Abstract
Objectives
Recombinant human thyroid-stimulating hormone (rhTSH) is widely used in radioactive iodine therapy (RIT) to avoid side effects caused by hypothyroidism during the therapy. Owing to RIT with rhTSH, serum thyroglobulin (Tg) is measured with high 131I concentrations. It is of concern that the relatively high energy of 131I could interfere with Tg measurement using the immunoradiometric assay (IRMA). We investigated the effect of 131I administration on Tg measurement with IRMA after RIT.
Methods
A total of 67 patients with thyroid cancer were analysed retrospectively. All patients had undergone rhTSH stimulation for RIT. The patients’ sera were sampled 2 days after 131I administration and divided into two portions: for Tg measurements on days 2 and 32 after 131I administration. The count per minute (CPM) of whole serum (200 μl) was also measured at each time point. Student’s paired t-test and Pearson’s correlation analyses were performed for statistical analysis.
Results
Serum Tg levels were significantly concordant between days 2 and 32, irrespective of the serum CPM. Subgroup analysis was performed by classification based on the 131I dose. No difference was noted between the results of the two groups.
Conclusions
IRMA using 125I did not show interference from 131I in the serum of patients stimulated by rhTSH.
Keywords: Immunoradiometric assay, Thyroglobulin, Radioactive iodine therapy, Recombinant human TSH
Introduction
Immunoradiometric assay (IRMA) is widely used for the measurement of thyroglobulin (Tg) levels [1]. Despite debates on the discordance between commercial assay kits [2–6], high-sensitivity human Tg IRMA can detect serum Tg concentrations of 0.2 ng/ml, and serum Tg concentrations <0.2 ng/ml are used to indicate complete remission [7]. Because the serum Tg level is used for surveillance after total thyroidectomy to evaluate the residual and recurrent differentiated thyroid cancer (DTC), accurate estimation of the serum Tg level is important [8].
Radioactive iodine therapy (RIT) is commonly used for the ablation of remnant tissue, treatment of functioning metastasis, and diagnostic purposes [9, 10]. RIT was conventionally performed with thyroid hormone withdrawal. However, recently, recombinant human thyroid-stimulating hormone (rhTSH) was approved for RIT for thyroid hormone stimulation, thereby substituting thyroid hormone withdrawal. Compared to the use of conventional thyroid hormone withdrawal, the use of rhTSH for TSH stimulation significantly improved the quality of life (QOL) during RIT, avoided iatrogenic hypothyroidism symptoms, and sustained the liver and kidney functions [11, 12].
131I emits beta and gamma rays simultaneously. Therefore, it can be used for simultaneous RIT and diagnostic imaging. 131I predominantly emits 363 and 637 keV of energy in a two-step decay process. On the other hand, 125I, which is used for IRMA, emits four kinds of gamma rays with a maximum energy of 35 keV. Because Tg measurement is performed a day after 131I administration, the relatively high energy of 131I can interfere with the measurement of Tg using IRMA. Serum Tg elevation is highest at 48 h after 131I administration, and optimal Tg measurement is obtained after 131I administration [13]. Therefore, measurement of Tg levels after 131I administration may be influenced by the high radioactivity of the serum 131I. However, to the best of our knowledge, no study has investigated the influence of 131I on the detection of 125I anti-human-Tg antibody. The present study aimed to evaluate the interference of 131I in the measurement of Tg using 125I anti-human-Tg antibody.
Materials and Methods
Patients
A total of 67 patients with pathologically confirmed papillary thyroid cancer (PTC), who underwent RIT between January and February 2014 were included in this study (Table 1). In all patients, TSH stimulation was performed with rhTSH.
Table 1.
