Abstract
Ischemic preconditioning (IPC) improves maximal exercise performance. However, the potential mechanism(s) underlying the beneficial effects of IPC remain unknown. The dynamics of pulmonary oxygen uptake (VO2) and muscle deoxygenation during exercise is frequently used for assessing O2 supply and extraction. Thus, this study examined the effects of IPC on systemic and local O2 dynamics during the incremental step transitions from low- to moderate- and from moderate- to severe-intensity exercise. Fifteen healthy, male subjects were instructed to perform the work-to-work cycling exercise test, which was preceded by the control (no occlusion) or IPC (3 × 5 min, bilateral leg occlusion at >300 mmHg) treatments. The work-to-work test was performed by gradually increasing the exercise intensity as follows: low intensity at 30 W for 3 min, moderate intensity at 90% of the gas exchange threshold (GET) for 4 min, and severe intensity at 70% of the difference between the GET and VO2 peak until exhaustion. During the exercise test, the breath-by-breath pulmonary VO2 and near-infrared spectroscopy-derived muscle deoxygenation were continuously recorded. Exercise endurance during severe-intensity exercise was significantly enhanced by IPC. There were no significant differences in pulmonary VO2 dynamics between treatments. In contrast, muscle deoxygenation dynamics in the step transition from low- to moderate-intensity was significantly faster in IPC than in CON (27.2 ± 2.9 vs. 19.8 ± 0.9 sec, P < 0.05). The present findings showed that IPC accelerated muscle deoxygenation dynamics in moderate-intensity exercise and enhanced severe-intensity exercise endurance during work-to-work test. The IPC-induced effects may result from mitochondrial activation in skeletal muscle, as indicated by the accelerated O2 extraction.
Keywords: Exercise, mitochondria, near-infrared spectroscopy, nitric oxide, skeletal muscle
Introduction
Ischemic preconditioning (IPC) by brief episodes of ischemia and reperfusion in the organs, especially the heart, provides protection from tissue damage such as myocardial injury (Murry et al. 1986). In the clinical setting, IPC is becoming an effective strategy for reducing the risk of myocardial injury and for improving the prognosis of patients after cardiac surgery (Thielmann et al. 2013). Alternatively, recent studies found that IPC can enhance endurance exercise performance (de Groot et al. 2010; Crisafulli et al. 2011; Bailey et al. 2012a,b). However, the potential mechanism(s) underlying the beneficial effects of IPC remain unknown.
Exercise performance is determined multifactorially, and oxidative metabolism is a major determinant. The dynamics of pulmonary oxygen uptake (VO2) and muscle deoxygenation during exercise are frequently used for assessing O2 supply and extraction (DeLorey et al. 2003; Wilkerson et al. 2004; Bailey et al. 2010). Those systemic and local O2 dynamics may be useful for understanding the beneficial effects of IPC on exercise performance. For example, the faster inductions of systemic and local O2 dynamics can be achieved using prior exercise, and these responses are closely related to cardiovascular and skeletal muscle activation (Burnley et al. 2000; Gurd et al. 2005; Marles et al. 2007; Faisal et al. 2009). Similarly, IPC also ameliorates the O2 response during ischemia and/or reperfusion in cardiac muscle (Xu et al. 1998; Zhu et al. 2007) and other organs (Koti et al. 2002; Mallick et al. 2005), potentially by increasing nitric oxide (NO) production. Importantly, an increase in NO levels induced by supplementation with NO metabolite compounds (e.g., L-arginine and nitrate) enhances exercise endurance and modifies systemic and local O2 response during exercise (Bailey et al. 2009, 2010; Larsen et al. 2011). Thus, one potential mechanism underlying IPC-induced effect may be the acceleration of systemic and local O2 dynamics due to NO-dependent mechanism. However, it remains unclear whether IPC ameliorates these O2 responses during exercise.
Previous studies have determined that the beneficial effects of IPC on exercise performance occurred independently of cardiac responses, as cardiac performance during exercise is not affected by IPC (de Groot et al. 2010; Crisafulli et al. 2011; Bailey et al. 2012a,b). Importantly, although a previous study reported that IPC can increase VO2 peak during a maximal ramp exercise test (de Groot et al. 2010), several other previous studies reported that IPC can increase the maximal exercise performance, not in VO2 peak (Crisafulli et al. 2011; Bailey et al. 2012a,b). Naturally, the VO2 peak is strongly affected by cardiovascular system in addition to peripheral system(s) (Åstrand et al. 1964), which suggests that IPC-induced improvement in exercise performance may result from peripheral adaptations, including those in the skeletal muscle rather than cardiovascular adaptation. Therefore, we proposed that the skeletal muscle adaptation, especially acceleration of muscle O2 response, may be necessary to achieve the beneficial effects of IPC on exercise performance.
Several recent studies have employed a unique work-to-work protocol that consists of double-step transitions from low- to moderate-intensity exercise and from moderate- to severe-intensity exercise (Williams et al. 2013; Da Boit et al. 2014). This protocol can help to observe the different modes of O2 response in a single exercise trial, and it can assess exercise endurance (i.e., the endurance time until exhaustion). Therefore, we hypothesized that IPC would enhance endurance exercise performance during a single work-to-work exercise, potentially by accelerating the muscular O2 dynamics. To test our hypothesis, we examined the effects of IPC on exercise endurance and the responses of pulmonary VO2 and muscle oxygenation during the work-to-work test.
