Abstract
The purpose of the present study was to elucidate the effect of increasing expiratory muscle work on sympathetic vasoconstrictor outflow and arterial blood pressure (BP) during dynamic exercise. We hypothesized that expiratory muscle fatigue would elicit increases in sympathetic vasomotor outflow and BP during submaximal exercise. The subjects performed four submaximal exercise tests; two were maximal expiratory pressure (PEmax) tests and two were muscle sympathetic nerve activity (MSNA) tests. In each test, the subjects performed two 10-min exercises at 40% peak oxygen uptake using a cycle ergometer in a semirecumbent position [spontaneous breathing for 5 min and voluntary hyperpnoea with and without expiratory resistive breathing for 5 min (breathing frequency: 60 breaths/min, inspiratory and expiratory times were set at 0.5 sec)]. PEmax was estimated before and immediately after exercises. MSNA was recorded via microneurography of the right median nerve at the elbow. PEmax decreased following exercise with expiratory resistive breathing, while no change was found without resistance. A progressive increase in MSNA burst frequency (BF) appeared during exercise with expiratory resistance (MSNA BF, without resistance: +22 ± 5%, with resistance: +44 ± 8%, P < 0.05), accompanied by an augmentation of BP (mean BP, without resistance: +5 ± 2%, with resistance: +29 ± 5%, P < 0.05). These results suggest that an enhancement of expiratory muscle activity leads to increases in sympathetic vasomotor outflow and BP during dynamic leg exercise.
Keywords: Dynamic leg exercise, metaboreflex, respiratory muscle, sympathetic outflow
Introduction
High-intensity whole body exercise induces inspiratory muscle (diaphragm) fatigue (Johnson et al. 1993; Romer and Polkey 2008). In healthy humans, this exercise-induced inspiratory muscle fatigue does not limit the hyperventilatory response to exercise. However, it has been thought that the fatiguing inspiratory muscle affects cardiovascular regulation and blood flow distribution during exercise (Harms et al. 1998; Dempsey et al. 2006, 2008). Voluntary breathing against inspiratory resistance at rest and during exercise augments an increase in muscle sympathetic nerve activity (MSNA), that is, sympathetic vasomotor outflow, with corresponding increase in arterial blood pressure (BP) (St Croix et al. 2000; Sheel et al. 2001; Katayama et al. 2012, 2013). This sympathoexcitation occurs through an inspiratory muscle fatigue-induced metaboreflex (Hill 2000).
Similar to the inspiratory muscle, expiratory muscle fatigue occurs following exercise (Loke et al. 1982; Fuller et al. 1996; Taylor et al. 2006). Fatiguing expiratory muscles could also affect cardiovascular regulation. Indeed, high-intensity contractions of expiratory muscles in the resting state cause increases in MSNA and BP (Derchak et al. 2002). Recently, Athanasopoulos et al. (2010) revealed an increase in blood flow in the expiratory muscle and a concomitant decrease in quadriceps muscle blood flow during leg exercise with expiratory muscle loading. Clinically, respiratory muscle fatigue could play an important role in cardiovascular regulation during exercise, because inspiratory and expiratory respiratory muscle works are enhanced during exercise in patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) (Sullivan et al. 1988; Musch 1993; Amann et al. 2010). Significant expiratory (abdominal) muscle fatigue occurs following exercise in patients with COPD (Hopkinson et al. 2010), although interindividual variability exists (Bachasson et al. 2013). Verges et al. (2007) reported that expiratory muscle fatigue impairs exercise performance as reported for inspiratory muscle fatigue. From these studies, it is assumed that an increase in expiratory muscle work activates metaboreflex resulting in increased sympathetic vasomotor outflow and thereby compromising blood flow and oxygen delivery during exercise. However, to the best of our knowledge, direct recording of sympathetic vasomotor outflow and cardiovascular variables during dynamic exercise with expiratory resistance has not been performed.
The purpose of the present study was to elucidate the effect of increasing expiratory muscle work on sympathetic vasoconstrictor outflow and BP during dynamic exercise. We recorded MSNA and cardiovascular variables during a leg-cycling exercise with expiratory resistance. We hypothesized that expiratory muscle fatigue would elicit increases in sympathetic vasomotor outflow and BP during submaximal exercise.
Methods
Subjects
Nine healthy males participated in the study; eight of these completed the study [means ± SE: age = 23 ± 1 year, height = 172 ± 2 cm, body mass = 68 ± 3 kg, forced vital capacity = 4.5 ± 0.1 L, forced expiratory volume in 1s = 4.0 ± 0.1 L, 89 ± 1%, maximal inspiratory pressure (PImax) = −133 ± 6 cmH2O]. All were nonsmokers with no history of respiratory or cardiovascular disease. Subjects were informed about the experimental procedures and potential risks involved, and written consent was obtained. This study was approved by the human research committee of the Research Center of Health, Physical Fitness and Sports, Nagoya University.
Experimental procedure
At the preliminary visit, the subjects were instructed on how to laterally extend both arms and how to hold their arms during leg-cycle exercise using an electromechanically braked ergometer in a semirecumbent position (Aerobike 75XL, Combi, Tokyo, Japan). Subjects reported to the laboratory on at least four occasions at the same time of day, and each visit was separated by 1 week.
