Abstract
Purpose
To evaluate the diagnostic performance of 68Ga-DOTATATE 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT), 18F-FDG PET/CT and 131I-MIBG scintigraphy in the mapping of metastatic pheochromocytoma and paraganglioma.
Materials and Methods
Seventeen patients (male = 8, female = 9; age range, 13–68 years) with clinically proven or suspicious metastatic pheochromocytoma or paraganglioma were included in this prospective study. Twelve patients underwent all three modalities, whereas five patients underwent 68Ga-DOTATATE and 131I-MIBG without 18F-FDG. A composite reference standard derived from anatomical and functional imaging findings, along with histopathological information, was used to validate the findings. Results were analysed on a per-patient and on per-lesion basis. Sensitivity and accuracy were assessed using McNemar’s test.
Results
On a per-patient basis, 14/17 patients were detected in 68Ga-DOTATATE, 7/17 patients in 131I-MIBG, and 10/12 patients in 18F-FDG. The sensitivity and accuracy of 68Ga-DOTATATE, 131I-MIBG and 18F-FDG were (93.3 %, 94.1 %), (46.7 %, 52.9 %) and (90.9 %, 91.7 %) respectively. On a per-lesion basis, an overall of 472 positive lesions were detected; of which 432/472 were identified by 68Ga-DOTATATE, 74/472 by 131I-MIBG, and 154/300 (patient, n = 12) by 18F-FDG. The sensitivity and accuracy of 68Ga-DOTATATE, 131I-MIBG and 18F-FDG were (91.5 %, 92.6 % p < 0.0001), (15.7 %, 26.0 % p < 0.0001) and (51.3 %, 57.8 % p < 0.0001) respectively. Discordant lesions were demonstrated on 68Ga-DOTATATE, 131I-MIBG and 18F-FDG.
Conclusions
Ga-DOTATATE PET/CT shows high diagnostic accuracy than 131I-MIBG scintigraphy and 18F-FDG PET/ CT in mapping metastatic pheochromocytoma and paraganglioma.
Keywords: 68Ga-DOTATATE, 18F-FDG, 131I-MIBG, PET/CT, Pheochromocytoma, Paraganglioma
Introduction
Pheochromocytoma (PCC) and paraganglioma (PGL) are rare chromaffin cell tumours that synthesise, but not always secrete catecholamines and their metabolites. Contrary to traditional belief that only 10 % of these tumours metastasise to non-chromaffin sites, such as lung, bone, liver or lymph nodes, higher prevalences of 26 and 35 % has been recently observed in malignant PCC and PGL respectively [1, 2].
Despite the recent discovery of certain germline mutations, such as succinate dehydrogenase subunit B (SDHB) in an aggressive tumour [3, 4], at present there is no single marker that can objectively distinguish between benign and malignant PCC/PGL [5–8]. The diagnosis of malignancy is solely based on the presence of distant metastasis. Hence, imaging techniques play a pivotal role in tumour localisation and mapping. In addition, surveillance imaging has often been incorporated as part of a monitoring tool in non-functional tumours.
Although anatomical imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) is often the first-line imaging modality [10], functional imaging is commonly needed to clarify equivocal lesions detected by anatomical imaging. Furthermore, functional imaging has been primarily recommended to exclude the possibility of multifocal or occult metastatic disease during initial workup in high risk groups, especially in young patients <40 years old and patients with adrenal PCC of >5 cm in size [9, 10].
Even though 123/131I-metaiodobenzylguanidine (123/131I-MIBG) scintigraphy is an excellent functional imaging tool in adrenal PCC, it has demonstrated low sensitivity in extra-adrenal PGL and metastatic PCC/PGL [11–13]. In recent years, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has been suggested as a preferred functional imaging tool in malignant PCC/PGL, particularly in SDH and von Hippel-Lindau (VHL) germline mutation tumours or when de-differentiated tumour is suspected [15–17].
The discovery of somatostatin receptors (SSTR) expression, especially subtypes 2A and 3, in PCC/PGL has promoted the use of 111In-octreotide or 99mTc-HYNIC-TOC scintigraphy to locate these tumours [18–21]. Despite the advancement of single-photon emission computed tomography (SPECT)/CT imaging, PET/CT imaging using 68Ga-DOTA-peptides (68Ga-DOTA-TOC, 68Ga-DOTA-NOC and 68Ga-DOTA-TATE) as radiotracers continues to provide better image quality [22].
Although the diagnostic yield of 68Ga-DOTA-peptides PET/CT in the detection of PCC/ PGL has been evaluated in several studies [23–26], its promising role in these tumours is still not largely accepted. 68Ga-DOTA-peptides are still considered as experimental tracers in recent guidelines [27]. Hence, the aim of this study was to evaluate the diagnostic performance of 68Ga-DOTA-TATE PET/CT in comparison to 131I-MIBG scintigraphy and 18F-FDG PET/CT in the mapping of these diseases. 123I-MIBG is not available in our centre.