Methods
Subjects
Fifteen habitually active, healthy male subjects (mean ± SE; age 24 ± 1 year, height 173 ± 1 cm, weight 68 ± 2 kg, systolic blood pressure 121 ± 2 mmHg) participated in this study. The subjects were informed about the experimental procedures and potential risks and gave written consent to participate in the study. All procedures were approved by the Ethics Committee of Ritsumeikan University (IRB-2013-050) and were conducted in accordance with the Declaration of Helsinki. Subjects were instructed to arrive at the laboratory in a rested and fully hydrated state, at least 4 h post prandial, and to avoid strenuous physical activity in the 24 h prior to each testing session. Each subject also abstained from caffeine and alcohol intake for 6 h and 24 h before each test, respectively. All tests were performed at the same time of day (±2 h).
Experimental design
Subjects were required to visit the laboratory on three occasions over a 2-weeks period. At the first visit, subjects completed a ramp-incremental exercise test to determine the VO2 peak and gas exchange threshold (GET). Thereafter, the subjects were asked to perform two trials: one with and one without IPC. The order of trials were randomly assigned and counterbalanced. Since the previous study reported that the beneficial effects of IPC continue for at least 48 h in human (Loukogeorgakis et al. 2005), it was ensured that testing sessions were performed with an interval at least 1 week to eliminate the late effects of IPC. IPC consisted of three cycles of 5 min of bilateral arterial occlusion to the lower limbs, with 5 min of separation (i.e., the reperfusion period). The cuffs for the IPC were placed proximally around the upper thighs, whereas subjects were in a sitting position and were inflated to >300 mmHg. Previous studies have applied ischemic pressure for IPC at the absolute pressure of 220 mmHg (de Groot et al. 2010; Bailey et al. 2012a,b). In the preliminary testing, this study also employed the ischemic pressure of 220 mmHg. However, the use of this method caused gradual increases of values of total-hemoglobin/myoglobin (Hb/Mb), measured by near-infrared spectroscopy (NIRS), over a 5-min ischemia period. The result represents arterial blood inflow to the lower limbs, despite the exposure to ischemia. Hence, based on the results of several examinations, this study applied an ischemic pressure of >300 mmHg, and the application of this pressure did not cause the increase in total-Hb/Mb during the ischemia period. The control (CON) treatment followed the same experimental time (i.e., 30 min) without cuff inflation (de Groot et al. 2010; Crisafulli et al. 2011). The work-to-work exercise test started 5 min after the CON or IPC trials.
Ramp-incremental test
At the first visit, all subjects performed a maximal incremental exercise test to determine the VO2 peak and GET on a cycling ergometer. Initially, subjects performed 3 min of baseline cycling at 30 W, after which the workload was increased at a rate of 30 W/min until the limit of tolerance. The subjects were asked to maintain a cadence of 60 rpm. The heights of saddle and bar in this test were recorded to reproduce the subsequent exercise tests. During the incremental test, breath-by-breath pulmonary gas exchange data were collected and averaged every 10 s. Heart rate (HR) was measured continuously via telemetry (RS400; Polar Electro Japan, Tokyo, Japan). The VO2 peak was determined as the highest 30-sec mean value attained prior to exhaustion. The exhaustion was assessed to be the maximum when three of the following criteria were obtained: (1) a plateau in the VO2 despite increasing workload (<100 mL/min), (2) a respiratory exchange ratio >1.10, (3) a heart rate within 10% of predicted maximal HR that was calculated as 220 – age, and (4) task failure of the pedaling rate of at least 55 rpm over 5 sec despite maximal effort. GET was determined using the V slope method, ventilatory equivalents, and end-tibial gas tension.
Work-to-work exercise test
The data of VO2 peak and GET collected during the ramp-incremental test were used to calculate the work rate for moderate- and severe-intensity exercises. The work rate for both intensities employed 90% of the VO2 at GET (90% GET) and 70% of the difference between the VO2 at the GET and VO2 peak (70%Δ), respectively (Da Boit et al. 2014). Prior to experimental day, all the subjects familiarized themselves with the moderate-intensity and severe-intensity loads used during the test, which was performed after an analysis of the ramp-incremental test completed. During the work-to-work test, the first-step transition consisted of baseline low-intensity exercise at 30 W for 3 min followed by moderate-intensity exercise at 90% GET for 4 min. Next, the second step transitioned from a moderate- to severe-intensity exercise at 70%Δ. Thereafter, the subjects continued the severe-intensity exercise until exhaustion, which was defined by the criteria in the ramp-incremental test. The endurance time during severe-intensity exercise was evaluated as exercise endurance. In a pilot study, we assessed the test–retest reliability on two separate days with 10 young subjects, and the intraclass correlation coefficient (ICC) score of exercise endurance during severe-intensity exercise of the work-to-work test was 0.990. During the work-to-work exercise test, the pulmonary gas exchanges and NIRS-derived signals were continuously recorded to determine to the dynamics of the pulmonary VO2 and muscle oxygenation.