On day 1, the subjects performed an incremental exercise test using the ergometer (maximal exercise test) to determine peak oxygen uptake (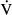 O2peak). The exercise test began at an initial power output of 90 W, and the workload was increased by 15 W every minute until exhaustion. The pedaling rate was maintained at 60 rpm with the aid of a metronome. Minute expired ventilation (
O2peak). The exercise test began at an initial power output of 90 W, and the workload was increased by 15 W every minute until exhaustion. The pedaling rate was maintained at 60 rpm with the aid of a metronome. Minute expired ventilation (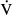 E), oxygen uptake (
E), oxygen uptake (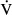 O2), heart rate (HR), and arterial oxygen saturation (SpO2) were recorded during the test and were averaged every 30s afterward. The highest
O2), heart rate (HR), and arterial oxygen saturation (SpO2) were recorded during the test and were averaged every 30s afterward. The highest 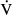 O2 value obtained was used as
O2 value obtained was used as 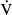 O2peak. Then, workload at 40%
O2peak. Then, workload at 40% 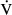 O2peak was calculated for the submaximal exercise test.
O2peak was calculated for the submaximal exercise test.
On day 2, subjects were again instructed on how to hold their right arm during submaximal exercise. Additionally, the subjects practiced controlling their breaths during exercise with or without the expiratory resistance by means of oscilloscope. The subjects also practiced measuring maximal expiratory pressure (PEmax) before and immediately after exercise.
On day 3, the subjects carried out two submaximal exercise tests with PEmax measurement (PEmax test), as shown in Fig.1. The PEmax tests were performed to assess expiratory muscle fatigue using expiratory resistance during exercise. The subjects rested for 30 min, and a PEmax measurement was taken before exercise. The subjects then rested for 5 min (rest-spontaneous breathing). Respiratory and cardiovascular variables were measured throughout the experiment. Submaximal exercise was then carried out for 10 min, and exercise intensity was set at 40% 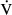 O2peak. The pedaling rate was kept at 60 rpm with the aid of a metronome. The subjects breathed spontaneously over the first 5 min of exercise (exercise-spontaneous breathing). During the next 5 min, the subjects were asked to control their breath with or without expiratory resistance (exercise-voluntary hyperpnoea): breathing frequency (fb) was maintained at 60 breaths/min and the inspiratory and expiratory time of one breath cycle was set at 0.5 s via auditory feedback from the metronome. Tidal volume (VT) was regulated at twice the resting VT via visual feedback from an oscilloscope marked with target VT levels (Sheel et al. 2001; Katayama et al. 2012, 2013). End-tidal partial pressure of CO2 (PETCO2) was maintained within ±3 mmHg of the level during exercise-spontaneous breathing by adding CO2 to the inspired air. The subjects were asked to report their rate of perceived exertion (RPE) (Borg 1982) for dyspnea during the last minute of each 5-min exercise, that is, the exercise-spontaneous breathing and the exercise-voluntary hyperpnoea. Immediately after exercise, PEmax measurement was performed. The procedure was repeated twice; that is, the exercise-voluntary hyperpnoea with or without expiratory resistance, with a 20-min interval between trials. In a preliminary study, we confirmed that the reduced PEmax and the enhanced MSNA after exercise with expiratory resistance did not return to pre-exercise levels within 20 min. Therefore, the exercise-voluntary hyperpnoea without expiratory resistance was performed first (First trial), and the exercise-voluntary hyperpnoea with expiratory resistance was done second (Second trial) (Fig.1). The resistance device was connected to the expiratory side via a tube. Expiratory resistance during exercise was set at 20% PEmax, because we confirmed that several subjects could not keep target VT levels for 5 min when expiratory resistance was set at 30% PEmax.
O2peak. The pedaling rate was kept at 60 rpm with the aid of a metronome. The subjects breathed spontaneously over the first 5 min of exercise (exercise-spontaneous breathing). During the next 5 min, the subjects were asked to control their breath with or without expiratory resistance (exercise-voluntary hyperpnoea): breathing frequency (fb) was maintained at 60 breaths/min and the inspiratory and expiratory time of one breath cycle was set at 0.5 s via auditory feedback from the metronome. Tidal volume (VT) was regulated at twice the resting VT via visual feedback from an oscilloscope marked with target VT levels (Sheel et al. 2001; Katayama et al. 2012, 2013). End-tidal partial pressure of CO2 (PETCO2) was maintained within ±3 mmHg of the level during exercise-spontaneous breathing by adding CO2 to the inspired air. The subjects were asked to report their rate of perceived exertion (RPE) (Borg 1982) for dyspnea during the last minute of each 5-min exercise, that is, the exercise-spontaneous breathing and the exercise-voluntary hyperpnoea. Immediately after exercise, PEmax measurement was performed. The procedure was repeated twice; that is, the exercise-voluntary hyperpnoea with or without expiratory resistance, with a 20-min interval between trials. In a preliminary study, we confirmed that the reduced PEmax and the enhanced MSNA after exercise with expiratory resistance did not return to pre-exercise levels within 20 min. Therefore, the exercise-voluntary hyperpnoea without expiratory resistance was performed first (First trial), and the exercise-voluntary hyperpnoea with expiratory resistance was done second (Second trial) (Fig.1). The resistance device was connected to the expiratory side via a tube. Expiratory resistance during exercise was set at 20% PEmax, because we confirmed that several subjects could not keep target VT levels for 5 min when expiratory resistance was set at 30% PEmax.
Figure 1.
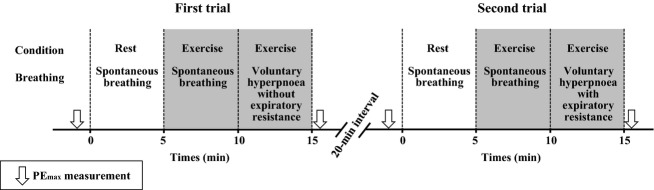
Time course of the experiment (PEmax test). MSNA test was done as the same of PEmax test, except PEmax measurement.
