Abstract
Purpose
The aim of this study was to investigate relationships between the immunohistochemical results and radioiodine scan and 18F-FDG PET findings in papillary thyroid cancer (PTC) patients with recurrent cervical nodal metastases.
Methods
A total of 46 PTC patients who had undergone a radioiodine scan and/or 18F-FDG PET/CT and a subsequent operation on recurrent cervical lymph nodes were enrolled. Twenty-seven patients underwent 18F-FDG PET/CT, 8 underwent radioiodine scans, and 11 underwent both scans. In all surgical specimens, the immunoexpressions of thyroglobulin (Tg), sodium-iodide symporter (NIS), glucose transporter 1 (Glut-1), and somatostatin receptor 1 and 2A (SSTR1 and SSTR2A) were assessed, and associations between these expressions and radioiodine scan and 18F-FDG PET findings were evaluated.
Results
Of the 38 patients who underwent 18F-FDG PET/CT, all patients with weak Tg expression had positive 18F-FDG uptake, while only 45 % of the patients with moderate or strong Tg expression showed positive uptake (p = 0.01). The proportion of patients with positive 18F-FDG uptake increased as the degree of Glut-1 expression with luminal accentuation increased. Of the 19 patients who underwent a radioiodine scan, the proportion with positive radioiodine uptake was greater among patients with strong NIS and SSTR2A expression than among patients expressing these markers at weak levels (p = 0.04 for all). All three patients with weak Tg expression were negative for radioiodine uptake.
Conclusion
The 18F-FDG uptakes of recurrent cervical nodes are related to strong Glut-1 expression with luminal accentuation and weak Tg expression, whereas radioiodine uptake is related to the strong expressions of NIS and SSTR2A.
Keywords: Thyroid cancer, 18F-fluorodeoxyglucose, Positron emission tomography, Radioiodine
Introduction
The prognosis of well-differentiated thyroid cancer (DTC) is favorable, with a 10-year survival rate exceeding 90 % [1]. However, up to 20 % of patients with DTC develop locoregional recurrence including cervical lymph node metastases, and 8 % of patients with recurrence will eventually succumb to the disease [2]. Although one of the main characteristics of DTC is its ability to trap radioiodine due to the expression of sodium-iodide symporter (NIS), approximately 15–30 % of metastatic cancer lesions lose this ability and thus show no radioiodine uptake on post-therapeutic 131I scans [3–5]. This lack of an ability to accumulate radioiodine is problematic in patients with recurrent lymph nodes; hence, imaging modalities other than a radioiodine scan might be necessary in DTC patients with recurrent cervical nodes. Furthermore, conventional imaging modalities such as neck ultrasonography and computed tomography (CT) have also shown moderate sensitivity with a range of 63–82 % [6, 7].
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is recommended in patients with radioiodine-negative DTC [8, 9]. Glucose transporter 1 (Glut-1) is known to be the most important glucose transporter in thyroid cancer cells and is also known to be related to 18F-FDG uptake in various cancers [10–12]. Furthermore, some previous reports have been issued on the use of somatostatin receptor (SSTR)-targeted imaging in DTC patients with negative radioiodine uptake [9, 13]. However, no consensus exists concerning optimal imaging modalities for the follow-up of patients with recurrent cervical nodes. Although findings of radioiodine scans and 18F-FDG PET can provide important information for planning further treatment and predicting prognosis, there are few studies to evaluate the relationship between the histopathological findings and radioiodine scan and 18F-FDG PET findings. We considered that a deeper understanding of the relationships between the expressions of the pathological markers of DTC and the uptakes of various radiotracers would facilitate the imaging modality choice for the follow-up of DTC patients with recurrent neck nodes.
The objectives of this study were to evaluate the relationships between the expression levels of pathological markers [thyroglobulin (Tg), NIS, Glut-1, SSTR1 and SSTR2A] and radioiodine scan and 18F-FDG PET findings in DTC patients with recurrent cervical nodal metastases.
Materials and Methods
Patients
This study was approved by our Institutional Review Board. The records of 185 patients with DTC who underwent surgical resection for recurrent cervical lymph nodal metastases between February 2006 and March 2009 were retrospectively reviewed. Of these patients, 46 were enrolled in this study after applying the following inclusion criteria: (1) a 131I scan and/or 18F-FDG PET/CT at most 6 months before surgery and (2) the availability of sufficient cancer tissue for immunohistochemical staining. The characteristics of the 46 study subjects are shown in Table 1. Of these 46 patients, 38 underwent 18F-FDG PET/CT and 19 a radioiodine scan. Eleven patients underwent both 18F-FDG PET/CT and radioiodine scans.
Table 1.
