Abstract
Radium-223 dichloride is an alpha-emitting radiopharmaceutical shown to prolong survival in patients with castrate-resistant prostate cancer (CRPC) and symptomatic skeletal metastases. This report describes in two patients the acute changes in bone metastatic activity detected by F-18 choline PET/CT imaging midway during treatment with radium-223 dichloride. In addition to visual and standardized uptake value analysis, changes in the whole-body tumor burden were quantified by measuring the difference in net metabolically active tumor volume (MATV) and total lesion activity (TLA) between pre- and mid-treatment PET scans. After the third dose of radium-223 dichloride, near-total disappearance of abnormal skeletal activity was observed in one case (net MATV change from 260.7 to 0.8 cc; net TLA change from 510.7 to 2.1), while a heterogeneous tumor response was observed in the other (net MATV change from 272.2 to 241.3 cc; net TLA change from 987.1 to 779.4). Corresponding normalization and persistent elevation in serum alkaline phosphatase levels were observed in these cases, respectively. Further research is needed to determine the predictive value of serial F-18 choline PET/CT imaging in patients receiving radium-223 dichloride for CRPC.
Keywords: Fluorocholine, PET/CT, Castrate-resistant prostate cancer, Radium-223 dichloride
Introduction
Radium-223 dichloride (223Ra) is an alpha-particle-emitting radiotherapeutic drug recently approved in the USA for treatment of castrate-resistant prostate cancer (CRPC) in patients with symptomatic bone metastases and no known visceral metastases. This calcium-mimetic radiopharmaceutical forms complexes with hydroxyapatite in areas of increased bone turnover such as skeletal metastases. The short path length and high energy transfer of the emitted alpha particles cause a localized anti-tumor effect mediated by double-strand DNA breaks. 223Ra was shown to prolong overall survival in patients with CRPC in a double-blind, randomized, multicenter clinical trial [1]. Currently, there is no diagnostic test known to reliably measure or predict the therapeutic response to 223Ra.
Conventional PET/CT using 18F-fluorodeoxglucose (FDG) has demonstrated poor sensitivity for the detection of prostate cancer [2]. A more recently developed PET tracer, 18F-fluoromethylcholine (18 F-choline), allows PET to measure tissue phospholipid membrane synthesis, a metabolic process distinctly different from glucose metabolism that is upregulated in a variety of cancers including prostate cancer [3]. In direct comparisons, 18F-choline has been shown to be superior to FDG for detecting both hormone-naïve and castrate-resistant prostate cancer [4].
We report the finding of acute changes in skeletal tumor activity on 18F-choline PET/CT in two patients undergoing treatment for CRPC with 223Ra. In addition to visual and standardized uptake value (SUV) assessments of the PET images, we objectively measured the volume of metastatic activity detected on PET by quantifying the net metabolically active tumor volume (MATV) and total lesion activity (TLA). Recently, this PET approach to measuring the whole-body tumor burden was applied as a potential prognostic index in a study of 18F-choline PET/CT in CRPC[5]. Since 18F-choline is currently not approved for clinical use in the USA, both patients gave written informed consent to undergo 18F-choline PET/CT as an investigational imaging procedure.
Case Report
Patient 1
This is a 63-year-old male diagnosed with Gleason 7 prostate adenocarcinoma. Upon diagnosis, he was treated with definitive external beam radiation therapy to the prostate and adjuvant goserelin. Within 2 years of diagnosis, his PSA level increased to 23.7 ng/ml, prompting treatment by chemical castration and subsequently antiandrogen therapy with bicalutamide. After 2 years of intermittent antiandrogen therapy, the PSA level increased to 363 ng/ml. At this point, a diagnosis of metastatic CRPC was made, and bone scintigraphy confirmed metastatic disease in the vertebral column, pelvis, and both upper and lower extremity long bones. Subsequent treatments for CRPC included docetaxel, cabazitaxel, abiraterone, and enzalutamide. At the time of presentation for 223Ra treatment, the PSA level was 2524 ng/ml, and morphine was required for control of significant bone pain.