Pulmonary VO2 dynamics
Pulmonary VO2 were measured by breath-by-breath using a gas analyzer (AE-310S; Minato Medical Science, Osaka, Japan). The breath-by-breath VO2 data collected during work-to-work exercise were edited by removing aberrant breath data (e.g., coughing, swallowing, sighing, etc.) that lay outside 4 SD of local mean. The VO2 data were interpolated to give second-by-second values and then were averaged into 5-sec bins. After this rectification, the VO2 data were used for determining the kinetics of pulmonary VO2 during a work-to-work exercise. To analyze the VO2 kinetics in moderate- and severe-intensity exercises, the first 20 sec of data during each step transition was deleted to remove the cardiodynamic response (i.e., phase I). A monoexponential model was used for moderate-intensity exercise, and a biexponential model was used for severe-intensity exercise, as described in the following equations:
| 1 |
| 2 |
where VO2 (t) represents VO2 at a given time t; VO2 baseline is the mean VO2 in the baseline period; Ap, TDp, and τp represent amplitude, time delay, and time constant, respectively, describing the primary response (i.e., phase II) in VO2 above baseline; and As, TDs, and τs represent amplitude of, time delay before the onset of, and time constant describing the development of, the slow phase response (i.e., phase III), respectively. Baseline VO2 values in moderate- and severe-intensity exercises were defined as the mean VO2 measured over the final 60 sec during low- and moderate-intensity exercises, respectively. End-exercise VO2 value in moderate-intensity exercise was defined as the mean VO2 measured over the final 30-sec during exercise. The VO2 value at 240 sec after the onset of severe-intensity exercise was taken as the mean VO2 between 210 and 240 sec. End-exercise VO2 value in severe-intensity exercise was defined as the mean VO2 measured over 30 sec before the exhaustion in the exercise. The amplitude of VO2 during the slow phase response was described as the increase in VO2 from slow phase time delay to the end of exercise (Burnley et al. 2000). The mean response time, an overall kinetics, was calculated as the sum of the time delay and time constant in the primary response. In a pilot study, ICC scores of VO2 kinetics parameters during moderate- and severe-intensity phases of the work-to-work test were 0.978 and 0.975 for the time delay, 0.943 and 0.975 for the time constant, and 0.983 and 0.991 for the mean response time, respectively.
Muscle oxygenation dynamics
Local tissue oxygenation profiles in the vastus lateralis (VL) muscle during exercise was measured using NIRS (NIRO 200; Hamamatsu Photonics, Shizuoka, Japan). During work-to-work exercise test, changes in oxy-Hb/Mb, deoxy-Hb/Mb, and total-Hb/Mb were sampled at 5 Hz and then averaged at 1 sec. This study predominantly analyzed the changes in deoxy-Hb/Mb, which is a marker of muscle deoxygenation. The muscle deoxygenation from deoxy-Hb/Mb is a reliable estimator of changes in intramuscular oxygenation states during exercise (De Blasi et al. 1994). The values of deoxy-Hb/Mb were calibrated using an arterial occlusion method (Hamaoka et al. 1996). To perform arterial occlusion, subjects completed a 3-min cool-down at 30 W after each exercise condition finished and then rested in a sitting position for 10 min. The arterial occlusion pressure was applied at the same pressure used for IPC. Arterial occlusion was continued for 10 min, at which time a maximal plateau value for deoxy-Hb/Mb was obtained for calibration. The baseline value for calibration was defined as the mean value measured over final 60 sec during low-intensity exercise in work-to-work test. The deoxy-Hb/Mb data during exercise were expressed as a percentage using baseline and maximal plateau values. To analyze the kinetics of deoxy-Hb/Mb during moderate- and severe-intensity exercises, deoxy-Hb/Mb were fitted with a monoexponential model in the following equation:
| 3 |
where deoxy-Hb/Mb (t) represents deoxy-Hb/Mb at a given time t; deoxy-Hb/Mb baseline is the baseline data before the onset of both step transients; A, TD, and τ represent amplitude, time delay, and time constant, respectively. This study employed a monoexponential model to determine the muscle deoxygenation dynamics in both exercise intensities (DeLorey et al. 2003). The time delays to an initial increase in deoxy-Hb/Mb after each step transition to moderate- and severe-intensity exercises were determined as the first point >1 SD above the mean of the baseline (DeLorey et al. 2003). Thereafter, deoxy-Hb/Mb was fitted from the time of initial increase to 120-sec during each intensity exercise. The basal information (e.g., amplitude) for deoxy-Hb/Mb was obtained with same time intervals to pulmonary VO2. The overall mean response time was calculated as the sum of the time delay and time constant. In a pilot study, ICC scores of the deoxy-Hb/Mb kinetics parameters during moderate- and severe-intensity phases of the work-to-work test were 0.883 and 0.897 for time delay, 0.910 and 0.973 for time constant, and 0.954 and 0.983 for the mean response time, respectively.
Blood glucose and lactate
The fingertip blood sample was collected into a capillary tube to determine the blood glucose and lactate levels. A basal blood glucose level was measured to check for the fasting state before the CON or IPC trials on each testing day using a glucose analyzer (Glutest Neo Alpha; Sanwa Kagaku Kenkyusho, Nagoya, Japan). The fasting blood glucose level was equal on each testing day for the CON (93 ± 3 mg/mL) and IPC (97 ± 5 mg/mL).
The blood [lactate] levels were measured five times throughout the experiment using a lactate analyzer (Lactate Pro 2; Arkray, Kyoto, Japan). Blood sampling was performed as follows: before each trial (i.e., rest), 30 sec preceding the onset of the low-intensity exercise (i.e., pre-exercise), 30 sec preceding the transition to moderate-intensity exercise (i.e., end of low-intensity exercise), 30 sec preceding the transition to severe-intensity exercises (i.e., end of moderate-intensity exercise), and as soon as possible (<10 sec) after exhaustion due to severe-intensity exercise (i.e., exhaustion).
Statistics
Statistical analysis for comparisons between CON and IPC was performed using a paired Student's t-test. The level of significance was set at P < 0.05. All statistical tests were performed using SPSS 16.0 (International Business Machines Corporation, NY, USA).