Patient characteristics (n = 46)
| Characteristics | Value (%) |
|---|---|
| Age (years) | 49 ± 12 (range: 24–73) |
| Sex | |
| Male | 10 (22 %) |
| Female | 36 (78 %) |
| Pathology | |
| Papillary | 46 (100 %) |
| Radioiodine scan (n = 19) | |
| Diagnostic 131I scan | 5 (26 %) |
| Post-therapeutic 131I scan | 14 (74 %) |
| Previous treatment | |
| TT only | 8 (17 %) |
| TT + RAI | 26 (57 %) |
| TT + neck LND + RAI | 12 (26 %) |
| Pathological stage at initial operation | |
| T1-3N0 | 4 (9 %) |
| T1-T3N1 | 37 (80 %) |
| T4N0 | 1 (2 %) |
| T4N1 | 4 (9 %) |
TT total thyroidectomy, RAI radioiodine treatment, LND lymph node dissection
18F-FDG PET/CT and 131I Scan Imaging
Mean time between an 18F-FDG PET/CT scan and surgery was 74 ± 59 days. Scans were performed using a Gemini PET/CT scanner (Philips, Milpitas, CA). All patients were normoglycemic and fasted for at least 6 h before scans. Patients were injected with 5.18 MBq/kg of 18F-FDG 1 h prior to imaging. Initially, a CT scan was performed at 80 mA and 140 kVp for attenuation correction, then an emission scan was performed from the skull base to the proximal thigh in one bed position for 3 min. Emission scan images were reconstructed onto a 128 × 128 matrix using an iterative algorithm (ordered subset expectation maximization), and attenuation correction was performed.
Mean time between the radioiodine scan and surgery was 124 ± 44 days. All patients discontinued replacement l-thyroxine (T4) therapy 4 weeks before radioiodine administration and received replacement l-triiodothyronine (T3) for up to 2 weeks before radioiodine administration. In addition, all patients followed a low-iodine diet from at least 2 weeks before radioiodine administration. At the time of radioiodine administration, serum thyroid-stimulating hormone (TSH) levels were higher than 30 IU/ml in all patients. Among 19 patients, 14 underwent a post-therapeutic 131I scan, and scanning was performed 3–5 days after an oral administration of a therapeutic activity of 131I ranged between 1.1 and 7.4 GBq (30–200 mCi). The remaining five patients underwent a diagnostic 131I scan, and scanning was performed 2 days after administering of 185 MBq (5 mCi) of 131I. All radioiodine scans were performed using a large-field-of-view gamma camera (ON 410, Ohio Nuclear, Solon, OH) equipped with a medium-energy parallel-hole collimator, and a 20 % symmetric window was centered at 364 KeV. Anterior and posterior images of the neck, chest and abdomen were obtained during all radioiodine scans, and a minimum of 100,000 counts were collected for each image.
All 18F-FDG PET/CT and radioiodine scan images were retrospectively reviewed by experienced nuclear medicine physicians with consensus. The 18F-FDG uptakes and radioiodine uptake of the resected cervical lymph nodes on the scan images were visually assessed. The neck lymph nodes that showed higher 18F-FDG uptake than surrounding neck tissue uptake were classified as lymph nodes with positive 18F-FDG uptake, and the neck lymph nodes that showed similar uptake to surrounding neck tissue uptake were classified as lymph nodes with negative 18F-FDG uptake. Furthermore, focal increased 131I uptake in the neck area was classified as lymph nodes with positive 131I uptake. In patients with multiple lymph nodal metastases who underwent 18F-FDG PET/CT, the lymph node that showed the most intense 18F-FDG uptake was selected for the analysis, and the lymph node specimen that corresponded to the anatomical location on PET/CT was selected for immunohistochemical analysis. In patients with multiple lymph nodal metastases who underwent a 131I scan, the lymph node that had the largest metastatic foci at the area of 131I uptake was selected for the analysis.
Immunohistochemistry
All specimens from the surgical resection of recurrent cervical lymph nodes were stained with hematoxylin and eosin and reviewed by an experienced pathologist to confirm the presence of recurrent thyroid cancer. Immunohistochemical staining in all 46 cases was performed automatically based on the conventional streptavidin-biotin-peroxidase method using the TechMate™ 500 Plus (DAKO, Glostrup, Denmark) according to the manufacturer’s protocol. The primary antibodies used were as follows: Tg (1:200, TGB04 + TGB05, Thermo Scientific, CA), anti-hNIS (1:200, Clone FP5A, Thermo Scientific, CA), Glut-1 (1:200, C-20, Santa Cruz Biotechnology, Heidelberg, Germany), SSTR1 (1:100, BioTrend, Cologne, Germany) and SSTR2A (1:150, BioTrend, Cologne, Germany). Cells were scored as 0, 1 or 2 (negative, weak, strong) for Glut-1 or 0, 1, 2 or 3 (negative, mild, moderate, strong) for Tg according to their staining intensities. For NIS, SSTR1 and SSTR2A, percentages of stained cells were determined, and a final histochemical score (H-score) was calculated by summing the products of staining intensities [scored as 0 or 1 (negative and positive, respectively) for SSTR2A and as 0, 1 or 2 (negative, weak, strong) for NIS and SSTR1] and their distributions (0–100 %). Tg, NIS, SSTR1 and SSTR2A antibodies showed positivity in the cytoplasm, and Glut was positive in the cytoplasm and cytoplasmic membranes with luminal accentuation. Normal thyroid follicular cells (for Tg and NIS), red blood cells (for Glut) and normal pancreatic islet cells (for SSTR1 and SSTR2A) served as internal positive controls.