Whole-body 18F-choline PET/CT and tumor burden quantitation were performed in accordance with a recent study [5]. The pretreatment scan performed 2 days before starting 223Ra therapy demonstrated increased focal activity in multiple thoracic and lumbar vertebrae as well as both humeri, scapulae, and femurs (Fig. 1a–c). The net MATV was 272.2 cm3. The net TLA was 987.1. Consistent with active bone remodeling, the pretreatment total alkaline phosphatase (ALP) level was elevated at 328 U/l, with an isoenzyme composition of 90 % bone, 7 % liver, and 3 % intestinal enzymes.
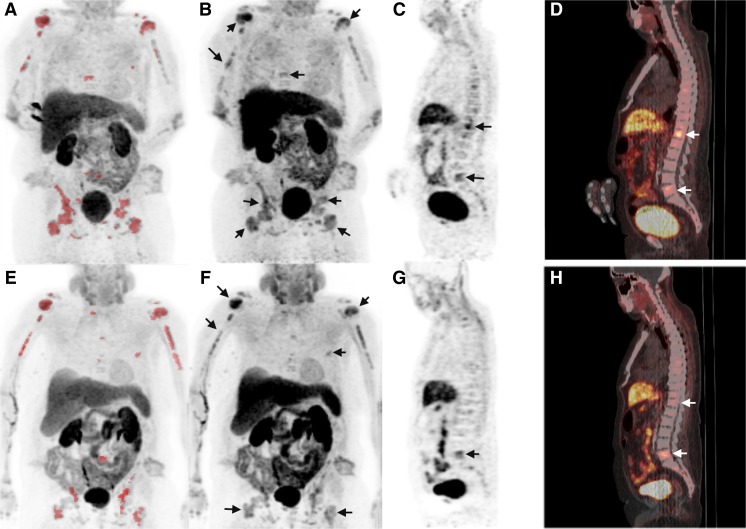
Fig. 1.
Case report 1 illustrating heterogeneous PET response to 223Ra. Panels a and e: Maximum intensity pixel projection (MIP) images depicting the overall tumor burden on 18F-choline PET before treatment with 223Ra (a) and at 13 weeks after the start of treatment (e). Red-colored regions on these MATV MIP images were defined by a 40 % volume-of-interest threshold of the tumor SUVmax in areas of skeletal activity exceeding 2 standard deviations above normal marrow. Thus, the color on this image does not reflect the intensity of tracer uptake but rather shows the voxels included in the net MATV calculation. Notice that the MATV dimensions in the thoracic spine, pelvis, and femurs decrease significantly between the two scans, while the MATV dimensions in the humeral heads and lumbar spine are essentially unchanged. Panels b and f: Corresponding conventional 18F-choline PET MIP images show heterogeneous changes in bone lesion uptake between the pre- (b) and mid-treatment (f) scans with persistently high uptake in the humeral bone lesions and heterogeneously diminishing uptake in the pelvis and femurs (arrows). Panels c, d, g, and h: Examples of sagittal PET and PET/CT images from the pre-treatment (c and d) and mid-treatment (g and h) scans show an interval decrease in 18F-choline uptake in the T12 vertebra (upper arrow) and persistent increased uptake in the L5 vertebra (lower arrow). These findings support speculation that the tumor response to 223Ra was heterogeneous
Another whole-body 18F-choline PET/CT scan was performed following the third administration of 223Ra (at 13 weeks). Visually, this subsequent PET scan demonstrated decreasing tumor activity in the thoracic spine, pelvis, and femurs and persistently increased activity in the humeri and lumbar spine (Fig. 1e–g). Corresponding to Fig. 1d and h, the maximum SUV (SUVmax) of a T12 vertebral lesion (arrowheads) decreased from 6.8 to 2.9, while the SUVmax of a L5 vertebral lesion (arrows) remained relatively unchanged from 5.2 to 4.8. The most active metastatic site was situated in the right humerus, demonstrating a persistent SUVmax of 7.1 on both scans. From baseline, the net MATV decreased by 11 % to 241.3 cm3. The net TLA decreased by 21 % to 779.4. The corresponding total ALP level at 13 weeks was 187 U/l (a 43 % decrease), with 83 % bone, 15 % liver, and 2 % intestinal isoenzyme composition. The corresponding PSA level decreased by 8 % to 2,313 ng/ml.