On day 4, the subjects performed the two submaximal exercise tests with MSNA measurement (MSNA test) to elucidate the influence of expiratory muscle fatigue on MSNA during exercise. The procedures and measurements of respiratory and cardiovascular parameters were identical to those of the PEmax test (Fig.1).
Initially, a total of nine subjects entered the study. MSNA recordings were completed for six of these, while the remaining three failed to record MSNA during exercise because of displacement of the electrodes from the muscle sympathetic nerve or bursts as a result of movement of the arm or body. In these three subjects, the MSNA test was repeated after a break of at least 1 month, at that point, MSNA recordings were completed in two of the three subjects. Consequently, eight subjects from whom we obtained nerve recordings were used in the analysis.
Expiratory muscle strength
PEmax, as an indicator of expiratory muscle strength, was measured using a handheld mouth pressure meter (AAM377; Minato Ikagaku, Osaka, Japan) connected to a computerized spirometry system (AS-507; Minato Ikagaku). All measurements were taken from total lung capacity, the subjects brought their hand to their cheeks and pressed forcefully (Suzuki et al. 1991; Fuller et al. 1996). For each measurement, five trials were completed, and the highest of three measurements with less than 5% variability was averaged and used as PEmax (Suzuki et al. 1991). If three measurements with less than 5% were not obtained within five trials, the procedure was repeated.
Respiratory variables
Subjects breathed through a mouthpiece with their nose occluded. The mouthpiece was attached to a hot wire flowmeter (RF-H; Minato Ikagaku), which was connected to a device equipped with a one-way low resistance valve. The dead space in this ventilatory system was ~130 mL. The flow signal from the flowmeter was connected to an oscilloscope, which indicated the target VT as a horizontal line for visual feedback. This system was similar to that in our previous studies (Katayama et al. 2012, 2013). Sample gas was drawn through a sampling tube connected to the mouthpiece in order to measure end-tidal O2 fraction (FETO2) and end-tidal CO2 fraction (FETCO2) by means of a gas analyzer (MG-360; Minato Ikagaku). 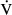 E, VT, fb, and
E, VT, fb, and 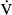 O2 were determined using an online system with a mixing chamber, as in our previous studies (Katayama et al. 2011, 2012, 2013). Expired gas volume was measured by a Fleisch pneumotachometer (PN-230; Arco Systems, Chiba, Japan), which was connected to the expiratory side of the valve via tube. Sample gas was drawn through a sampling tube inserted into the pneumotachometer to measure expired gas fractions. The expired gas fractions were analyzed using a mass spectrometer (ARCO-1000; Arco Systems) that was calibrated and confirmed before each test. Breath-by-breath data were analyzed continuously using customized computer software (PC-9821Ra40; NEC, Tokyo, Japan). SpO2 was measured using a finger pulse oximeter (Biox 3740; Ohmeda, Madison, WI). The signals from the flowmeter, gas analyzer, and pulse oximeter were sampled at a frequency of 200 Hz through an analog-to-digital converter (CSI-320416; Interface, Hiroshima, Japan) and were stored in a computer (CF-F8; Panasonic, Osaka, Japan). The signals were analyzed afterward using our own computer software.
O2 were determined using an online system with a mixing chamber, as in our previous studies (Katayama et al. 2011, 2012, 2013). Expired gas volume was measured by a Fleisch pneumotachometer (PN-230; Arco Systems, Chiba, Japan), which was connected to the expiratory side of the valve via tube. Sample gas was drawn through a sampling tube inserted into the pneumotachometer to measure expired gas fractions. The expired gas fractions were analyzed using a mass spectrometer (ARCO-1000; Arco Systems) that was calibrated and confirmed before each test. Breath-by-breath data were analyzed continuously using customized computer software (PC-9821Ra40; NEC, Tokyo, Japan). SpO2 was measured using a finger pulse oximeter (Biox 3740; Ohmeda, Madison, WI). The signals from the flowmeter, gas analyzer, and pulse oximeter were sampled at a frequency of 200 Hz through an analog-to-digital converter (CSI-320416; Interface, Hiroshima, Japan) and were stored in a computer (CF-F8; Panasonic, Osaka, Japan). The signals were analyzed afterward using our own computer software.
Cardiovascular variables
An electrocardiogram (ECG) was measured using a three-lead electrocardiograph (AB-621; Nihon Koden, Tokyo, Japan), and HR was calculated from each R-R interval obtained from the ECG. Beat-to-beat arterial BP was acquired using finger photoplethysmography from the middle finger of the left hand (Finometer, Finapres Medical Systems BV, Amsterdam, The Netherlands). ECG and BP signals were sampled and analyzed using a method similar to that for respiratory variables. Arterial systolic and diastolic BP (SBP and DBP) were determined from the BP wave form signal, and mean arterial BP (MBP) was calculated using the following equation: MBP = (SBP-DBP) / 3+ DBP.