Statistical Analyses
For the purpose of statistical analysis, the expressions of Tg, Glut-1, NIS, SSTR1 and SSTR2A were dichotomized; Tg immunoexpression was divided into two groups of staining intensities: ≥ 2 (score = 2, 3) or ≤1 (score = 0, 1). Glut-1 expression was categorized as positive (score = 1, 2) or negative (score = 0). In addition, to evaluate the significance of the luminal accentuation of Glut-1 expression, the expression of Glut-1 was also categorized as membranous with or without luminal accentuation. The expressions of NIS, SSTR1 and SSTR2A were dichotomized about the half maximal H-scores for each marker (100 for SSTR1 and 50 for SSTR2A). The cutoff value used for NIS was determined by considering the mean H-score because NIS expressions tended to be weak. The chi-square test and Fisher’s exact test were performed to determine differences between the frequency of positive 18F-FDG or radioiodine uptakes in the two groups for each pathological marker. All statistical tests were performed using SPSS (version 15.0; SPSS Inc.). P-values < 0.05 were considered statistically significant.
Results
18F-FDG PET/CT and Radioiodine Scan Results and Immunohistochemical Findings
All 46 patients had a diagnosis of recurrent papillary thyroid cancer (PTC). In the 38 patients who underwent 18F-FDG PET/CT, 21 (55 %) showed positive 18F-FDG uptake in recurrent cervical nodes, while the remaining 17 (45 %) were negative for 18F-FDG uptake. In the 19 patients who underwent a radioiodine scan, 6 (32 %) showed positive radioiodine uptake in recurrent lesions, and the remaining 13 (68 %) showed negative uptake. Of these six patients with positive radioiodine uptake, four underwent a post-therapeutic scan, and the other two underwent a diagnostic scan. Furthermore, of the 13 patients with negative uptake, 10 underwent a post-therapeutic scan, and 3 underwent a diagnostic scan.
The results of immunohistochemical staining for Tg, NIS, Glut-1, SSTR1 and SSTR2A are shown in Table 2. Among 46 study subjects, 39 (85 %) showed moderate or strong (staining intensity = 2 or 3) Tg expression, and only 2 and 9 showed strong Glut-1 expression in the cytoplasm and cytoplasmic membrane, respectively. NIS expression tended to be weak among study subjects (mean H-score = 54 ± 42), and a cutoff H-score of 50 was used for NIS. SSTR2A expression was only mild (staining intensity = 0 or 1), and overall expression of SSTR2A in enrolled patients was weaker than that of SSTR1.
Table 2.
Immunohistochemical results (n = 46)
| Biologic marker (staining intensities) | Value (%) |
|---|---|
| Thyroglobulin | |
| 0 | 0 (0 %) |
| 1 | 7 (15 %) |
| 2 | 29 (63 %) |
| 3 | 10 (22 %) |
| Glut-1 (Cyt) | |
| 0 | 0 (0 %) |
| 1 | 44 (96 %) |
| 2 | 2 (4 %) |
| Glut-1 (Memb) | |
| 0 | 13 (28 %) |
| 1 | 7 (15 %) |
| 1 with luminal accentuation* | 17 (37 %) |
| 2 | 0 (0 %) |
| 2 with luminal accentuation* | 9 (20 %) |
| NIS (H-score) | 55 ± 41 (range: 0–160) |
| SSTR1(H-score) | 114 ± 47 (range: 50–200) |
| SSTR2A (H-score) | 70 ± 23 (range: 25–100) |
Cyt cytoplasm, Memb cytoplasmic membrane, *positive in the cytoplasmic membrane with luminal accentuation, Glut-1: glucose transporter 1, NIS: sodium-iodide symporter, SSTR: somatostatin receptor
Relationships Between 18F-FDG PET/CT and Immunohistochemical Results
Relationships between 18F-FDG PET/CT study and immunohistochemical results are summarized in Table 3. Recurrent cervical node 18F-FDG uptake was found to be related to Tg expression and Glut-1 membranous expression with luminal accentuation. All patients with weak Tg expression (staining intensity = 0 or 1) showed positive 18F-FDG uptake (Fig. 1), whereas only 45 % of patients with strong Tg expression (staining intensity = 2 or 3) showed positive 18F-FDG uptake (p = 0.01). Cytoplasmic Glut-1 expression was not found to be associated with positive 18F-FDG uptake. However, although a marginally significant relationship was found between Glut-1 expression with luminal accentuation and the proportion of patients with positive 18F-FDG uptake (p = 0.06), the proportion of patients positive for 18F-FDG uptake among patients with Glut-1 membranous expression (staining intensity = 2) with luminal accentuation (75 %; Fig. 2) was two-fold higher than the proportion of patients with Glut-1 expression without luminal accentuation (38 %). In contrast, irrespective of luminal accentuation, the intensity of Glut-1 membranous expression was not found to be related to 18F-FDG uptake (p = 0.3). Furthermore, no relationship was found between the expressions of NIS, SSTR1 or SSTR2 and the number of patients with positive 18F-FDG uptake (p > 0.05). In patients with strong SSTR1 or SSTR2A expression, 40 and 38 %, respectively, were negative for 18F-FDG uptake.