Patient 2
This is a 76-year-old male diagnosed remotely with Gleason 9 prostate adenocarcinoma. He received primary treatment with radical prostatectomy followed by adjuvant radiation therapy. Biochemical recurrence had been noted 3 years before, prompting systemic treatments with leuprolide, bicalutamide, and goserelin. The patient was subsequently diagnosed with CRPC after his PSA level had risen to 41.5 ng/ml despite complete androgen blockade. Bone scintigraphy at that time confirmed metastatic disease in the axial skeleton and proximal left femur. Subsequent treatments included sipuleucel-T, denosumab, and degarelix. The patient also received palliative external beam radiation to the lumbar spine approximately 14 months prior to 223Ra therapy. After radiation therapy, there was bone scintigraphic evidence of metastatic progression, leading to treatment with abiraterone. Progressively worsening bone pain, particularly in the back and left shoulder, prompted treatment with 223Ra.
Whole-body 18F-choline PET/CT performed 2 days before 223Ra treatment demonstrated multiple areas of increased activity in the thoracic spine and pelvic bones (Fig. 2a and c). There was also focal increased activity in the left femur, bilateral humeri, and clavicles. Of note, these metastatic lesions showed significant uptake on the baseline scan despite ongoing treatment with abiraterone. The net MATV was 260.7 cm3. The net TLA was 510.7. The corresponding PSA level was 25.0 ng/ml, and the ALP level was mildly elevated at 160 U/l, with an isoenzyme composition of 77 % bone, 20 % liver, and 3 % intestinal enzymes.
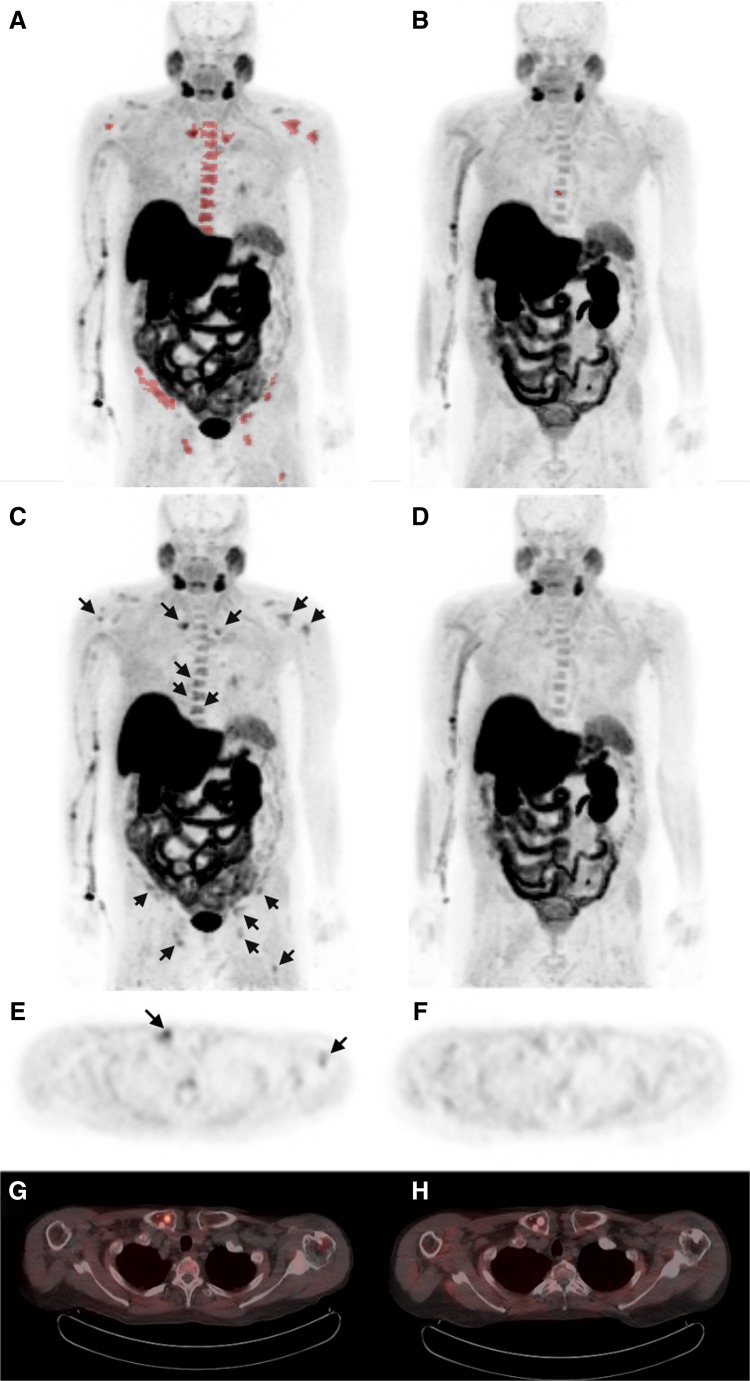
Fig. 2.