Results
Mean values of VO2 peak and GET, as measured by the ramp-incremental test in the subjects, were 3.19 ± 0.13 L/min and 1.98 ± 0.11 L/min, respectively. On the basis of GET and VO2 peak, the mean workloads of the moderate- and severe-intensity exercises in the work-to-work test were 138 ± 3 W and 233 ± 7 W, respectively.
The result of exercise endurance is shown in Figure1. As expected, exercise endurance (i.e., the time to exhaustion in severe-intensity exercise) was significantly greater in IPC than in CON. Results of the dynamics of pulmonary VO2 during the work-to-work cycling exercise test are shown in Table1 and Figure2. Mean values of pulmonary VO2 at the end-exercise of the work-to-work exercise in CON and IPC conditions reached 95.4 ± 4.2% and 97.0 ± 4.2%, respectively, suggesting that volitional fatigue was achieved in both conditions. Alternatively, changes in the pulmonary VO2 throughout the work-to-work test were not significantly different between CON and IPC conditions. Moreover, the kinetics of pulmonary VO2 during the moderate- and severe-intensity exercises were not significantly different between the two trials.
Figure 1.
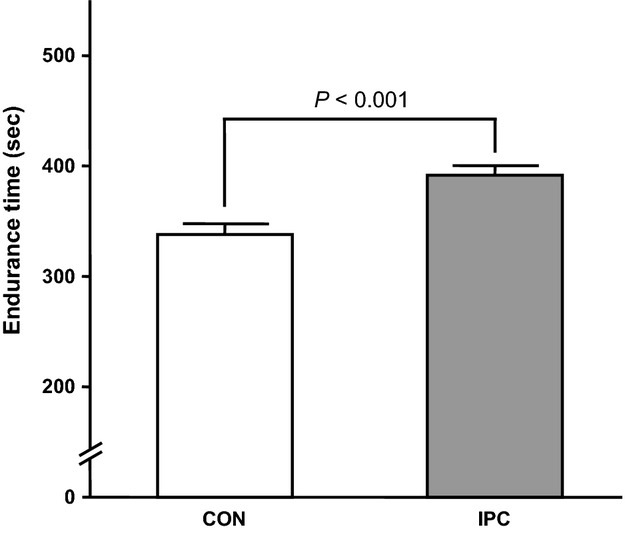
Endurance time to exhaustion during severe-intensity exercise in the work-to-work cycling exercise test. Values are presented as mean ± SE.
Table 1.
Kinetics parameters of pulmonary oxygen uptake during work-to-work exercise
| CON | IPC | |
|---|---|---|
| Moderate-intensity exercise | ||
| Baseline, L/min | 0.64 ± 0.02 | 0.67 ± 0.02 |
| End-exercise, L/min | 1.66 ± 0.03 | 1.73 ± 0.03 |
| Primary amplitude, L/min | 1.02 ± 0.03 | 1.07 ± 0.03 |
| Primary time delay, sec | 22.0 ± 1.7 | 20.3 ± 1.3 |
| Primary time constant, sec | 30.6 ± 3.1 | 31.7 ± 2.8 |
| Mean response time, sec | 52.6 ± 4.6 | 52.0 ± 3.9 |
| Severe-intensity exercise | ||
| Baseline, L/min | 1.66 ± 0.03 | 1.65 ± 0.03 |
| Primary amplitude, L/min | 0.89 ± 0.05 | 0.93 ± 0.06 |
| 240 sec, L/min | 2.80 ± 0.06 | 2.79 ± 0.06 |
| Primary time delay, sec | 36.3 ± 4.6 | 31.2 ± 4.1 |
| Primary time constant, sec | 64.9 ± 6.2 | 54.3 ± 7.3 |
| Mean response time, sec | 101.2 ± 9.3 | 85.5 ± 11.0 |
| Slow phase time delay sec | 138.7 ± 8.1 | 146.0 ± 7.0 |
| Slow phase amplitude, L/min | 0.43 ± 0.03 | 0.44 ± 0.05 |
| End-exercise, L/min | 2.97 ± 0.07 | 3.03 ± 0.08 |
Values are presented as mean ± SE.
Figure 2.