Table 3.
Relationships between 18F-FDG PET/CT and immunohistochemical results (n = 38)
| Biologic marker (staining intensities) | PET (+) | PET (-) | p-value |
|---|---|---|---|
| Thyroglobulin | |||
| 2, 3 (n = 31) | 14 (45 %) | 17 (55 %) | |
| 0, 1 (n = 7) | 7 (100 %) | 0 (0 %) | 0.01 |
| Glut-1 (Memb) | |||
| 2 with luminal accentuation (n = 8) | 6 (75 %) | 2 (25 %) | |
| 1 with luminal accentuation (n = 14) | 9 (64 %) | 5 (36 %) | |
| No luminal accentuation (n = 16) | 6 (38 %) | 10 (62 %) | 0.06 |
| Glut-1 (Memb) | |||
| Positive (1, 2) (n = 28) | 17 (61 %) | 11 (39 %) | |
| Negative (0) (n = 10) | 4 (40 %) | 6 (60 %) | 0.3 |
| Glut-1 (Cyt) | |||
| 1 (n = 36) | 20 (56 %) | 16 (44 %) | |
| 2 (n = 2) | 1 (50 %) | 1 (50 %) | 1.0 |
| NIS | |||
| H-score >50 (n = 17) | 11 (65 %) | 6 (35 %) | |
| H-score ≤50 (n = 21) | 10 (48 %) | 11 (52 %) | 0.3 |
| SSTR1 | |||
| H-score >100 (n = 20) | 12 (60 %) | 8 (40 %) | |
| H-score ≤100 (n = 18) | 9 (50 %) | 9 (50 %) | 0.8 |
| SSTR2A | |||
| H-score >50 (n = 26) | 16 (62 %) | 10 (38 %) | |
| H-score ≤50 (n = 12) | 5 (42 %) | 7 (58 %) | 0.4 |
Memb cytoplasmic membrane, Cyt cytoplasm, Glut-1 glucose transporter 1, NIS sodium-iodide symporter, SSTR somatostatin receptor
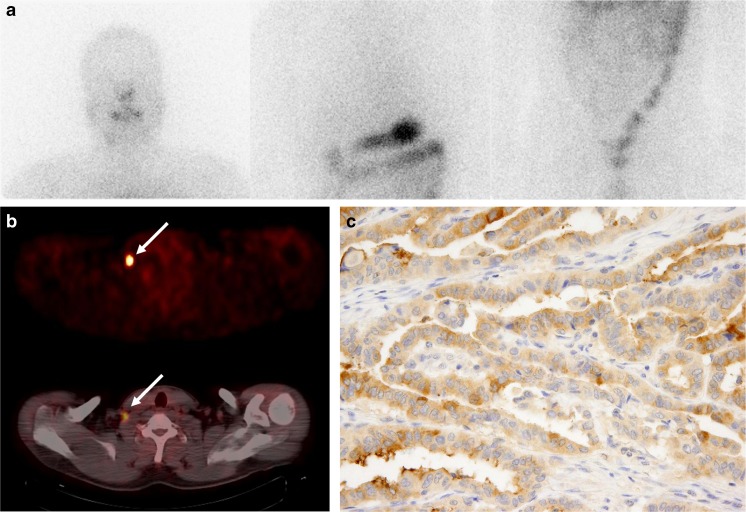
Fig. 1.
Anterior post-therapeutic 131I scan image (a) and 18F-FDG PET and fused PET/CT images (b) of a 42-year-old male patient with papillary thyroid cancer. 18F-FDG PET and PET/CT images (b) showing focal intense 18F-FDG uptake in the right lower neck lymph node (arrow), whereas the 131I scan image (a) shows no abnormal 131I uptake. Immunostaining for thyroglobulin (Tg) in the surgical specimen of the right lower neck node (c) showed weak Tg expression
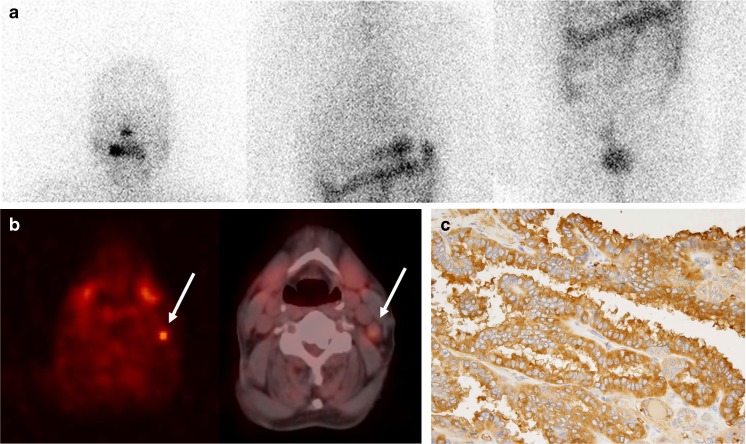
Fig. 2.