Case report 2 illustrating the near-total PET response to 223Ra. Panels a and b: MIP images depicting the overall tumor burden on 18F-choline PET performed before treatment (a) and at 13 weeks after starting treatment (b). Tumor activity identified on the pre-treatment scan (demarcated as in Fig. 1 by automated MATV segmentation and colored red) has been nearly eliminated at the mid-point of 223Ra therapy. Panels c and d: Corresponding conventional 18F-choline PET MIP images showing near complete resolution of focally increased uptake in the axial skeleton and long bones (arrows). Some mild diffuse thoracic spine activity is noticeable on the mid-treatment scan (d). This activity did not meet the threshold of MATV segmentation and could reflect either reactive bone marrow or attenuated metastatic disease. Panels e–h: Example of axial PET images from the pre-treatment (e) and mid-treatment (f) scans show resolution of abnormal 18F-choline uptake in the right clavicle and left humerus (arrows). PET/CT images from the pre-treatment (g) and mid-treatment (h) scans show corresponding increased bone sclerosis suggestive of a treatment effect over this interval
After the third administration of 223Ra, PET revealed a significant decrease in 18F-choline uptake in all areas noticed as having abnormal uptake on the baseline scan. The net MATV decreased to 0.8 cm3. The net TLA decreased to 2.1. The only lesion identified by MATV threshholding on this second scan was a portion of the T7 vertebra (Fig. 2b and d). It demonstrated a pretreatment SUVmax of 3.7, which decreased to 2.8 on subsequent scanning. The most active lesion on the pre-treatment scan was localized to the right clavical (Fig. 2e and g). It demonstrated a decrease in SUVmax from 4.8 to 2.4 (Fig. 2f and h). The corresponding PSA level decreased to 2.8 ng/ml. The total alkaline phosphatase level also decreased to 50 U/l (a 69 % reduction to within normal range), with isoenzyme makeup of 73 % bone, 27 % liver, and 3 % intestinal.
Discussion
Bone metastases are a prime factor contributing to CRPC morbidity and mortality. One problem in treating CRPC is the lack of reliable methods for assessing skeletal treatment response in real time. Indeed, bone lesions are generally considered non-measurable by the Response Evaluation Criteria in Solid Tumors (RECIST) [6]. This real difficulty in assessing skeletal tumor response in CRPC not only hampers clinical practice, but also impedes drug development. An imaging method capable of assessing tumor response in a timely fashion may not only speed the discovery of new treatments for CRPC, but also provide potentially predictive information that could help tailor therapy for individual patients.
The standard regimen for 223Ra is every 4 weeks for six cycles. This is the first report to describe the detection of acute changes in tumor metabolic activity by 18F-choline PET/CT early in the course of treatment with 223Ra. In addition to the significant changes observed on PET after three cycles, a significant 12-week ALP response was noted in both patients. In the 223Ra phase 3 trial, a total ALP response (≥30 % decline or >50 % reduction from baseline) was observed more frequently in the 223Ra treated arm versus placebo [1]. While there is evidence suggesting that bone ALP is a tumor response marker in CRPC [7], it is not possible to form generalizable inferences from the current case reports. However, the patient demonstrating near resolution of abnormal PET activity also had normalization of his ALP level, while the patient demonstrating heterogeneous changes on PET continued to have significantly elevated levels of both ALP and PSA. Thus, 18F-choline PET/CT could potentially complement other biomarkers by annotating the specific anatomic sites of disease activity.