Patient characteristics
| Patient number | ||
|---|---|---|
| Sex | M | 17 |
| F | 50 | |
| Age | <35 years | 13 |
| >35 years | 54 | |
| RAI dose | 148 MBq | 20 |
| 1,110 MBq | 47 | |
| PTC stage | I | 29 |
| II | 12 | |
| III | 26 | |
| IV | 0 |
PTC papillary thyroid carcinoma
Study Protocol
TSH stimulation was performed with rhTSH, as per the instructions of the manufacturer (Genzyme Corp., Cambridge, MA, USA), and patient sera were collected after 131I administration. Two intra-muscular injections of rhTSH, each of 0.9 mg, were given at 24-h intervals and 131I was administered 48 h after the first rhTSH injection. Then, patients’ sera were collected after 48 h of 131I administration and divided in two portions for Tg concentration measurements at two different time points: at day 2 after administration and at day 32 (the serum was stored at −20 °C) (Fig. 1).
Fig. 1.
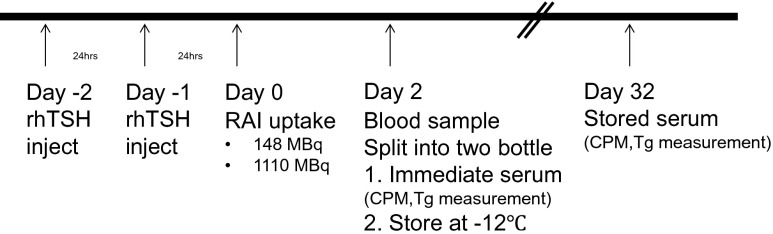
The study protocol
Tg Measurement Protocol Using IRMA
Tg was measured using the IRMA commercial kit (Tg-plus; Brahms Diagnostica, Berlin, Germany), having an analytical sensitivity of 0.08–250 ng/ml, as per the manufacturer’s instructions. Briefly, all kit components and patient samples were stored at room temperature. All liquid reagents including patients’ sera were agitated gently before use. Standard solution, controls, Tg-free serum, and patients’ sera (100 μl) were pipetted into test tubes coated with anti-h-Tg (polyclonal, rabbit). The tubes were briefly agitated on a sample mixer and incubated overnight (18 ± 4 h) at room temperature. Next, 2 ml of the washing solution was added and the tube was washed twice. The tubes were placed upside down on adsorbent paper for a minimum of 10 min. Finally, 200 μl tracer of 125I-labeled monoclonal Tg antibody was pipetted into each tube. The tubes were incubated for 2–3 h at room temperature with shaking (170–300 rpm) and washed again with 2 ml of the washing solution. All tubes were placed upside down on adsorbent paper for at least 10 min twice. Radioactivity of each tube was measured using a gamma counter (Gamma-10; Shinjin Medics, Goyang, Korea) with a countable isotope energy of 15–2,000 keV.
Statistical Analysis
The relationship between the Tg levels of days 2 and 32 were analysed by Student’s paired t-test and Pearson’s correlation analysis. Statistical analyses were performed using SPSS (version 18.0; IBM Software, Chicago, IL, USA).
Results
Patients’ Demographic Information
Patients’ information is summarised in Table 1. A total of 67 patients (age range: 27–69 years) pathologically diagnosed with PTC after surgery were included. The patients received either 148 MBq or 1,110 MBq of 131I for remnant thyroid ablation. The stages of PTC were distributed from I to III, with no patient having distant metastasis.