MSNA
Multiunit muscle sympathetic nerve discharges were recorded using the microneurographic technique. A recording system similar to that in our previous studies (Katayama et al. 2011, 2012, 2013, 2014) was utilized. A tungsten microelectrode with shaft diameter of 0.1 mm (impedance 1–5 MΩ) was inserted manually by an experimenter into the right median nerve at the cubital fossa (Saito et al. 1993; Katayama et al. 2011, 2012, 2013, 2014). The right arm was fixed using equipment to prevent arm movement artifacts during the leg cycling exercise. The electrode was adjusted until MSNA was recorded after insertion. Identification of MSNA was based on the following criteria: spontaneous burst discharge synchronized with the heartbeat and enhanced by Valsalva maneuver or breath holding, but showing no change in response to sensory stimuli, such as a loud noise or cutaneous touch (Delius et al. 1972; Vallbo et al. 1979; Fagius and Wallin 1980). Furthermore, we asked the subjects to hold their breath to identify MSNA at the middle phase during the exercise-spontaneous breathing and after exercise (at least 5 sec). The neurogram was fed to a differential amplifier and amplified 100,000 times through a band-pass filter (700–2000 Hz). The neurogram was full-wave rectified and integrated by a capacitance-integrated circuit with a time constant of 0.1 sec. The mean voltage neurogram was continuously digitized through an analog-to-digital converter with a sampling frequency of 200 Hz for storage on a computer in a manner similar to that for used respiratory and cardiovascular variables. MSNA bursts were identified from the mean voltage neurogram using a customized computer program-assisted inspection (Katayama et al. 2011, 2012, 2013, 2014), which accounted for the latency from the ECG-R wave to the sympathetic burst (Fagius and Wallin 1980). MSNA was quantified as burst frequency (BF, bursts/min) and burst incidence (BI, bursts/100HR) (Katayama et al. 2011, 2012, 2013, 2014). Since electromyographic efferent and afferent nerve activities altered the baseline of the integrated neurogram during dynamic leg cycling in most recordings (Saito et al. 1993; Katayama et al. 2011, 2012, 2013, 2014), we could not calculate MSNA burst amplitude and total activity.
Statistical analysis
Values are expressed as means ± SE. The respiratory and cardiovascular variables, and MSNA BF values were averaged every 1 min throughout the experiment. The assumption of normal distribution for all data was verified using a Kolmogorov-Smirnov test. Changes in parameters during the experiment in each trial were analyzed using a Dunnett's test, that is, vs. at 5 min at the rest-spontaneous breathing or vs. at 5 min during the exercise-spontaneous breathing. Comparisons of parameters between the nonresistance and resistance trials were performed using paired t-test (parametric test) when the distribution was regular. When the distribution was not regular, a Wilcoxon test (nonparametric test) was used. The StatView (5.0; SAS Institute, Tokyo, Japan) and the SPSS (11.5; SPSS, Tokyo, Japan) statistical packages were used for the analyses. A P < 0.05 was considered to indicate significance.
Results
Maximal exercise test
Cardiorespiratory variables and workload at exhaustion during maximal exercise test are as follows: 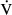 O2 = 3.1 ± 0.1 L/min, 46 ± 2 mL/kg/min,
O2 = 3.1 ± 0.1 L/min, 46 ± 2 mL/kg/min, 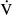 E = 122 ± 8 L/min, HR = 185 ± 1 beats/min, SpO2 = 96 ± 4%, and workload = 255 ± 4W.
E = 122 ± 8 L/min, HR = 185 ± 1 beats/min, SpO2 = 96 ± 4%, and workload = 255 ± 4W.
Submaximal exercise test
Baseline descriptive data
No significant differences were found in any of the respiratory and cardiovascular variables throughout the experiment between the PEmax and MSNA tests. Therefore, we report only the data during the MSNA test. No differences in these variables were found at the rest-spontaneous breathing between the first and second trials. Workload during submaximal exercise was 75 ± 5W.
Expiratory muscle strength
In the first trial, PEmax was unchanged after exercise (182 ± 12 to 183 ± 13 cmH2O). On the contrary, a significant reduction of PEmax appeared in the second trial (188 ± 13 to 158 ± 12 cmH2O, P < 0.05).
RPE
RPE dyspnea scores increased from the exercise-spontaneous breathing to the exercise-voluntary hyperpnoea in the first (10.5 ± 0.3 to 11.8 ± 0.3, P < 0.05) and second (10.5 ± 0.3 to 14.0 ± 0.5, P < 0.05) trials. RPE dyspnea in the exercise-voluntary hyperpnoea with expiratory resistance was higher (P < 0.05) than the hyperpnoea without resistance.
Respiratory variables
Representative flow and partial pressure of CO2 (PCO2) during the first and second trials are shown in Fig.2, and mean values of respiratory parameters were shown in Table1. 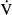 E, VT, and fb and PETCO2 increased (P < 0.05) during the exercise-spontaneous breathing, and
E, VT, and fb and PETCO2 increased (P < 0.05) during the exercise-spontaneous breathing, and  E, and fb increased (P < 0.05) further during the exercise-voluntary hyperpnoea. A small but significant increase in SpO2 appeared during the exercise-voluntary hyperpnoea. There were no significant differences in all respiratory parameters between the first and second trials throughout the experiment.
E, and fb increased (P < 0.05) further during the exercise-voluntary hyperpnoea. A small but significant increase in SpO2 appeared during the exercise-voluntary hyperpnoea. There were no significant differences in all respiratory parameters between the first and second trials throughout the experiment.
Figure 2.
Representative records of flow, PCO2, ECG, BP, and MSNA in the first and second trials.
Table 1.