Anterior diagnostic 131I scan image (a) and 18F-FDG PET and fused PET/CT images (b) in a 70-year-old male patient with papillary thyroid cancer. 18F-FDG PET and PET/CT images (b) showing focal intense 18F-FDG uptake in a left neck lymph node (arrow), whereas the 131I scan image (a) shows no abnormal 131I uptake. Immunostaining for glucose-transporter 1 (Glut-1) in the surgical specimen of the left neck node (c) showed strong Glut-1 expression in cytoplasmic membranes with luminal accentuation
Relationship Between Radioiodine Scans and Immunohistochemistry
Relationships between radioiodine scan results and immunohistochemical results are shown in Table 4. The proportion of patients with positive radioiodine uptake in recurrent cervical nodes was found to be significantly related to NIS and SSTR2A expression (p = 0.04 for all; Fig. 3) and to be marginally related to SSTR1 expression (p = 0.06). Over 90 % of patients with weak NIS and SSTR1 expression and 100 % of patients with weak SSTR2 expression showed negative radioiodine uptake, but only 50–60 % of patients strongly expressing these markers showed positive radioiodine uptake. All three patients with weak Tg expression (staining intensity = 0 or 1) were negative for radioiodine uptake. In addition, no relationship was found between the cytoplasmic or membrane expressions of Glut-1 and the proportion of patients showing radioiodine uptake (p > 0.05).
Table 4.
Relationships between radioiodine scan (RI) and immunohistochemical results (n = 19)
| Biologic marker (staining intensities) | RI (+) | RI (-) | p-value |
|---|---|---|---|
| Thyroglobulin | |||
| 2, 3 (n = 16) | 6 (37 %) | 10 (63 %) | |
| 0, 1 (n = 3) | 0 (0 %) | 3 (100 %) | 0.5 |
| Glut-1 (Memb) | |||
| Positive (1, 2) (n = 12) | 4 (33 %) | 8 (67 %) | |
| Negative (0) (n = 7) | 2 (30 %) | 5 (70 %) | 1.0 |
| Glut-1 (Cyt) | |||
| 1 (n = 19) | 6 (32 %) | 13 (68 %) | |
| 2 (n = 0) | 0 | 0 | |
| NIS | |||
| H-score >50 (n = 8) | 5 (63 %) | 3 (37 %) | |
| H-score ≤50 (n = 11) | 1 (9 %) | 10 (91 %) | 0.04 |
| SSTR1 | |||
| H-score >100 (n = 9) | 5 (56 %) | 4 (44 %) | |
| H-score ≤100 (n = 10) | 1 (10 %) | 9 (90 %) | 0.06 |
| SSTR2A | |||
| H-score >50 (n = 12) | 6 (50 %) | 6 (50 %) | |
| H-score ≤50 (n = 7) | 0 (0 %) | 7 (100 %) | 0.04 |
Memb cytoplasmic membrane, Cyt cytoplasm, Glut-1 glucose transporter 1, NIS sodium-iodide symporter, SSTR somatostatin receptor
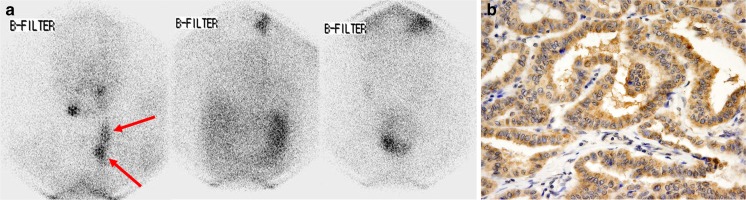
Fig. 3.
Anterior diagnostic 131I scan image (a) in a 27-year-old female patient with papillary thyroid cancer. 131I scan image (a) showing multifocal 131I uptake in the left neck area (arrow). The patient underwent left neck lymph node dissection. Immunostaining for sodium-iodide symporter (NIS) in the surgical specimen showed increased NIS expression with an H-score 100 (b)
Discussion
This study shows that all patients with weak Tg expression had positive 18F-FDG uptake or negative radioiodine uptake, and positive radioiodine uptake was found to be related to the expressions of NIS and SSTR2A, which suggests that the uptakes of 18F-FDG and radioiodine are associated with the differentiation of thyroid cancer cells. Furthermore, 18F-FDG uptake was found to be related to the luminal accentuation of Glut-1 expression rather than the membrane expression of Glut-1.