The Relationship Between Serum Tg Levels at Days 2 and 32
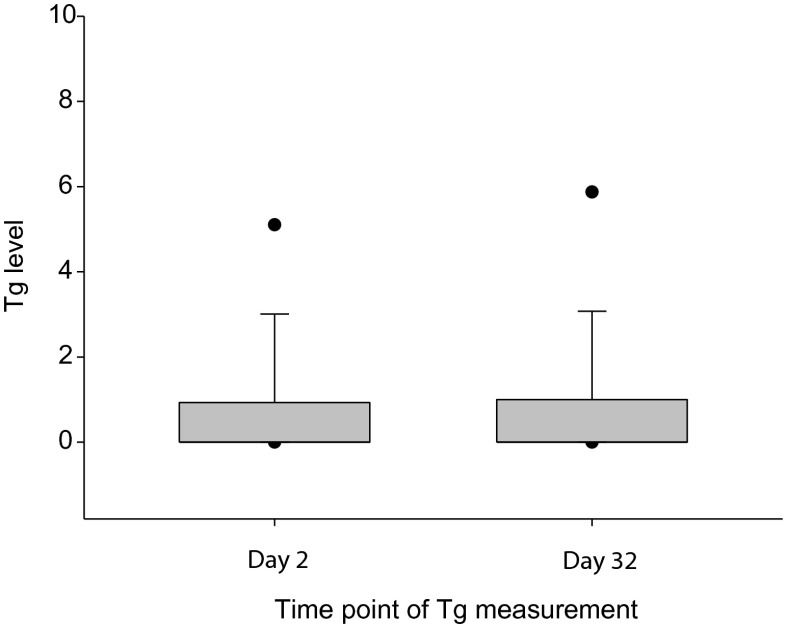
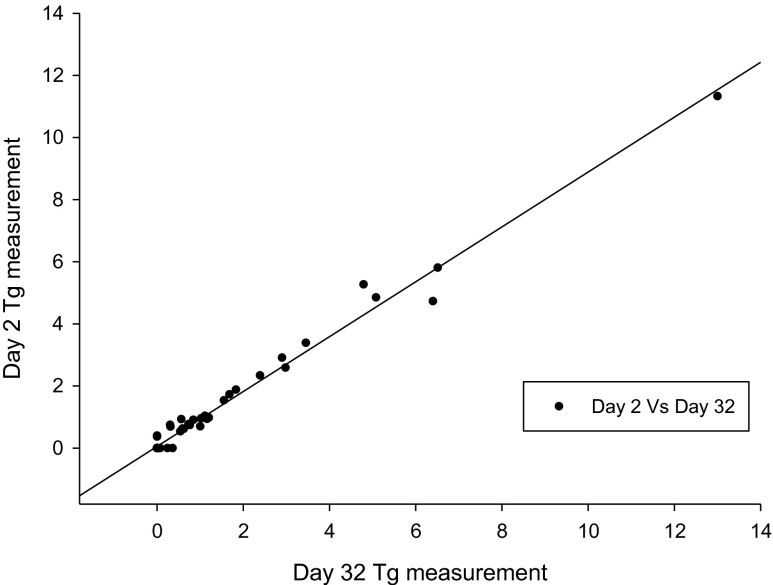
The serum Tg level of each sample was measured on days 2 and 32; levels <0.2 ng/ml were regarded as “undetected”. The mean Tg levels of days 2 and 32 were 0.90 ± 1.88 ng/ml and 0.95 ± 2.11 ng/ml, respectively. No significant difference was noted between the two samples (p = 0.201; Fig. 2). Furthermore, a significant correlation was found between the two samples (r = 0.992, p < 0.001; Fig. 3).
Fig. 2.
Box plot of Tg measurements on days 2 and 32. The mean Tg value was 0.90 ± 1.88.ng/ml and 0.95 ± 2.11 ng/ml in each group, with no statistically significant difference, as determined by the paired t-test (p = 0.201)
Fig. 3.
Significant correlation was noted between the Tg values on days 2 and 32 (r = 0.992, p = 0.000)
The serum count per minute (CPM) determined at the two different time points showed a significant decline from day 2 to day 32 (mean CPM on days 2 and 32 were 1,004 ± 834 and 116 ± 63, respectively; p < 0.001). In addition, decay correction performed between day-2 serum CPM and day-32 serum CPM was significantly correlated (p < 0.001).