Materials and Methods
Patients
Seventeen consecutive patients (males:females = 8:9; aged 13–68 years, median age 40 years) with histopathologically proved PCC/PGL followed-up in Putrajaya Hospital from December 2011 to June 2013 were recruited in this prospective study. Inclusion criteria were as follows: (1) histologically proven metastatic disease for further restaging; (2) suspicion of metastasis due to (a) suspicious metastatic lesion(s) on CT, MRI or 18F-FDG PET/CT, (b) primary adrenal mass >5 cm, (c) raised 24-h urine catecholamine, or (d) persistent uncontrolled blood pressure.
All patients were subjected to both 68Ga-DOTATATE PET/CT at the National Cancer Institute and 131I-MIBG scintigraphy in Kuala Lumpur Hospital. No therapeutic interventions between two examinations. The interval examination between studies was within 3 months, except for one patient with the interval of 23 months. This particular patient had shown negative finding on three consecutive 131I-MIBG imagings but was found to have extensive metastatic disease on 18F-FDG PET/CT. A repeat 131I-MIBG study was ethically unjustifiable.
Preceding 131I-MIBG and 68Ga-DOTATATE examinations, 18F-FDG PET/CT had been performed on 12 patients, with 4 patients having additional CT. Of the remaining five patients without prior 18F-FDG PET/CT, two patients had CT, whereas three patients had both CT and MRI. The median interval between the 18F-FDG PET/CT and the 131I-MIBG/68Ga-DOTATATE study was 4 months.
The study protocol was approved by the local ethics committee, and written informed consent was obtained from all patients.
131I-MIBG Imaging Protocol
To minimise 131I uptake in the thyroid gland, 1 ml Lugol’s iodine 3 times per day were administered orally 3 days prior to injection of 131I-MIBG and continued 3 more days after injection. A dose of 0.015 mCi/kg (range, 0.5–1.2 mCi) of 131I-MIBG was injected intravenously. Whole-body (from head to toes) planar images with spot views of head, chest, abdomen and pelvis were obtained 48 h, 72 h, and 96 h after injection of 131I-MIBG, using a dual-head camera with a high-energy parallel-hole collimator (ECAM; Siemens, Erlangen, Germany) with the patient supine. In view of lower administered dose, static images were acquired for total counts of 1,200 k per view with a matrix size of 256 × 256.
68Ga-DOTATATE PET/CT Imaging Protocol
PET-CT imaging was performed on the dedicated GE Discovery ST scanner (GE Healthcare, Chalfont St Giles, Bucks., UK), combining a PET unit and an eight-slice CT unit. No specific patient preparation was instructed prior to this examination. Patients were administered 148–185 MBq (4–5 mCi) of 68Ga-DOTATATE intravenously. Image acquisition was performed 45–60 min post-injection with a whole-body field of view (vertex to mid thigh).
Transmission CT images for attenuation correction were performed with the exposure factors for all examinations of 120 kVp and 80 mA in 0.8 s. Emission PET images were obtained in two-dimensional mode, at a rate of 4 min per bed position with a three-slice overlap between consecutive bed positions. Transaxial PET data were reconstructed using filtered backprojection. The CT data for PET were reconstructed to axial slices of 3.3-mm thick.
18F-FDG PET/CT Imaging Protocol
Patients were fasted for at least 4 h prior to examination. Upon admission, the patient’s body weight was taken and blood glucose level was recorded. An activity of 6 MBq/kg (range, 285–460 MBq) of 18F-FDG was administered intravenously. PET-CT imaging was performed on the dedicated GE Discovery ST scanner (GE Healthcare), combining a PET unit and an eight-slice CT unit. Image acquisition was performed 45–60 min post-injection with a whole-body field of view (vertex to mid-thigh).
Transmission CT images for attenuation correction were performed with the exposure factors for all examinations of 120 kVp and 80 mA in 0.8 s. No intravenous CT contrast was administered. Emission PET images were obtained in two-dimensional mode, at a rate of 4 min per bed position with a three-slice overlap between consecutive bed positions. Transaxial PET data were reconstructed using filtered backprojection. The CT data for PET were reconstructed to axial slices of 3.3 mm thick.
Image Interpretation
All 68Ga-DOTATATE and 18F-FDG PET/CT images were reviewed on a GE Advantage 4.2 workstation (GE Healthcare), whereas 131I-MIBG scintigraphy images were reviewed on an ESOFT Syngo workstation (Siemens Healthcare) by two experienced nuclear medicine physicians. CT and MRI images were re-evaluated on a Mac-based OsiriX workstation by an experienced radiologist. The site and number of lesions were evaluated.
Positive uptakes on PET findings were based on visual assessment and maximum standardised uptake values (SUVmax) with correction for body weight. 131I-MIBG uptake in the adrenals was considered to be normal if it was mild, symmetrical and not enlarged. Positive findings on CT and MRI were based on specific appearance of malignant disease.