In the present study, all cervical nodal metastases with weak Tg expression showed positive 18F-FDG uptake or negative radioiodine uptake. It has been previously reported that the production and expression of Tg suggests a differentiated thyroid cancer phenotype and that anaplastic thyroid cancer shows significantly lower Tg expression than DTC [14, 15]. Papillary thyroid carcinomas can be classified as relatively well differentiated or as relatively less differentiated, and the relatively well-differentiated cancers show higher levels of NIS and Tg expression and a lower level of Glut-1 expression [16]. Accordingly, decreased Tg expression could imply an anaplastic change in papillary cancer. Thus, 18F-FDG PET rather than a radioiodine scan should be adopted to follow-up patients with weak Tg expression.
Glut-1 expression is known to be related to a poor prognosis in thyroid cancer and to be significantly elevated in papillary thyroid cancers without radioiodine uptake [17, 18]. Moreover, previous studies have shown that Glut-1 expression in DTC is more often cytoplasmic than membranous [17, 19]. In the present study, it was also found that all 46 patients exhibited positive cytoplasmic Glut-1 expression and that 70 % exhibited positive membranous Glut-1 expression. Membranous Glut-1 expression is most prominent around necrotic cancer areas, and hypoxia has been shown to result in the translocation of Glut-1 to the plasma membrane [19, 20]. It is reasonable to assume that membranous Glut-1 expression is more important and significant than its cytoplasmic expression when evaluating tumor aggressiveness [21]. In a previous study by Haber et al. [22], two Glut-1 staining patterns were described on the membranes of thyroid cancer cells: circumferential and asymmetric membranous staining (referred to as luminal accentuation in the present study). According to our results, membranous Glut-1 expression is not related to 18F-FDG uptake, but membranous Glut-1 expression with luminal accentuation was found to show a marginally significant relationship, which suggests that this pattern of Glut-1 positivity might play a significant role in 18F-FDG uptake in DTC.
SSTRs are often expressed in human endocrine tumors, such as parafollicular C cell-derived medullary thyroid carcinomas, but the expression of SSTR in DTC has rarely been reported and remains controversial. Pisarek et al. [23] reported that SSTR1 and SSTR2A were expressed in 89 and 44 % of DTC cases, respectively; however, Druckenthaner et al. [24] reported that SSTR2 was predominantly expressed in DTC. In the present study, the immunoexpression of SSTR1 was more intense than that of SSTR2. Furthermore, in patients with strong SSTR1 expression, only 50 and 60 % showed positive radioiodine uptake and 18F-FDG uptake, respectively, suggesting that a selective SSTR1-targeted imaging agent might be useful in patients with negative radioiodine uptake or 18F-FDG uptake. Currently most of the radiotracers available for SSTR imaging, such as 111In-DTPA-octreotide, 99mTc-depreotide and 68Ga-DOTATOC, target SSTR2 [9, 13, 25]; we also assessed the expression of SSTR2A, along with SSTR1, to determine whether a radiotracer for SSTR2 imaging could be used for the detection of recurrent PTC. The results of our study showed that the expression of SSTR2A was weaker than that of SSTR1 in enrolled patients, implying that the use of radiotracers for SSTR2 might not be suitable for PTC patients with recurrent neck lymph node metastasis. Furthermore, over 50 % of patients with a negative radioiodine scan (7 of 13 patients) had weak SSTR2A expression, suggesting that SSTR2A expression is correlated with the degree of differentiation in DTC. Radiotracers for SSTR2A would be of limited use for the imaging of less-differentiated DTC with negative radioiodine uptake.
Although NIS expression and Glut-1 expression with luminal accentuation were found to be related to 131I and 18F-FDG uptake, respectively, some patients showed negative radioiodine uptake with strong NIS expression, and other showed positive 18F-FDG uptake with negative or weak membranous Glut-1 expression without luminal accentuation. These findings could be the result of various factors that influence radioiodine uptake or 18F-FDG uptake. A previous study showed attenuated thyroperoxidase and pendrin expressions in cancers with no 131I uptake and suggested that low expressions of the genes involved in the radioiodine organification process might result in a short radioiodine retention time and negative findings for these cancers on radioiodine scans [18]. Glut-3 and hexokinase I could also be associated with 18F-FDG uptake in DTC. Glut-3 expression appears to predominate in more differentiated thyroid tumor cells, whereas Glut-1 overexpression appears to predominate in more de-differentiated thyroid cancers [10]. Additionally, it has also been reported that 18F-FDG uptake is associated with the overexpression of hexokinase I and that reciprocal staining patterns of NIS and hexokinase I are observed in thyroid cancer cells [19, 26].