Subgroup Analysis Classified by Radioactive Iodine Dose
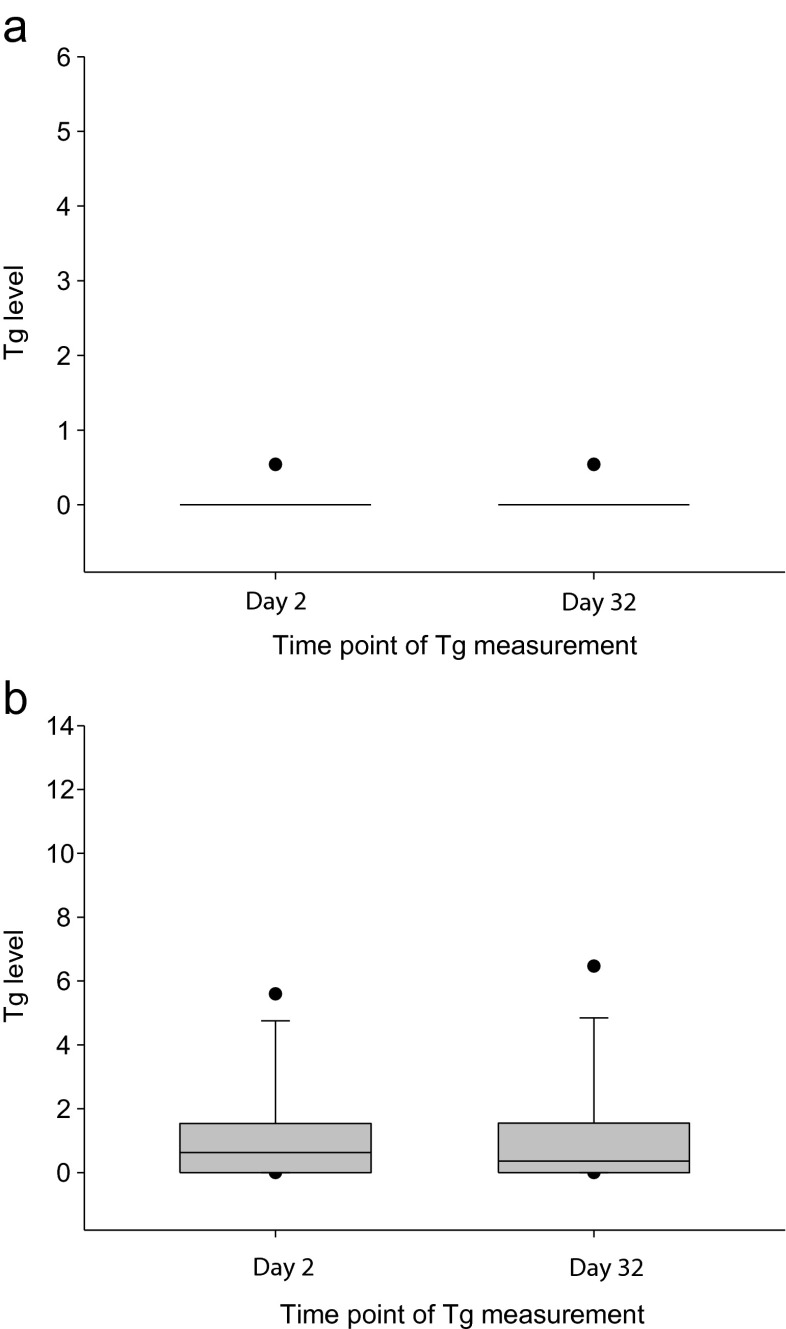
From among 67 patients, 20 patients underwent RIT with 131I 148 MBq, and 47 patients underwent RIT with 131I 1,100 MBq. In the 131I 148 MBq group, the day-2 and day-32 Tg levels were equal for all samples (mean value: 0.27 ± 0.03 ng/ml). In the 131I 1,100 MBq group, the mean Tg values of day 2 and day 32 were 1.27 ± 0.31 ng/ml and 1.34 ± 0.35 ng/ml, respectively. Conclusively, the mean Tg level in each group showed no significant difference (p = 1.000 in the 131I 148 MBq group and p = 0.202 in the 131I 1,100 MBq group; Fig. 4).
Fig. 4.