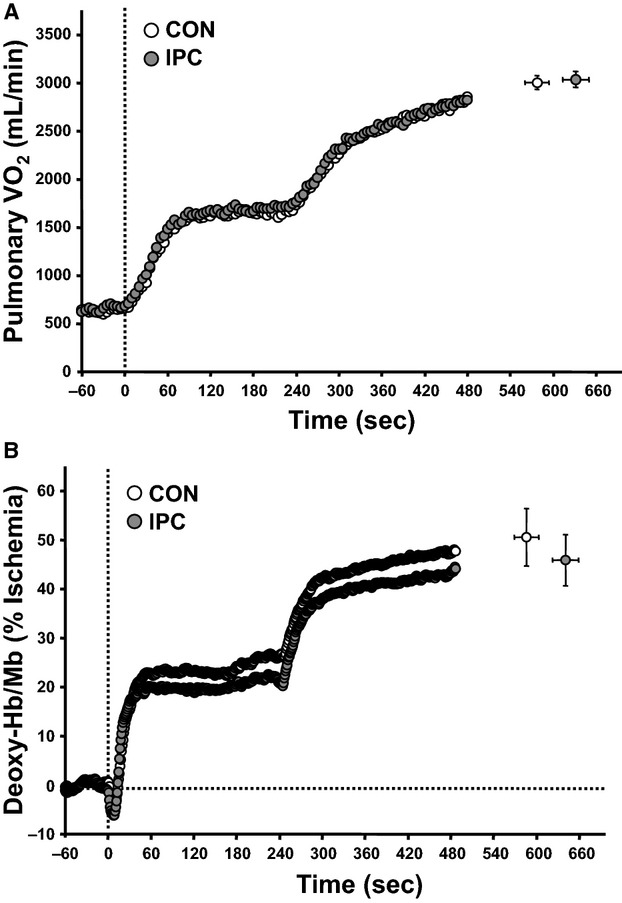
Pulmonary oxygen uptake (VO2) and deoxy-Hb/Mb responses during work-to-work exercise. Panel A illustrates the pulmonary VO2 responses during work-to-work exercise. Data are shown as 5-sec mean values after rectification. Panel B illustrates the deoxy-Hb/Mb responses during work-to-work exercise. Data are expressed as a percentage of the highest plateau value during 10-min arterial occlusion. The systemic and local O2 responses following the CON and IPC trials are presented as open and solid circles, respectively. The dotted vertical line at zero in panels A and B indicates the transition from low-intensity exercise to moderate-intensity exercise. The dotted horizontal line in panel B indicates the baseline in deoxy-Hb/Mb. End-exercise values for pulmonary VO2 and deoxy-Hb/Mb are presented as mean ± SE.
The results of dynamics of deoxy-Hb/Mb are shown in Table2, Figures2 and 3. The amplitude of deoxy-Hb/Mb during moderate-intensity exercise was significantly smaller in IPC than in CON. However, the same trend was not observed during severe-intensity exercise. The time delay and time constant for muscle deoxygenation after the transition from low- to moderate-intensity exercise tended to be shorter in IPC than in CON, although the difference was not statistically significant. More importantly, the mean response time for muscle deoxygenation during moderate-intensity exercise was significantly shorter in IPC than in CON. In contrast, the kinetics of the transition in severe-intensity exercise were unaffected by IPC.
Table 2.
Kinetics parameters of deoxy-hemoglobin/myoglobin (Hb/Mb) during work-to-work exercise
| CON | IPC | |
|---|---|---|
| Moderate-intensity exercise | ||
| Amplitude, % | 27.3 ± 4.1 | 21.4 ± 3.5* |
| Time delay, sec | 14.8 ± 1.6 | 11.9 ± 0.7 |
| Time constant, sec | 12.4 ± 2.2 | 7.8 ± 1.0 |
| Mean response time, sec | 27.2 ± 2.9 | 19.8 ± 0.9* |
| Severe-intensity exercise | ||
| Baseline, % | 26.7 ± 4.0 | 21.2 ± 3.4* |
| 240 sec, % | 49.3 ± 5.6 | 42.8 ± 4.6 |
| End-exercise, % | 51.6 ± 5.8 | 45.6 ± 4.9 |
| Amplitude, % | 24.9 ± 3.5 | 24.4 ± 3.0 |
| Time delay, sec | 7.2 ± 1.8 | 5.3 ± 1.6 |
| Time constant | 24.4 ± 4.3 | 29.7 ± 4.7 |
| Mean response time, sec | 31.6 ± 4.5 | 35.1 ± 4.8 |
Values are presented as mean ± SE. The values for deoxy-Hb/Mb are expressed as a percentage of the highest plateau value during 10-min arterial occlusion.
Significant difference from the CON trial: P < 0.05.
Figure 3.
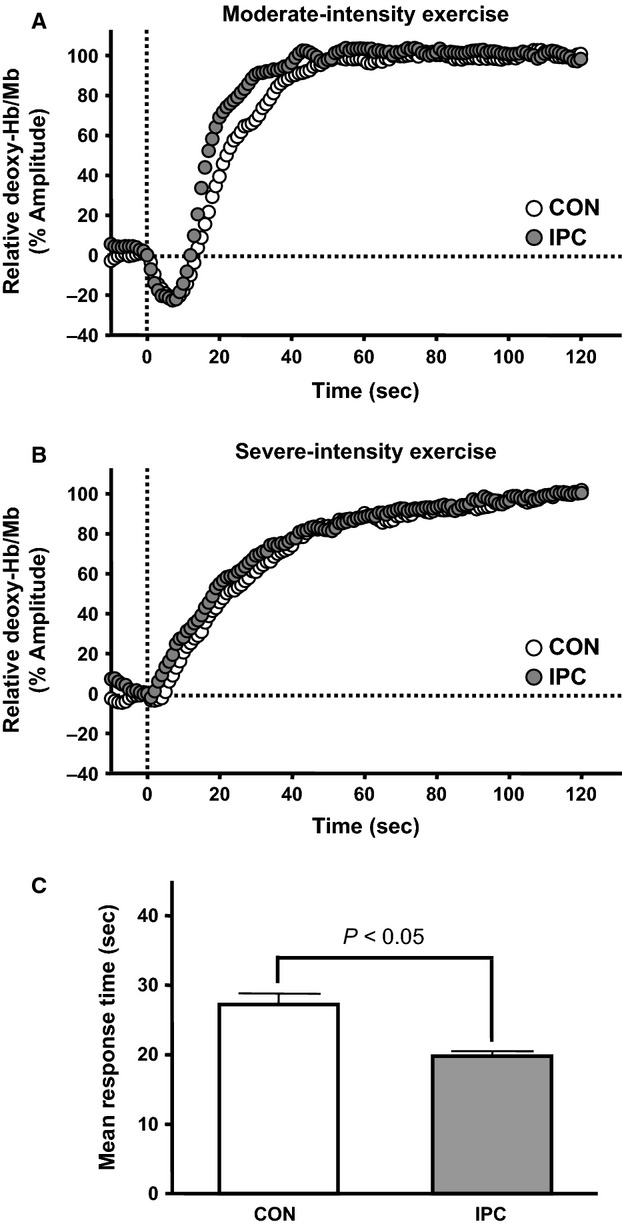
Muscle deoxygenation responses during moderate- and severe-intensity exercises in work-to-work exercise. Panels A and B illustrate the relative changes of deoxy-Hb/Mb during moderate- and severe-intensity exercises, respectively. Data are normalized relative to the end-exercise amplitude. Panel C shows the mean response time of muscle deoxygenation during moderate-intensity exercise. Values are presented as mean ± SE.