While skeletal tumor activity decreased significantly in case 2, some noticeable diffuse mild increased marrow activity remained on the mid-treatment 18F-choline PET/CT (Fig. 2d). The majority of this activity was below the MATV segmentation threshold (Fig. 2b). Since 223Ra is a bone-seeking radiopharmaceutical, hematologic side effects leading to a reactive bone marrow response could account for this increased marrow uptake of 18F-choline [8]. Alternatively, some residual uptake could represent attenuated metastases. Further research is needed to better understand the effects of 223Ra on bone marrow physiology. It is also worth noting that in case 2, the pre-treatment PET demonstrated significant abnormalities despite ongoing treatment with abiraterone. Therefore, trials investigating the potential of synergy among treatments for CRPC may benefit from the use of 18F-choline PET/CT to discern areas of resistant disease over the course of different treatments.
The clinical value of measuring treatment-related changes in tumor activity on 18F-choline PET/CT is not known at this time. Through these case reports, we demonstrate the feasibility of globally quantifying the changes on 18F-choline PET/CT by applying serial MATV measurements. In another study, this approach to whole-body PET quantitation was applied successfully to produce tumor burden indices that correlated significantly with overall survival in patients with CRPC[5]. The current report builds on that approach by illustrating its potential application as a treatment biomarker. Given that dramatic changes in tumor 18F-choline uptake can be detected early in the course of 223Ra therapy, such an approach to quantifying tumor response could potentially give useful information to help make real-time adjustments to treatment, particularly in scenarios involving combination therapy (e.g., 223Ra plus antiandrogen) or sequenced therapies.
Conclusion
These case reports illustrate the potential use of 18F-choline PET/CT to monitor the effects of 223Ra on skeletal tumor metabolic activity. Acute changes in skeletal 18F-choline uptake (which included nearly complete disappearance of abnormal tumor uptake in one case) were noted early in the course of 223Ra treatment. Clinical outcome studies in a sufficient number of patients will be needed to ascertain the therapeutic predictive value of such measurements.
Acknowledgments
Disclosure
Kyle S. Miyazaki, Yu Kuang, and Sandi A. Kwee declare that they have no conflicts of interest. All study procedures were in accordance with an institutional review board-approved human subject research protocol that complies with the ethical standards set forth in the 1964 Declaration of Helsinki and all subsequent amendments thereof. Written informed consent and authorization for the use and reporting of de-identified clinical information were obtained from the patients included in this study.
References
- 1.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 2.Schoder H, Herrmann K, Gonen M, Hricak H, Eberhard S, Scardino P, et al. 2-[18 F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res: Off J Am Assoc Cancer Res. 2005;11(13):4761–9. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 3.Glunde K, Jacobs MA, Bhujwalla ZM. Choline metabolism in cancer: implications for diagnosis and therapy. Expert Rev Mol Diagn. 2006;6(6):821–9. doi: 10.1586/14737159.6.6.821. [DOI] [PubMed] [Google Scholar]
- 4.Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168(1):273–80. doi: 10.1016/S0022-5347(05)64906-3. [DOI] [PubMed] [Google Scholar]
- 5.Kwee SA, Lim J, Watanabe A, Kromer-Baker K, Coel MN. Prognosis related to metastatic burden measured by 18 F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med: Off Publ Soc Nucl Med. 2014;55(6):905–10. doi: 10.2967/jnumed.113.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30(5):607–13. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Balogova S, Huchet V, Egrot C, Michaud L, Paycha F, Kerrou K, et al. Effect of erythropoietin on bone marrow uptake of 18 F-fluorocholine in prostate cancer: comparison with 18 F-fluoride uptake. Clin Nucl Med. 2013;38(3):200–2. doi: 10.1097/RLU.0b013e31827a2294. [DOI] [PubMed] [Google Scholar]