Subgroup analysis of the Tg level measurement. a The 148 MBq 131I group, which shows no significant change in the Tg value between days 2 and 32. b The 1,110 MBq 131I group, which shows no significant change in the Tg value between days 2 and 32
Discussion
IRMA is a reliable method for the measurement of Tg in the follow-up of DTC after total thyroidectomy [1]. Our study evaluated whether 131I administration interferes with Tg measurement using IRMA because of the relatively high energy of 131I compared to 125I. Usage of rhTSH for stimulation of TSH is becoming easy because of insurance coverage in Korea. On using rhTSH for TSH stimulation, the serum Tg elevation was highest at 48 h after rhTSH administration, which is a day after 131I administration [13]. As a result, the optimal Tg measurement was performed after 131I administration. So when we use rhTSH for stimulation of TSH, optimal Tg measurement is done with high serum background radioactivity due to the administration of 131I. If the administered 131I interferes with the serum Tg measurement, the Tg levels determined by IRMA can be inaccurate. However, in our study, the Tg levels of serum samples with high 131I and decayed 131I showed no significant difference. Therefore, we conclude that the 131I radioactivity of serum does not interfere with the measurement of Tg by IRMA.
The Tg level was correlated with the remnant tumour burden and prognosis. A high pre-ablation Tg level is known to be the most significant predictor of therapeutic failure [14]. The stimulated Tg level is related to the prediction with stimulation using rhTSH. The first rhTSH stimulation Tg level showed excellent prediction of remission [15]. Furthermore, Tg level measurement is a sensitive method for monitoring response and recurrence during the treatment process. As mentioned earlier, knowledge of the Tg level is important to make precise clinical decisions; therefore, the Tg value should be accurately measured.
The half-life of 131I is 8.01 days and that of 125I is 59.4 days. Therefore, we believe that, after a month, the radioactivity of 131I significantly decreases to <7 % of the initial activity, although the radioactivity of 125I decreases to approximately 30 %. The biological half-life of 131I may be 18 h because of its excretion from the body [16]. Although considering the biological half-life of 131I, the sera on day 2 still contain high radioactivity of 131I. Therefore, our study confirmed the reliability of immediate Tg measurement in sera with high radioactivity of 131I.
TSH stimulation for RIT is conventionally performed with thyroid hormone withdrawal. Hypothyroidism is inevitable before RIT, and hypothyroidism decreases patient QOL. rhTSH improves a patient’s QOL by avoiding long-term hypothyroidism [11]. rhTSH is expensive; hence, there is considerable controversy surrounding the cost-effectiveness of using rhTSH [17]. However, the ablation success rate by the proposed method is comparable to that obtained using thyroid hormone withdrawal, even in high-risk patients with metastatic thyroid cancer; the long-term outcome is also similar between both protocols [18–20]. Therefore, it is recommended that rhTSH be applied routinely for RIT to improve patient QOL. Furthermore, diagnostic monitoring is feasible using rhTSH as compared with that using thyroid hormone withdrawal because of not only the efficacy of rhTSH but also its effect on improving the QOL [21, 22].
The radioactive iodine dose was different in each patient. For diagnostic purposes, the remnant thyroid tissue was examined and very low dose of 131I was administered (148 MBq). However, for treatment purposes, a low dose of 131I was administered for remnant ablation (1,110 MBq) [9]. As the administered dose could influence the serum CPM at the time of blood collection, subgroup analysis classified by the radioactive iodine dose may affect our data interpretation. However, no significant difference was noted between the Tg values of days 2 and 32 in each group. The limitation of our study is that it did not include high-dose RAI patients. However, no difference was noted between the results of doses of 148 MBq and 1,110 MBq, suggesting that the binding force between the antibody and Tg was strong enough to neglect the serum CPM.
A serum Tg level <0.2 was regarded as “undetectable”. The cutoff level of Tg for surveillance remains controversial; however, the cutoff level of 0.2 could be rational for the surveillance of DTC after total thyroidectomy [23–26]. In our study, the Tg level of most patients was undetectable. There was a concern that an increase in Tg level may occur between days 2 and 32. However, a significant correlation was observed between the dual time points for detectable Tg levels, and the results were significant when analysed by the paired t-test. Thus, IRMA has a high reproducibility irrespective of the administered dose of 131I and the serum Tg level.