Respiratory variables in the MSNA test.
| Trials | Rest-spontaneous breathing | Exercise- spontaneous breathing | Exercise- voluntary hyperpnoea | ||
|---|---|---|---|---|---|
 E (L/min) E (L/min) |
First | 10.2 ± 0.4 | 27.8 ± 1.11 | Without resistance | 72.1 ± 2.31,3 |
| Second | 11.8 ± 0.8 | 29.3 ± 1.52 | With resistance | 70.4 ± 3.12,4 | |
| VT (L) | First | 0.61 ± 0.03 | 1.23 ± 0.091 | Without resistance | 1.23 ± 0.041 |
| Second | 0.64 ± 0.02 | 1.32 ± 0.132 | With resistance | 1.19 ± 0.062 | |
| fb (breaths/min) | First | 13.6 ± 1.4 | 24.1 ± 1.51 | Without resistance | 59.6 ± 0.21,3 |
| Second | 14.4 ± 1.4 | 24.3 ± 2.42 | With resistance | 59.8 ± 0.12,4 | |
| PETO2 (mmHg) | First | 105.2 ± 1.6 | 106.7 ± 1.7 | Without resistance | 134.0 ± 1.41,3 |
| Second | 108.0 ± 1.1 | 107.3 ± 2.0 | With resistance | 134.9 ± 1.32,4 | |
| PETCO2 (mmHg) | First | 44.4 ± 0.7 | 45.5 ± 0.61 | Without resistance | 45.5 ± 0.61 |
| Second | 44.0 ± 0.8 | 45.8 ± 0.72 | With resistance | 45.1 ± 0.72 | |
| SpO2 (%) | First | 97.3 ± 0.3 | 97.3 ± 0.3 | Without resistance | 98.7 ± 0.21,3 |
| Second | 97.7 ± 0.2 | 97.5 ± 0.2 | With resistance | 99.0 ± 0.32,4 | |
Values are mean ± SE. 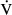 E, expired minute ventilation; VT, tidal volume; fb, breathing frequency; PETO2, end-tidal partial pressure of O2; PETCO2, end-tidal partial pressure of CO2; SpO2, arterial oxygen saturation.
E, expired minute ventilation; VT, tidal volume; fb, breathing frequency; PETO2, end-tidal partial pressure of O2; PETCO2, end-tidal partial pressure of CO2; SpO2, arterial oxygen saturation.
Significant from Rest-spontaneous breathing in the first trial, P < 0.05.
Significant from Rest-spontaneous breathing in the second trial, P < 0.05.
Significant from Exercise-spontaneous breathing in the first trial, P < 0.05.
Significant from Exercise-spontaneous breathing in the second trial, P < 0.05.
Cardiovascular variables
Typical recordings of ECG and BP are indicated in Fig.2. Mean values of HR, SBP, and DBP are shown in Fig.3. These variables increased (P < 0.05) during the exercise-spontaneous breathing in the first and second trials. Significant increases in HR, SBP, and DBP occurred during the exercise-voluntary hyperpnoea with expiratory resistance. During the exercise-voluntary hyperpnoea without expiratory resistance, a small but significant increase in HR occurred during, whereas SBP and DBP were unchanged. HR, SBP, and DBP during the latter part of the exercise-voluntary hyperpnoea with expiratory resistance were higher (P < 0.05) than those in the hyperpnoea without expiratory resistance.
Figure 3.

Changes in HR (A), SBP (B), and DBP (C) during the experiment. *P < 0.05 vs. at 5 min during rest-spontaneous breathing in the first trial. †P < 0.05 vs. at 5 min during rest-spontaneous breathing in the second trial. #P < 0.05 vs. at 5 min during exercise-spontaneous breathing in the first trial. ‡at 5 min during exercise-spontaneous breathing in the second trial. §P < 0.05 between the first and second trials.
MSNA
Representative MSNA recordings are indicated in Fig.2, and mean MSNA BF and BI values are shown in Fig.4. MSNA BF did not change during the exercise-spontaneous breathing in both trials. A progressive increase in MSNA BF appeared during the exercise-voluntary hyperpnoea with expiratory resistance. MSNA BF exhibited a small but significant increase by voluntary hyperpnoea without expiratory resistance. The extent of MSNA BF during the latter part of the exercise-voluntary hyperpnoea with expiratory resistance was larger (P < 0.05) than the hyperpnoea without resistance. MSNA BI decreased significantly during the exercise-spontaneous breathing. During the exercise-voluntary hyperpnoea, MSNA BI increased in both with and without expiratory resistance, but the magnitude of an increase in MSNA BI was higher in the exercise-voluntary hyperpnoea with resistance.
Figure 4.
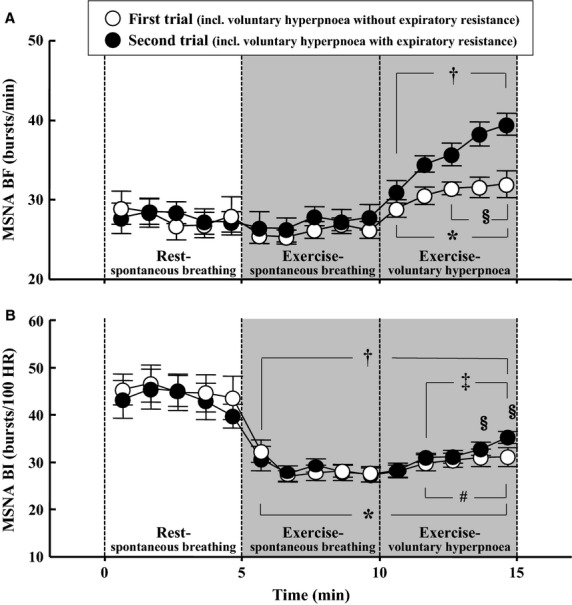
Changes in MSNA BF (A) and MSNA BI (B) during the experiment. *P < 0.05 vs. at 5 min during rest-spontaneous breathing in the first trial. †P < 0.05 vs. at 5 min during rest-spontaneous breathing in the second trial. #P < 0.05 vs. at 5 min during exercise-spontaneous breathing in the first trial. ‡at 5 min during exercise-spontaneous breathing in the second trial. §P < 0.05 between the first and second trials.