Results of the heart rate and blood [lactate] responses are shown in Table3. There was no significant difference in the changes in HR throughout the exercise between CON and IPC. Additionally, no significant difference was observed in the changes in the blood lactate level during exercise between both trials.
Table 3.
Heart rate and blood [lactate] responses during work-to-work exercise
| CON | IPC | |
|---|---|---|
| Heart rate, beats/min | ||
| Rest | 68 ± 3 | 67 ± 2 |
| Pre-exercise | 69 ± 2 | 64 ± 4 |
| End of low-intensity exercise | 81 ± 2 | 81 ± 1 |
| End of moderate-intensity exercise | 127 ± 4 | 127 ± 2 |
| Exhaustion | 179 ± 4 | 180 ± 3 |
| Blood [lactate], mM | ||
| Rest | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Pre-exercise | 1.3 ± 0.2 | 1.4 ± 0.1 |
| End of low-intensity exercise | 1.8 ± 0.3 | 2.1 ± 0.2 |
| End of moderate-intensity exercise | 3.7 ± 0.3 | 3.8 ± 0.4 |
| Exhaustion | 13.6 ± 0.9 | 13.8 ± 1.3 |
| Δblood [lactate] | 12.2 ± 0.9 | 12.4 ± 1.2 |
Values are presented as mean ± SE. Rest, before each trial; Pre-exercise, 30 sec preceding the onset of the low-intensity exercise; End of low-intensity exercise, 30 sec preceding the transition to moderate-intensity exercise, End of moderate-intensity exercise, 30 sec preceding the transition to severe-intensity exercises; Exhaustion, as soon as possible (<10 sec) after exhaustion from severe-intensity exercise; [Lactate], lactate concentration; Δ, change between pre-exercise and exhaustion.
Discussion
The principal findings in this study were that IPC accelerated muscle deoxygenation in moderate-intensity exercise and enhanced severe-intensity exercise endurance during a work-to-work cycling exercise. Previous studies have reported that IPC enhances maximal exercise performance, such as VO2 peak and maximal workload (de Groot et al. 2010; Crisafulli et al. 2011). The present findings extended these previous findings by showing that IPC can enhance submaximal endurance exercise performance. Alternatively, our findings showed that the dynamics of pulmonary VO2 during the exercise was unaffected by IPC. Moreover, the VO2 values at exhaustion during severe-intensity exercise did not increase because of IPC treatment. A previous study reported that the VO2 peak during the maximal exercise test was increased by IPC (de Groot et al. 2010). In contrast, several other previous studies showed that IPC increased the maximal workload during the incremental ramp exercise test, but it did not affect the VO2 peak (Crisafulli et al. 2011; Bailey et al. 2012a,b). Although the integrity of the effect of IPC on the pulmonary VO2 response during exercise remains unknown, the present findings may support the fact that IPC has no impact on systemic O2 responses during exercise. Pulmonary O2 dynamics are mainly affected by cardiovascular and skeletal muscle responses. For example, a faster induction of pulmonary O2 dynamics induced by prior exercise results from cardiovascular activation in addition to skeletal muscle activation (Burnley et al. 2000; Gurd et al. 2005; Marles et al. 2007; Faisal et al. 2009). Compared with prior exercise, IPC did not have a beneficial effect on the cardiovascular response during exercise (de Groot et al. 2010; Crisafulli et al. 2011; Bailey et al. 2012a,b). In this study, the changes in HR during exercise was also unaffected by IPC. Therefore, these findings suggest that the effect of IPC on exercise performance may be achieved by the adaptation of local skeletal muscle rather than the systemic cardiovascular system.
To our knowledge, this study is the first to determine that IPC accelerates the muscular O2 response during systemic whole-body exercise. We evaluated muscle deoxygenation using NIRS-derived deoxy-Hb/Mb. A part of the NIRS signal parameters indicate muscular O2 extraction (DeLorey et al. 2003), which reflects the regional balance between O2 utilization and O2 availability. Therefore, the observed IPC-induced acceleration of muscle oxygenation dynamics may result from accelerated O2 extraction in skeletal muscle. Previous studies have shown that prior muscle contractions in rodent and Xenopus muscles can speed up muscle vascular O2 dynamics during subsequent muscle contractions (Hogan 2001; Behnke et al. 2002). The findings of these previous models clearly showed that prior muscle contraction induces an accelerated muscle O2 response, suggesting that IPC may be able to achieve a beneficial effect similar to prior exercise treatment without muscle contractions. Moreover, prior exercise-induced acceleration of the muscle O2 response is associated with mitochondrial enzymatic activation in skeletal muscle (Gandra et al. 2012). The mitochondrial enzyme activity in skeletal muscle is acutely enhanced by a single bout of exercise (Leek et al. 2001; Fernström et al. 2004), and it may be related to the accelerated O2 metabolism. Therefore, we propose that IPC enhances exercise performance with skeletal muscle mitochondrial activation, as indicated by the accelerated O2 extraction.