Conclusion
The 131I in the serum of the patients stimulated by rhTSH did not interfere with the IRMA using 125I. In the follow-up of DTC, IRMA can be used for disease surveillance, irrespective of the RIT used.
Acknowledgments
Declaration of Interest
The authors fully declare any financial or other potential conflict of interest.
Conflict of Interest
Sohyun Park, Ji-In Bang, Ho-Young Lee, and Sang-Eun Kim declare that they have no conflict of interest.
References
- 1.Schlumberger M, Fragu P, Gardet P, Lumbroso J, Violot D, Parmentier C. A new immunoradiometric assay (IRMA) system for thyroglobulin measurement in the follow-up of thyroid cancer patients. Eur J Nucl Med. 1991;18:153–157. doi: 10.1007/BF02262724. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari L, Seregni E, Aliberti G, Martinetti A, Pallotti F, Villano C, et al. Comparative evaluation of two methods to assay thyroglobulin serum concentrations in patients with differentiated thyroid carcinomas. Q J Nucl Med Mol Imaging. 2004;48:237–242. [PubMed] [Google Scholar]
- 3.Taylor KP, Parkington D, Bradbury S, Simpson HL, Jefferies SJ, Halsall DJ. Concordance between thyroglobulin antibody assays. Ann Clin Biochem. 2011;48:367–369. doi: 10.1258/acb.2011.010248. [DOI] [PubMed] [Google Scholar]
- 4.Crane MS, Strachan MW, Toft AD, Beckett GJ. Discordance in thyroglobulin measurements by radioimmunoassay and immunometric assay: a useful means of identifying thyroglobulin assay interference. Ann Clin Biochem. 2013;50:421–432. doi: 10.1177/0004563213480492. [DOI] [PubMed] [Google Scholar]
- 5.Tortajada-Genaro LA, Cozar MP, Frigols JL, de Avila CR. Comparison of immunoradiometric assays for determination of thyroglobulin: a validation study. J Clin Lab Anal. 2007;21:147–153. doi: 10.1002/jcla.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weightman DR, Mallick UK, Fenwick JD, Perros P. Discordant serum thyroglobulin results generated by two classes of assay in patients with thyroid carcinoma: correlation with clinical outcome after 3 years of follow-up. Cancer. 2003;98:41–47. doi: 10.1002/cncr.11472. [DOI] [PubMed] [Google Scholar]
- 7.Giovanella L, Ceriani L. High-sensitivity human thyroglobulin (hTG) immunoradiometric assay in the follow-up of patients with differentiated thyroid cancer. Clin Chem Lab Med. 2002;40:480–484. doi: 10.1515/CCLM.2002.083. [DOI] [PubMed] [Google Scholar]
- 8.Iervasi A, Iervasi G, Ferdeghini M, Solimeo C, Bottoni A, Rossi L, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2007;67:434–441. doi: 10.1111/j.1365-2265.2007.02907.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 10.Boelaert K. Thyroid gland: revised guidelines for the management of thyroid cancer. Nat Rev Endocrinol. 2010;6:185–186. doi: 10.1038/nrendo.2010.17. [DOI] [PubMed] [Google Scholar]
- 11.Rani D, Kaisar S, Awasare S, Kamaldeep O, Abhyankar A, Basu S. Examining recombinant human TSH primed I therapy protocol in patients with metastatic differentiated thyroid carcinoma: comparison with the traditional thyroid hormone withdrawal protocol. Eur J Nucl Med Mol Imaging. 2014;41:1767–1780. doi: 10.1007/s00259-014-2737-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Lee HY, Lee WW, Kim SE. The effect of recombinant human thyroid stimulating hormone on sustaining liver and renal function in thyroid cancer patients during radioactive iodine therapy. Nucl Med Commun. 2014;35:727–732. doi: 10.1097/MNM.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 13.David A, Blotta A, Bondanelli M, Rossi R, Roti E, Braverman LE, et al. Serum thyroglobulin concentrations and (131)I whole-body scan results in patients with differentiated thyroid carcinoma after administration of recombinant human thyroid-stimulating hormone. J Nucl Med. 2001;42:1470–1475. [PubMed] [Google Scholar]