Discussion
The primary finding of the present study was that an increase in MSNA BF appeared during a leg-cycling exercise with expiratory resistive breathing, accompanied by an augmentation of BP. This finding supports our hypothesis that the expiratory muscle fatigue may lead to increase in sympathetic vasomotor outflow and BP during submaximal exercise. The results from this study should provide information regarding the significance of expiratory muscle activity on circulatory regulation during dynamic exercise.
Effect of expiratory resistance on expiratory muscle strength
To estimate the effect of expiratory resistive breathing on expiratory muscle fatigue, we measured PEmax, as an index of expiratory muscle strength, before and after submaximal exercise. Consequently, PEmax decreased significantly following the exercise-voluntary hyperpnoea with expiratory resistance, indicating that expiratory muscle fatigue had occurred. This result is in agreement with the data of previous studies, which demonstrated reductions in PEmax after resistive breathing (Suzuki et al. 1991; Orozco-Levi et al. 2001; Derchak et al. 2002). A reduction in the gastric pressure using magnetic nerve stimulation (Taylor et al. 2006) and large declines of electromyogram of the abdominal muscle during maximal expiratory efforts (Fuller et al. 1996) after high-intensity exercise have been reported. Abdominal muscles have a relatively high percentage of fast-twitch fibers (Johnson et al. 1973), and the oxidative capacity in abdominal muscles is lower than that in the diaphragm (Uribe et al. 1992). In addition, there is evidence that muscle endurance is lower in expiratory muscles compared with in inspiratory muscles (Gandevia et al. 1983). In our previous study (Katayama et al. 2012), inspiratory resistance during exercise was set at 29–40% PImax under similar VT and fb, and the all subjects completed the inspiratory resistive breathing for 5 min. In contrast to this, in our preliminary study several subjects could not keep target VT levels for 5 min during exercise with expiratory resistive breathing at 30% PEmax. From these observations, it is assumed that the expiratory muscle is more fatigable than the inspiratory muscle.
Effect of expiratory resistance on MSNA and BP response during dynamic exercise
The exercise pressor reflex, the afferent arm of which is composed of type III and IV muscle afferents, plays a role in the cardiovascular adjustments (Adreani et al. 1997). The diaphragm has an abundance of type IV afferent fiber, and activity in the fiber increases during fatiguing diaphragm contractions (Hill 2000). Although there is no available data concerning innervation of the abdominal muscles of expiration by type III and IV afferents, many such fibers identify in the internal intercostal nerve that subserves the rib-cage expiratory muscles (Duron 1981). The ubiquity of these fibers in other skeletal muscle and the diaphragm suggests that type III and IV afferents are present in expiratory muscles (Derchak et al. 2002). At resting, it was revealed that high-intensity contractions of expiratory muscles cause an increase in MSNA (Derchak et al. 2002). In the present study, expiratory resistive breathing during leg cycling did cause a progressive increase in MSNA BF with an enhancement of BP. Collectively, it is plausible that a metaboreflex arising in the expiratory muscles is an important determinant of sympathetic vasomotor outflow and BP during submaximal exercise.
Other possible mechanisms affecting MSNA during exercise with expiratory resistive breathing should be considered. First, it is necessary to consider the increased central respiratory motor output (central command). The increased RPE dyspnea score indicates a higher central respiratory motor output during the exercise-voluntary hyperpnoea with expiratory resistance, and this may be a cause of the observed sympathoexcitation. Derchak et al. (2002) reported that an enhanced central respiratory motor output produced an increased HR but not MSNA and BP. HR is more vulnerable to central command influences (Victor et al. 1989). Similarly, St Croix et al. (2000) reported that an increase in central respiratory output led immediate an sustained increases in HR without significant effect on sympathetic outflow. However, in their studies (St Croix et al. 2000; Derchak et al. 2002), exceptional subjects demonstrated increases in MSNA during nonfatiguing, resistive breathing. Additionally, in the present study, a small but significant increase in MSNA BF was noted during the exercise-voluntary hyperpnoea without expiratory resistance (Fig.4). From these results, the effect of augmented central expiratory effort may involve the reasons for an increase in MSNA. Second, it has been demonstrated that heavy expiratory resistance causes not only PEmax reduction but also a decrease in PImax as an index of inspiratory muscle fatigue (Suzuki et al. 1991; Haverkamp et al. 2001). However, expiratory resistance for short duration does not induce diaphragm fatigue (Derchak et al. 2002). Accordingly, it is conceivable that the inspiratory muscle fatigue did not appear and thereby would have only minor effects on MSNA via inspiratory muscle metaboreflex in the present study. Third, stimulation of mechanoreceptors in abdominal viscera may induce an increase in vagal afferent nerve traffic and BP, suggesting increased sympathetic vasomotor outflow (Gieroba et al. 1995). This is unlikely because the receptors respond mainly to distension, and the abdominal viscera were compressed, not distended, during expiratory efforts (Derchak et al. 2002). Fourth, cardiopulmonary baroreceptor reflex may affect the change in MSNA during exercise. During mild dynamic leg exercise, cardiopulmonary baroreflex could play an important role for sympathetic vasomotor outflow (Ray et al. 1993; Fadel and Raven 2012; Katayama et al. 2014). Enhanced intrathoracic pressure during expiratory resistance induces a large decrease in central blood volume, which may induce unloading cardiopulmonary receptors (Ray et al. 1993; Miller et al. 2007). Accordingly, cardiopulmonary baroreflex may be included as one of the reasons for the increased MSNA during exercise with expiratory resistance. Fifth, sympathetic vasomotor outflow is influence by mental stress (Callister et al. 1992). We asked the subjects to control their breath during exercise-voluntary hyperpnoea. This may affect MSNA response, especially with expiratory resistance, although we do not have enough data for how much stress the subjects had during the task. Finally, it is possible that pain may be associated with the increased MSNA during resistive breathing (St Croix et al. 2000; Sheel et al. 2001; Derchak et al. 2002). However, it was reported that exercise-induced increased MSNA disappeared after exercise while persistent muscle pain (Vissing et al. 1998). None of the subjects complained of pain following exercise with resistive breathing. Therefore, it is unlikely that such distress is related to the increased MSNA during the exercise-voluntary hyperpnoea with expiratory resistance.