The present study has several limitations. First, different types (diagnostic and post-therapeutic) of radioiodine scans were performed on the enrolled patients. Of the 19 patients who underwent a radioiodine scan, 26 % underwent diagnostic 131I scans, and previous studies have shown that diagnostic 131I scans have lower sensitivity for the detection of metastatic lesions than post-therapeutic 131I scans in patients with DTC [27, 28]. Second, only 11 patients underwent both 18F-FDG PET and radioiodine scans; thus, relationships between the immunohistochemical results and 18F-FDG PET and radioiodine scans were evaluated separately. Third, because the present study was performed retrospectively, a selection bias is inevitable. Furthermore, the exact location of the metastatic lymph node was not always clearly identified in the surgical reports; therefore, there might be some mismatches between imaging and histopathological findings. Lastly, because of insufficient cancer tissue for immunohistochemical staining in most of metastatic lymph nodes and poor resolution of 131I scans, a lesion-based analysis could not be performed.
In conclusion, the present study demonstrates that positive radioiodine uptake by recurrent neck nodes is related to the strong expressions of NIS and SSTR2A and that 18F-FDG uptake is associated with the luminal accentuation of membranous Glut-1 expression. Furthermore, all recurrent cervical nodal lesions with weak Tg expression showed positive 18F-FDG uptake or negative radioiodine uptake. The evaluations of the expressions of pathological markers in thyroid cancer cells can facilitate choices regarding optimal imaging and therapeutic modalities during the follow-up of thyroid cancer patients with recurrent cervical nodes.
Acknowledgments
This work was supported by the Korean Science and Engineering Foundation (KOSEF) through the Tumor Immunity Medical Research Center at Seoul National University College of Medicine. (20100028340)
Conflict of Interest
Jeong Won Lee, Hye Sook Min, Sang Mi Lee, Hyun Woo Kwon and June-Key Chung declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. The study design and exemption of informed consent were approved by the Institutional Review Board of Seoul National University Hospital.
References
- 1.Pelizzo MR, Merante BI, Toniato A, Pagetta C, Casal IE, Mian C, et al. Diagnosis, treatment, prognostic factors and long-term outcome in papillary thyroid carcinoma. Minerva Endocrinol. 2008;33:359–79. [PubMed] [Google Scholar]
- 2.Stokkel MP, Duchateau CS, Dragoiescu C. The value of FDG-PET in the follow-up of differentiated thyroid cancer: a review of the literature. Q J Nucl Med Mol Imag. 2006;50:78–87. [PubMed] [Google Scholar]
- 3.Arturi F, Russo D, Giuffrida D, Schlumberger M, Filetti S. Sodium-iodide symporter (NIS) gene expression in lymph-node metastases of papillary thyroid carcinomas. Eur J Endocrinol. 2000;143:623–7. doi: 10.1530/eje.0.1430623. [DOI] [PubMed] [Google Scholar]
- 4.Arturi F, Russo D, Schlumberger M, du Villard JA, Caillou B, Vigneri P, et al. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab. 1998;83:2493–6. doi: 10.1210/jcem.83.7.4974. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Baudin E. Serum thyroglobulin determination in the follow-up of patients with differentiated thyroid carcinoma. Eur J Endocrinol. 1998;138:249–52. doi: 10.1530/eje.0.1380249. [DOI] [PubMed] [Google Scholar]
- 6.Wu LM, Gu HY, Qu XH, Zheng J, Zhang W, Yin Y, et al. The accuracy of ultrasonography in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: a meta-analysis. Eur J Radiol. 2012;81:1798–805. doi: 10.1016/j.ejrad.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39:191–6. doi: 10.1016/j.ejso.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 8.Miller ME, Chen Q, Elashoff D, Abemayor E, St JM. Positron emission tomography and positron emission tomography-CT evaluation for recurrent papillary thyroid carcinoma: meta-analysis and literature review. Head Neck. 2010;33:562–5. doi: 10.1002/hed.21492. [DOI] [PubMed] [Google Scholar]
- 9.Middendorp M, Selkinski I, Happel C, Kranert WT, Grunwald F. Comparison of positron emission tomography with [(18)F]FDG and [(68)Ga]DOTATOC in recurrent differentiated thyroid cancer: preliminary data. Q J Nucl Med Mol Imag. 2010;54:76–83. [PubMed] [Google Scholar]
- 10.Ciampi R, Vivaldi A, Romei C, Del Guerra A, Salvadori P, Cosci B. Expression analysis of facilitative glucose transporters (GLUTs) in human thyroid carcinoma cell lines and primary tumors. Mol Cell Endocrinol. 2008;291:57–62. doi: 10.1016/j.mce.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Usuda K, Sagawa M, Aikawa H, Ueno M, Tanaka M, Machida Y, et al. Correlation between glucose transporter-1 expression and 18F-fluoro-2-deoxyglucose uptake on positron emission tomography in lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:405–10. doi: 10.1007/s11748-010-0603-1. [DOI] [PubMed] [Google Scholar]