- 14.Park H, Jeong G-C, Kwon S, Min J-J, Bom H-S, Park K, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;1–7. [DOI] [PMC free article] [PubMed]
- 15.Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Wartofsky L. Does an undetectable rhTSH-stimulated Tg level 12 months after initial treatment of thyroid cancer indicate remission? Clin Endocrinol (Oxf) 2011;74:111–117. doi: 10.1111/j.1365-2265.2010.03898.x. [DOI] [PubMed] [Google Scholar]
- 16.Smallridge RC, Diehl N, Bernet V. Practice trends in patients with persistent detectable thyroglobulin and negative diagnostic radioiodine whole body scans: a survey of American Thyroid Association members. Thyroid. 2014;24:1501–1507. doi: 10.1089/thy.2014.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Peng W, Huang R, Tian R, Zeng Y, Kuang A. Thyroid cancer: radiation safety precautions in 131I therapy based on actual biokinetic measurements. Radiology. 2014;132234. [DOI] [PubMed]
- 18.Wang TS, Cheung K, Mehta P, Roman SA, Walker HD, Sosa JA. To stimulate or withdraw? A cost-utility analysis of recombinant human thyrotropin versus thyroxine withdrawal for radioiodine ablation in patients with low-risk differentiated thyroid cancer in the United States. J Clin Endocrinol Metab. 2010;95:1672–1680. doi: 10.1210/jc.2009-1803. [DOI] [PubMed] [Google Scholar]
- 19.Pak K, Cheon GJ. The effectiveness of recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal prior to radioiodine remnant ablation in thyroid cancer: a meta-analysis of randomized controlled trials. 2014;29:811–17. [DOI] [PMC free article] [PubMed]
- 20.Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity 131I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metab. 2013;98:2693–2700. doi: 10.1210/jc.2012-4137. [DOI] [PubMed] [Google Scholar]
- 21.Bartenstein P, Calabuig EC, Maini CL, Mazzarotto R, Muros de Fuentes MA, Petrich T, et al. High-risk patients with differentiated thyroid cancer T4 primary tumors achieve remnant ablation equally well using rhTSH or thyroid hormone withdrawal. Thyroid. 2014;24:480–487. doi: 10.1089/thy.2013.0157. [DOI] [PubMed] [Google Scholar]
- 22.Sherman SI. The role of recombinant human thyrotropin for diagnostic monitoring of patients with differentiated thyroid cancer. Endocr Pract. 2013;19:157–161. doi: 10.4158/EP12315.RA. [DOI] [PubMed] [Google Scholar]
- 23.Rosario PW, Furtado Mde S, Mineiro Filho AF, Lacerda RX, Calsolari MR. Value of diagnostic radioiodine whole-body scanning after initial therapy in patients with differentiated thyroid cancer at intermediate and high risk for recurrence. Thyroid. 2012;22:1165–1169. doi: 10.1089/thy.2012.0026. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:1433–1441. doi: 10.1210/jc.2002-021702. [DOI] [PubMed] [Google Scholar]
- 25.Cappelli C, Rotondi M, Pirola I, De Martino E, Gandossi E, Agosti B, et al. Usefulness of repeated recombinant human thyrotropin-stimulated thyroglobulin test in the post-surgical follow-up of very low-risk patients with differentiated thyroid carcinoma. J Endocrinol Investig. 2012;35:459–463. doi: 10.3275/8057. [DOI] [PubMed] [Google Scholar]
- 26.Ciappuccini R, Hardouin J, Heutte N, Vaur D, Quak E, Rame JP, et al. Stimulated thyroglobulin level at ablation in differentiated thyroid cancer: the impact of treatment preparation modalities and tumor burden. Eur J Endocrinol. 2014;171:247–252. doi: 10.1530/EJE-14-0192. [DOI] [PubMed] [Google Scholar]