Technical considerations and limitations
Several technical considerations and limitations should be noted. One limitation was the use of PEmax as an index of expiratory muscle strength; we could not utilize a more definitive method to assess expiratory muscle function; that is., using gastric pressure response to magnetic stimulation (Kyroussis et al. 1996; Taylor et al. 2006). In the present study, five measurements were performed, and the highest of three measurements with less than 5% variability was averaged and used as PEmax (Suzuki et al. 1991). The maneuvers were repeated if three measurements with less than 5% were not obtained. Consequently, within-day coefficient of variation for PEmax, which was calculated from the data before each trial, was 4.3%. Thus, PEmax data presented in the present study are valid and the PEmax values were comparable before and after exercise with or without expiratory resistance.
It is necessary to consider exercise intensity. As in our previous studies (Katayama et al. 2012, 2013, 2014), we utilized mild exercise intensity (40% 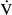 O2peak), because the percentage of successful MSNA recordings is high when the exercise intensity is mild. Another reason is that MSNA BF is not altered during leg cycling at 40%
O2peak), because the percentage of successful MSNA recordings is high when the exercise intensity is mild. Another reason is that MSNA BF is not altered during leg cycling at 40%  O2peak exercise compared with when at rest (Katayama et al. 2012, 2013). In the present study, we confirmed this as shown in Fig.4. Therefore, we assumed that an increase in MSNA BF with expiratory resistive breathing would become apparent under these conditions.
O2peak exercise compared with when at rest (Katayama et al. 2012, 2013). In the present study, we confirmed this as shown in Fig.4. Therefore, we assumed that an increase in MSNA BF with expiratory resistive breathing would become apparent under these conditions.
MSNA was assessed as BF and BI in the present study. We could not estimate burst amplitude because electromyographic and efferent and afferent nerve activities altered the baseline of the integrated neurogram during dynamic leg cycling (Saito et al. 1993; Katayama et al. 2012, 2013). However, there is a positive correlation between BF and burst amplitudes (Mark et al. 1985) and parallel increases in BF and burst amplitude during exercise (Seals et al. 1991). Therefore, it seems reasonable to suppose that our MSNA BF values are valid and MSNA levels were comparable during leg cycling with or without resistive breathing.
Perspective and significance
In this study, a progressive increase in MSNA BF appeared during exercise with expiratory resistance accompanied by an augmentation of BP. These results suggest that an enhancement of expiratory muscle activity leads to increases in sympathetic vasomotor outflow and BP during dynamic leg exercise. The results in this study support the idea that fatiguing respiratory muscle affects cardiovascular regulation during exercise (Harms et al. 1998; Dempsey et al. 2006, 2008). Fatigue of the expiratory muscle causes a sympathetically mediated vasoconstriction, similar to that described previously for fatiguing inspiratory muscle work (St Croix et al. 2000; Sheel et al. 2001; Katayama et al. 2012, 2013). In animal study, infusion of lactic acid into the deep circumflex iliac artery resulted in a reduction in hind limb blood flow (Rodman et al. 2003). Recently, it was revealed that blood flow in the expiratory muscle increases, quadriceps muscle blood flow decrease, during leg exercise with expiratory muscle loading (Athanasopoulos et al. 2010). A reduction in blood flow (oxygen transport) to working limb muscles would be expected to increase locomotor muscle fatigue (Taylor and Romer 2008). Indeed, it is confirmed that expiratory muscle fatigue impairs exercise performance (Verges et al. 2007). Clinically, respiratory muscle activity cold play an important role in limiting oxygen delivery during exercise, since it has been reported that patients with COPD and CHF have a large amount of respiratory muscle work (Sullivan et al. 1988; Musch 1993; Amann et al. 2010). Several studies demonstrated expiratory (abdominal) muscle fatigue following exercise in COPD patients (Hopkinson et al. 2010), although wide interindividual variability exists (Bachasson et al. 2013). Therefore, it is assumed that vasoconstriction, which is induced by sympathoexcitation via expiratory fatigue-induced metaboreflex during exercise, affects locomotor oxygen transport and consequent locomotor muscle fatigue and exercise tolerance in patients.
Acknowledgments
The authors acknowledge Professor J. A. Dempsey at the University of Wisconsin–Madison for informative discussions of the manuscript.
Conflict of Interest
None declared.
