,, Milner R. Isolation and culture of primary pericytes from mouse brain. Methods Mol Biol. 2014; 1135: 383–392. [DOI] [PubMed] [Google Scholar]
- 46. Herman IM,, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985; 101: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He Q,, Spiro MJ. Isolation of rat heart endothelial cells and pericytes: evaluation of their role in the formation of extracellular matrix components. J Mol Cell Cardiol. 1995; 27: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 48. Newcomb PM,, Herman IM. Pericyte growth and contractile phenotype: modulation by endothelial-synthesized matrix and comparison with aortic smooth muscle. J Cell Physiol. 1993; 155: 385–393. [DOI] [PubMed] [Google Scholar]
- 49. Hirschi KK,, Rohovsky SA,, Beck LH,, Smith SR,, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999; 84: 298–305. [DOI] [PubMed] [Google Scholar]
- 50. Papetti M,, Shujath J,, Riley KN,, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: A role for myf-5 and smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003; 44: 4994–5005. [DOI] [PubMed] [Google Scholar]
- 51. Kolyada AY,, Riley KN,, Herman IM. Rho GTPase signaling modulates cell shape and contractile phenotype in an isoactin-specific manner. Am J Physiol Cell Physiol. 2003; 285: C1116–C1121. [DOI] [PubMed] [Google Scholar]
- 52. Johnstone S,, Isakson B,, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009; 278: 69–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bobbie MW,, Roy S,, Trudeau K,, Munger SJ,, Simon AM,, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010; 51: 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li AF,, Sato T,, Haimovici R,, Okamoto T,, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci. 2003; 44: 5376–5382. [DOI] [PubMed] [Google Scholar]
- 55. Gariano RF,, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005; 438: 960–966. [DOI] [PubMed] [Google Scholar]
- 56. Gerhardt H,, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003; 314: 15–23. [DOI] [PubMed] [Google Scholar]
- 57. Murata T,, Ishibashi T,, Inomata H,, Sueishi K. Media conditioned by coculture of pericytes and endothelial cells under a hypoxic state stimulate in vitro angiogenesis. Ophthalmic Res. 1994; 26: 23–31. [DOI] [PubMed] [Google Scholar]
- 58. Kimura T,, Watanabe T,, Sato K,, et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, edg-1 and edg-3. Biochem J. 2000; 348: 71–76. [PMC free article] [PubMed] [Google Scholar]
- 59. Kim BS,, Park H,, Ko SH,, Lee WK,, Kwon HJ. The sphingosine-1-phosphate derivative NHOBTD inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2011; 413: 189–193. [DOI] [PubMed] [Google Scholar]
- 60. Pyne NJ,, Pyne S. Selectivity and specificity of sphingosine 1-phosphate receptor ligands: “off-targets” or complex pharmacology? Front Pharmacol. 2011; 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011; 36: 97–107. [DOI] [PubMed] [Google Scholar]
- 62. Afzal A,, Shaw LC,, Ljubimov AV,, Boulton ME,, Segal MS,, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc Res. 2007; 74: 131–144. [DOI] [PubMed] [Google Scholar]
- 63. Schmid MC,, Bisoffi M,, Wetterwald A,, et al. Insulin-like growth factor binding protein-3 is overexpressed in endothelial cells of mouse breast tumor vessels. Int J Cancer. 2003; 103: 577–586. [DOI] [PubMed] [Google Scholar]
- 64. Kim JH,, Choi DS,, Lee OH,, Oh SH,, Lippman SM,, Lee HY. Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and egr-1-mediated transcriptional events. Blood. 2011; 118: 2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Granata R,, Trovato L,, Lupia E,, et al. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost. 2007; 5: 835–845. [DOI] [PubMed] [Google Scholar]
- 66. Granata R,, Trovato L,, Garbarino G,, et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 2004; 18: 1456–1458. [DOI] [PubMed] [Google Scholar]
- 67. Podesta F,, Romeo G,, Liu WH,, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000; 156: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Romeo G,, Liu WH,, Asnaghi V,, Kern TS,, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002; 51: 2241–2248. [DOI] [PubMed] [Google Scholar]
- 69. Joussen AM,, Poulaki V,, Le ML,, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18: 1450–1452. [DOI] [PubMed] [Google Scholar]
- 70. Ridley AJ,, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992; 70: 389–399. [DOI] [PubMed] [Google Scholar]
- 71. Zhang YW,, Morita I,, Ikeda M,, Ma KW,, Murota S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene. 2001; 20: 4138–4149. [DOI] [PubMed] [Google Scholar]
- 72. Inoki I,, Takuwa N,, Sugimoto N,, et al. Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun. 2006; 346: 293–300. [DOI] [PubMed] [Google Scholar]
- 73. Osada M,, Yatomi Y,, Ohmori T,, Ikeda H,, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun. 2002; 299: 483–487. [DOI] [PubMed] [Google Scholar]
- 74. Igarashi J,, Erwin PA,, Dantas AP,, Chen H,, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A. 2003; 100: 10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Markiewicz M,, Nakerakanti SS,, Kapanadze B,, Ghatnekar A,, Trojanowska M. Connective tissue growth factor (CTGF/CCN2) mediates angiogenic effect of S1P in human dermal microvascular endothelial cells. Microcirculation. 2011; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bolz SS,, Vogel L,, Sollinger D,, et al. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003; 108: 342–347. [DOI] [PubMed] [Google Scholar]
- 77. Spoerri PE,, Ellis EA,, Tarnuzzer RW,, Grant MB. Insulin-like growth factor: receptor and binding proteins in human retinal endothelial cell cultures of diabetic and non-diabetic origin. Growth Horm IGF Res. 1998; 8: 125–132. [DOI] [PubMed] [Google Scholar]
- 78. Kim JH,, Choi DS,, Lee OH,, Oh SH,, Lippman SM,, Lee HY. Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and egr-1-mediated transcriptional events. Blood. 2011; 118: 2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schmid MC,, Bisoffi M,, Wetterwald A,, et al. Insulin-like growth factor binding protein-3 is overexpressed in endothelial cells of mouse breast tumor vessels. Int J Cancer. 2003; 103: 577–586. [DOI] [PubMed] [Google Scholar]
- 80. Chang KH,, Chan-Ling T,, McFarland EL,, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A. 2007; 104: 10595–10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dulmovits BM,, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012; 44: 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]