- 12.Hiyoshi Y, Watanabe M, Imamura Y, Nagai Y, Baba Y, Yoshida N, et al. The relationship between the glucose transporter type 1 expression and F-fluorodeoxyglucose uptake in esophageal squamous cell carcinoma. Oncology. 2009;76:286–92. doi: 10.1159/000207505. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues M, Li S, Gabriel M, Heute D, Greifeneder M, Virgolini I. 99mTc-depreotide scintigraphy versus 18F-FDG-PET in the diagnosis of radioiodine-negative thyroid cancer. J Clin Endocrinol Metab. 2006;91:3997–4000. doi: 10.1210/jc.2006-0825. [DOI] [PubMed] [Google Scholar]
- 14.Ordonez NG, El-Naggar AK, Hickey RC, Samaan NA. Anaplastic thyroid carcinoma. immunocytochemical study of 32 cases. Am J Clin Pathol. 1991;96:15–24. doi: 10.1093/ajcp/96.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Wiseman SM, Griffith OL, Deen S, Rajput A, Masoudi H, Gilks B, et al. Identification of molecular markers altered during transformation of differentiated into anaplastic thyroid carcinoma. Arch Surg. 2007;142:717–27. doi: 10.1001/archsurg.142.8.717. [DOI] [PubMed] [Google Scholar]
- 16.Chung JKYH, Kang JH, Lee HY, Kang KW. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl Med Mol Imaging. 2010;44:4–14. doi: 10.1007/s13139-009-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonberger J, Ruschoff J, Grimm D, Marienhagen J, Rummele P, Meyringer R, et al. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: an immunohistochemical study. Thyroid. 2002;12:747–54. doi: 10.1089/105072502760339307. [DOI] [PubMed] [Google Scholar]
- 18.Mian C, Barollo S, Pennelli G, Pavan N, Rugge M, Pelizzo MR, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 2008;68:108–16. doi: 10.1111/j.1365-2265.2007.03008.x. [DOI] [PubMed] [Google Scholar]
- 19.Hooft L, van der Veldt AA, van Diest PJ, Hoekstra OS, Berkhof J, Teule GJ. [18F]fluorodeoxyglucose uptake in recurrent thyroid cancer is related to hexokinase i expression in the primary tumor. J Clin Endocrinol Metab. 2005;90:328–34. doi: 10.1210/jc.2004-0779. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JZ, Behrooz A, Ismail-Beigi F. Regulation of glucose transport by hypoxia. Am J Kidney Dis. 1999;34:189–202. doi: 10.1016/S0272-6386(99)70131-9. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda M, Ogane N, Hayashi H, Kameda Y, Miyagi Y, Iida T, et al. Glucose transporter-1 expression in the thyroid gland: clinicopathological significance for papillary carcinoma. Oncol Rep. 2005;14:1499–504. doi: 10.3892/or.14.6.1499. [DOI] [PubMed] [Google Scholar]
- 22.Haber RS, Weiser KR, Pritsker A, Reder I, Burstein DE. GLUT1 glucose transporter expression in benign and malignant thyroid nodules. Thyroid. 1997;7:363–7. doi: 10.1089/thy.1997.7.363. [DOI] [PubMed] [Google Scholar]
- 23.Pisarek H, Stepien T, Kubiak R, Borkowska E, Pawlikowski M. Expression of somatostatin receptor subtypes in human thyroid tumors: the immunohistochemical and molecular biology (RT-PCR) investigation. Thyroid Res. 2009;2:1. doi: 10.1186/1756-6614-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Druckenthaner M, Schwarzer C, Ensinger C, Gabriel M, Prommegger R, Riccabona G, et al. Evidence for Somatostatin receptor 2 in thyroid tissue. Regul Pept. 2007;138:32–9. doi: 10.1016/j.regpep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Kurdziel K, Ravizzini G, Croft B, Tatum J, Choyke P, Kobayashi H. The evolving role of nuclear molecular imaging in cancer. Expert Opin Med Diagn. 2008;2:829–42. doi: 10.1517/17530059.2.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung YH, Hah JH, Sung MW, Kim KH, Cho SY, Jeon YK. Reciprocal immunohistochemical expression of sodium/iodide symporter and hexokinase I in primary thyroid tumors with synchronous cervical metastasis. Laryngoscope. 2009;119:541–8. doi: 10.1002/lary.20073. [DOI] [PubMed] [Google Scholar]
- 27.Pacini F, Lippi F, Formica N, Elisei R, Anelli S, Ceccarelli C, et al. Therapeutic doses of iodine-131 reveal undiagnosed metastases in thyroid cancer patients with detectable serum thyroglobulin levels. J Nucl Med. 1987;28:1888–91. [PubMed] [Google Scholar]
- 28.Iwano S, Kato K, Nihashi T, Ito S, Tachi Y, Naganawa S. Comparisons of I-123 diagnostic and I-131 post-treatment scans for detecting residual thyroid tissue and metastases of differentiated thyroid cancer. Ann Nucl Med. 2009;23:777–82. doi: 10.1007/s12149-009-0303-z. [DOI] [PubMed] [Google Scholar]