References
- Adreani CM, Hill JM. Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J. Appl. Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos D, Louvaris Z, Cherouveim E, Andrianopoulos V, Roussos C, Zakynthinos S, et al. Expiratory muscle loading increases intercostal muscle blood flow during leg exercise in healthy humans. J. Appl. Physiol. 2010;109:388–395. doi: 10.1152/japplphysiol.01290.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachasson D, Wuyam B, Pepin JL, Tamisier R, Levy P. Verges S. Quadriceps and respiratory muscle fatigue following high-intensity cycling in COPD patients. PLoS ONE. 2013;8:e83432. doi: 10.1371/journal.pone.0083432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GAV. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Callister R, Suwarno NO. Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J. Physiol. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A. Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol. Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J. Smith C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Amann M, Romer LM. Miller JD. Respiratory system determinants of peripheral fatigue and endurance performance. Med. Sci. Sports Exerc. 2008;40:457–461. doi: 10.1249/MSS.0b013e31815f8957. [DOI] [PubMed] [Google Scholar]
- Derchak PA, Sheel AW, Morgan BJ. Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J. Appl. Physiol. 2002;92:1539–1552. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- Duron B. intercostal and diaphragmatic muscle afferents. In: Hornbein TF, editor. Regulation of breathing. New York: Dekker; 1981. pp. 473–540. in , ed. . [Google Scholar]
- Fadel PJ. Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp. Physiol. 2012;97:39–50. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J. Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J. Neurol. Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fuller D, Sullivan J. Fregosi RF. Expiratory muscle endurance performance after exhaustive submaximal exercise. J. Appl. Physiol. 1996;80:1495–1502. doi: 10.1152/jappl.1996.80.5.1495. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Neering IR. Endurance properties of respiratory and limb muscles. Respir. Physiol. 1983;53:47–61. doi: 10.1016/0034-5687(83)90015-4. [DOI] [PubMed] [Google Scholar]
- Gieroba ZJ, Messenger JP. Blessing WW. Abdominal vagal stimulation excites bulbospinal barosensitive neurons in the rostral ventrolateral medulla. Neuroscience. 1995;65:355–364. doi: 10.1016/0306-4522(94)00509-4. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Haverkamp HC, Metelits M, Hartnett J, Olsson K. Coast JR. Pulmonary function subsequent to expiratory muscle fatigue in healthy humans. Int. J. Sports Med. 2001;22:498–503. doi: 10.1055/s-2001-17612. [DOI] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–244. doi: 10.1016/s0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Hopkinson NS, Dayer MJ, Moxham J. Polkey MI. Abdominal muscle fatigue following exercise in chronic obstructive pulmonary disease. Respir. Res. 2010;11:15. doi: 10.1186/1465-9921-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D. Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Babcock MA, Suman OE. Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J. Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T. Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R456–R464. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- Katayama K, Iwamoto E, Ishida K, Koike T. Saito M. Inspiratory muscle fatigue increases sympathetic vasomotor outflow and blood pressure during submaximal exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1167–R1175. doi: 10.1152/ajpregu.00006.2012. [DOI] [PubMed] [Google Scholar]
- Katayama K, Yamashita S, Ishida K, Iwamoto E, Koike T. Saito M. Hypoxic effects on sympathetic vasomotor outflow and blood pressure during exercise with inspiratory resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R374–R382. doi: 10.1152/ajpregu.00489.2012. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ishida K, K Saito MT, Hirasawa A. Ogoh S. Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol. Rep. 2014;2:e12070. doi: 10.14814/phy2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyroussis D, Mills GH, Polkey MI, Hamnegard CH, Koulouris N, Green M, et al. Abdominal muscle fatigue after maximal ventilation in humans. J. Appl. Physiol. 1996;81:1477–1483. doi: 10.1152/jappl.1996.81.4.1477. [DOI] [PubMed] [Google Scholar]
- Loke J, Mahler DA. Virgulto JA. Respiratory muscle fatigue after marathon running. J. Appl. Physiol. 1982;52:821–824. doi: 10.1152/jappl.1982.52.4.821. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C. Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ. Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Miller JD, Smith CA, Hemauer SJ. Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H580–H592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am. J. Physiol. 1993;265:H1721–H1726. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Gea J, Ferrer A, Mendez R, Ramirez-Sarmiento A, Maldonado D, et al. Expiratory muscle endurance in middle-aged healthy subjects. Lung. 2001;179:93–103. doi: 10.1007/s004080000049. [DOI] [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP. Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am. J. Physiol. 1993;264:H1–H7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Henderson KS, Smith CA. Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J. Appl. Physiol. 2003;95:1159–1169. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- Romer LM. Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J. Appl. Physiol. 2008;104:879–888. doi: 10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D. Mano T. Muscle sympathetic nerve responses to graded leg cycling. J. Appl. Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG. Fregosi RF. Hypoxia potentiates exercise-iduced sympathetic neural activation in humans. J. Appl. Physiol. 1991;71:1032–1040. doi: 10.1152/jappl.1991.71.3.1032. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ. Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J. Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ. Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J. Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Higginbotham MB. Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77:552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Suzuki J. Okubo T. Expiratory muscle fatigue in normal subjects. J. Appl. Physiol. 1991;70:2632–2639. doi: 10.1152/jappl.1991.70.6.2632. [DOI] [PubMed] [Google Scholar]
- Taylor BJ. Romer LM. Effect of expiratory muscle fatigue on exercise tolerance and locomotor muscle fatigue in healthy humans. J. Appl. Physiol. 2008;104:1442–1451. doi: 10.1152/japplphysiol.00428.2007. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, How SC. Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J. Appl. Physiol. 2006;100:1554–1562. doi: 10.1152/japplphysiol.01389.2005. [DOI] [PubMed] [Google Scholar]
- Uribe JM, Stump CS, Tipton CM. Fregosi RF. Influence of exercise training on the oxidative capacity of rat abdominal muscles. Respir. Physiol. 1992;88:171–180. doi: 10.1016/0034-5687(92)90038-x. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE. Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol. Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Verges S, Sager Y, Erni C. Spengler CM. Expiratory muscle fatigue impairs exercise performance. Eur. J. Appl. Physiol. 2007;101:225–232. doi: 10.1007/s00421-007-0491-y. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH. Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ. Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Vissing J, Vissing SF, MacLean DA, Saltin B, Quistorff B. Haller RG. Sympathetic activation in exercise is not dependent on muscle acidosis. Direct evidence from studies in metabolic myopathies. J. Clin. Invest. 1998;101:1654–1660. doi: 10.1172/JCI555. [DOI] [PMC free article] [PubMed] [Google Scholar]