In previous studies, potential molecule(s) underlying IPC-induced enhancement of exercise performance remain unknown. It is well known that NO is produced from vascular endothelial cells following an increase in shear stress (Kooijman et al. 2008; Green et al. 2014), which is induced by the raid increase in blood flow, such as that occurs with reperfusion of IPC. In fact, a recent study determined that IPC increased the levels of the blood NO metabolites in human (Rassaf et al. 2014). The study also clearly showed that the beneficial effects of IPC are strongly regulated by NO produced from endothelial NO synthase in mice. Previous studies have reported that supplementation with NO metabolite compounds improves exercise performance (Bailey et al. 2009, 2010; Larsen et al. 2011). Moreover, enhanced NO levels reduce the local muscular O2 cost in addition to the systemic O2 cost during exercise (Bailey et al. 2009, 2010), by improving energy efficiency in the mitochondria of the skeletal muscle (Larsen et al. 2011). In this study, IPC did not affect the systemic pulmonary O2 cost during the work-to-work exercise test. In contrast, IPC reduced the amplitude of deoxy-Hb/Mb during moderate-intensity exercise, but not during severe-intensity exercise. This finding suggests that IPC may decrease the O2 cost in moderate exercising muscles, which is consistent with the beneficial effects of supplementation with NO metabolite compounds. Importantly, Bailey et al. (2012a,b) demonstrated that IPC enhanced running performance during strenuous exercise and prevented flow-mediated dilation (FMD)-measured endothelial dysfunction after this exercise, suggesting that IPC-induced enhancement of exercise performance may be related to NO bioavailability. Therefore, we suggest that one potential molecule underlying IPC-induced effects may result from increased NO synthase.
This study has several limitations. First, this study finding showed that dynamics of deoxygenation in skeletal muscle during moderate-intensity in the work-to-work test was accelerated by IPC; however, this was not observed during severe-intensity exercise. It is difficult to explain the discrepancy between the moderate- and severe-intensity phases from only the present findings. Nevertheless, in a recent study, Patterson et al. (in press2015) reported that IPC enhanced the power output during the early phase in repeated sprint cycling exercise, which suggests that IPC-induced effects on exercise performance may be dissolved over the time course from IPC. In fact, the response in FMD during reperfusion, which is induced by increasing NO production, reverses within 5 min (Nishiyama et al. 2007). Hence, a benefit from IPC may disappear until the time point (i.e., 12 min after the last ischemia has finished) of the transition from moderate- to severe-intensity; however, the advantage during moderate-intensity exercise may be useful enough for enhancing severe-intensity exercise endurance. Alternatively, in contrast to our speculation, Bailey et al. (Bailey et al. 2012a,b) reported that IPC-induced enhancement of exercise performance was observed over 1 h after IPC. Thus, further study would be needed to examine the duration of IPC-induced effect.
Next, previous studies analyzed the dynamics of pulmonary VO2 and muscle deoxygenation by measuring several bouts over a short-term period (DeLorey et al. 2003; Bailey et al. 2010), and they were averaged to rectify the signal-to-noise ratio. However, this study analyzed the dynamics of pulmonary VO2 and muscle deoxygenation by using only a single bout of a work-to-work test. Previous studies determined that IPC provides protection from tissue damage for up to 24 h in rodents (Marber et al. 1993; Xuan et al. 2007). Moreover, a previous study reported that the beneficial effects of IPC continue for at least 48 h in human (Loukogeorgakis et al. 2005). As the length of the late effects of IPC remains unclear, each testing session was separated by an interval of at least 1 week to eliminate bias. Thus, a relatively long period is required when performing multiple measurements; however, it is not easy to control the physiological states of the subjects. In addition, the analysis of the dynamics of pulmonary VO2 and muscle deoxygenation has been performed in many previous studies not only using multiple bouts but also a single bout (Wilkerson et al. 2004). In a previous pilot study, we examined test–retest reliability of systemic and local O2 dynamics during the work-to-work test, and the ICC showed a reasonably high value. Thus, we believe that IPC-induced acceleration of muscle deoxygenation dynamics during the exercise is reliable.
In conclusion, the findings of this study showed that IPC accelerated muscle deoxygenation dynamics in moderate-intensity exercise and endurance in severe-intensity exercise during a work-to-work test. The IPC-induced effects may result from mitochondrial activation in skeletal muscle, as indicated by the accelerated O2 extraction, which may have the enhanced NO synthase. Further study is needed to clarify the biological and molecular mechanisms underlying the effects of IPC on exercise performance.
Acknowledgments
The authors are grateful to all subjects who gave of their time and effort to participate in this study.
Conflict of Interest
None declared.
References
- Åstrand PO, Cuddy TE, Saltin B. Stenberg J. Cardiac output during submaximal and maximal work. J. Appl. Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard PG, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, et al. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010;109:1394–1403. doi: 10.1152/japplphysiol.00503.2010. [DOI] [PubMed] [Google Scholar]
- Bailey TG, Birk GK, Cable NT, Atkinson G, Green DJ, Jones H, et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am. J. Physiol. Heart Circ. Physiol. 2012a;303:H533–H538. doi: 10.1152/ajpheart.00272.2012. [DOI] [PubMed] [Google Scholar]
- Bailey TG, Jones H, Gregson W, Atkinson G, Cable NT. Thijssen DH. Effect of ischemic preconditioning on lactate accumulation and running performance. Med. Sci. Sports Exerc. 2012b;44:2084–2089. doi: 10.1249/MSS.0b013e318262cb17. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Kindig CA, Musch TI, Sexton WL. Poole DC. Effects of prior contractions on muscle microvascular oxygen pressure at onset of subsequent contractions. J. Physiol. 2002;539:927–934. doi: 10.1113/jphysiol.2001.013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnley M, Jones AM, Carter H. Doust JH. Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J. Appl. Physiol. 2000;89:1387–1396. doi: 10.1152/jappl.2000.89.4.1387. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Tangianu F, Tocco F, Concu A, Mameli O, Mulliri G, et al. Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. J. Appl. Physiol. 2011;111:530–536. doi: 10.1152/japplphysiol.00266.2011. [DOI] [PubMed] [Google Scholar]
- Da Boit M, Bailey SJ, Callow S, Dimenna FJ. Jones AM. Effects of interval and continuous training on O2 uptake kinetics during severe-intensity exercise initiated from an elevated metabolic baseline. J. Appl. Physiol. 2014;116:1068–1077. doi: 10.1152/japplphysiol.01365.2013. [DOI] [PubMed] [Google Scholar]
- De Blasi RA, Ferrari M, Natali A, Conti G, Mega A. Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J. Appl. Physiol. 1994;76:1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM. Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J. Appl. Physiol. (1985) 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- Faisal A, Beavers KR, Robertson AD. Hughson RL. Prior moderate and heavy exercise accelerate oxygen uptake and cardiac output kinetics in endurance athletes. J. Appl. Physiol. 2009;106:1553–1563. doi: 10.1152/japplphysiol.91550.2008. [DOI] [PubMed] [Google Scholar]
- Fernström M, Tonkonogi M. Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J. Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra PG, Nogueira L. Hogan MC. Mitochondrial activation at the onset of contractions in isolated myofibres during successive contractile periods. J. Physiol. 2012;590:3597–3609. doi: 10.1113/jphysiol.2012.232405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Dawson EA, Groenewoud HM, Jones H. Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension. 2014;63:376–382. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- de Groot PC, Thijssen DH, Sanchez M, Ellenkamp R. Hopman MT. Ischemic preconditioning improves maximal performance in humans. Eur. J. Appl. Physiol. 2010;108:141–146. doi: 10.1007/s00421-009-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd BJ, Scheuermann BW, Paterson DH. Kowalchuk JM. Prior heavy-intensity exercise speeds VO2 kinetics during moderate-intensity exercise in young adults. J. Appl. Physiol. 2005;98:1371–1378. doi: 10.1152/japplphysiol.01028.2004. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, et al. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J. Appl. Physiol. 1996;81:1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- Hogan MC. Fall in intracellular PO2 at the onset of contractions in Xenopus single skeletal muscle fibers. J. Appl. Physiol. 2001;90:1871–1876. doi: 10.1152/jappl.2001.90.5.1871. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, et al. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J. Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koti RS, Seifalian AM, McBride AG, Yang W. Davidson BR. The relationship of hepatic tissue oxygenation with nitric oxide metabolism in ischemic preconditioning of the liver. FASEB J. 2002;16:1654–1656. doi: 10.1096/fj.01-1034fje. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O. Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R441–R447. doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE. MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mallick IH, Yang W, Winslet MC. Seifalian AM. Ischaemic preconditioning improves microvascular perfusion and oxygenation following reperfusion injury of the intestine. Br. J. Surg. 2005;92:1169–1176. doi: 10.1002/bjs.4988. [DOI] [PubMed] [Google Scholar]
- Marber MS, Latchman DS, Walker JM. Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Marles A, Perrey S, Legrand R, Blondel N, Delangles A, Betbeder D, et al. Effect of prior heavy exercise on muscle deoxygenation kinetics at the onset of subsequent heavy exercise. Eur. J. Appl. Physiol. 2007;99:677–684. doi: 10.1007/s00421-007-0395-x. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB. Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nishiyama SK, Wray DW, Berkstresser K, Ramaswamy M. Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J. Appl. Physiol. 2007;103:843–851. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Bezodis NE, Glaister M. Pattison JR. The effect of ischemic preconditioning on repeated sprint cycling performance. Med. Sci. Sports Exerc. 2015 doi: 10.1249/MSS.0000000000000576. , and in press. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G. Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Campbell IT. Jones AM. Influence of nitric oxide synthase inhibition on pulmonary O2 uptake kinetics during supra-maximal exercise in humans. J. Physiol. 2004;561:623–635. doi: 10.1113/jphysiol.2004.071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Paterson DH. Kowalchuk JM. High-intensity interval training speeds the adjustment of pulmonary O2 uptake, but not muscle deoxygenation, during moderate-intensity exercise transitions initiated from low and elevated baseline metabolic rates. J. Appl. Physiol. 2013;114:1550–1562. doi: 10.1152/japplphysiol.00575.2012. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Endoh H, Ishihata A, Takahashi E. Doi K. Effect of ischemic preconditioning on myocardial oxygen consumption during ischemia. J. Mol. Cell. Cardiol. 1998;30:2165–2174. doi: 10.1006/jmcc.1998.0771. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G. Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription1/3 pathway. Circulation. 2007;116:535–544. doi: 10.1161/CIRCULATIONAHA.107.689471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, et al. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1442–H1450. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]