Statistical Analysis
Out of the 17 patients, 12 patients underwent all three modalities, whereas 5 patients underwent 68Ga-DOTATATE and 131I-MIBG without 18F-FDG. Therefore, analysis on 131I-MIBG scintigraphy and 68Ga-DOTATATE PET/CT was based on a total of 17 patients, whereas analysis on 18F-FDG PET-CT was evaluated in 12 patients by excluding patients with no 18F-FDG PET-CT study.
Histopathological confirmation is used as the “gold standard” for most of the diagnostic studies. In the presence of extensive metastases, where biopsy or surgical excision of all lesions or previous surgical sites was not feasible, a composite reference standard was adopted to validate the findings by incorporating both anatomical and functional imaging information.
Analysis was assessed on a per-patient and on per-lesion basis. In the per-lesion analysis, lesion was considered true positive when (1) there were positive histopathological results or (2) there was an unequivocal focus of abnormally increased tracer uptake detected by either 18F-FDG PET/CT, 68Ga-DOTATATE PET/CT or 131I-MIBG imaging in a non-physiological site, which was confidently judged as disease by the reporting physicians. A true-negative lesion was considered when no uptake was seen by either 18F-FDG PET/CT, 68Ga-DOTATATE PET/CT or 131I-MIBG imaging in a lesion detected by contrasted/non-contrasted CT or MRI. Lesions that were judged insufficient to establish the diagnosis (equivocal focus) were excluded from the analysis. In the per-patient analysis, metastatic disease was considered positive when at least one positive lesion per patient was detected.
With regard to histopathological correlation, all patients had undergone primary tumour resection with histopathological confirmation. In 13 out of 17 patients, apart from primary removal, 5 patients had undergone three or more metasectomies, whereas 6 patients had two matasectomies and 2 patients had one metasectomy. Out of the four patients without histopathogical confirmation of metastasis, two patients were found to have all negative scans, whereas two patients showed extensive metastases, where biopsy or metasectomy in these two patients was technically impossible and not justified.
SPSS Software (version 19.0) was used to perform statistical analysis. Sensitivity and accuracy were calculated, and significance between the values determined using McNemar’s test. A p value of less than 0.05 was considered statistical significant.
Results
Baseline Characteristics
The distribution of patients’ characteristics is summarised in Table 1. Based on World Health Organisation (WHO) criteria [3], ten patients were diagnosed as PCC and seven patients as PGL. Prior to the study, 76 % (13/17) of patients had the metastatic status confirmed by CT, MRI or metasectomy. In the remaining patients, suspicion of metastasis was based on either equivocal lesion(s) on contrasted CT or MRI, primary adrenal mass of >5 cm in size or uncontrolled high blood pressure on more than two anti-hypertensive medications.
Table 1.
Baseline distribution of sociodemographic and subjects’ characteristics
| Characteristic | Number, n (%) | |
|---|---|---|
| Gender | Female Male |
9 (53 %) 8 (47 %) |
| Age in year, median (range) | 40 (13–68) | |
| Type of tumour | Pheochromocytoma Paraganglioma |
10 (59 %) 7 (41 %) |
| Primary site | Adrenal Extra-adrenal |
10 (59 %) 7 (41 %) |
| Known metastases | Yes Suspicious |
13 (76 %) 4 (24 %) |
| Catecholamine secretion | Yes No Unknown |
9 (53 %) 6 (35 %) 2 (12 %) |
| Genetic preposition | Hereditary Sporadic |
3 (18 %) 14 (82 %) |
| Modalities | MIBG + DOTATATE + FDG MIBG + DOTATATE |
12 5 |
Out of 15 patients that underwent 24-h catecholamine urine test to confirm the functional status of PCC/PGL tumour, 9 patients demonstrated raised urinary catecholamine level. Based on pedigree analysis, three (18 %) patients were diagnosed as familial syndrome; whereas 82 % of patients were considered as sporadic cases. Genetic confirmation was unavailable during the study.
Per-Patient Analysis
Fifteen out of 17 patients were positive of having metastatic disease. 68Ga-DOTATATE PET/CT correctly identified 14 positive patients, whereas only 7 patients were detected by 131I-MIBG scintigraphy. In the 18F-FDG PET/CT subgroup (patient, n = 12), 10 out of 11 positive patients were correctly detected by 18F-FDG PET/CT.
On the basis of per-patient analysis, the sensitivity and accuracy of 68Ga-DOTATATE PET/CT, 131I-MIBG scintigraphy and 18F-FDG PET/CT were (93.3 %, 94.1 %), (46.7 %, 52.9 %) and (90.9 %, 91.7 %) respectively (Table 2). Since any unequivocal lesion detected by either 68Ga-DOTATATE PET/CT, 131I-MIBG scintigraphy or 18F-FDG PET/CT was considered true-positive, the specificities of all three modalities are thus, by definition, 100 %.
Table 2.
Diagnostic performance of all three modalities based on per-patient and per-lesion analysis
| 68Ga-DOTATATE PET/CT | 131I-MIBG scintigraphy | 18F-FDG PET/CT | |
|---|---|---|---|
| Per-patient | |||
| Sensitivity | 93.3 % | 46.7 % | 90.9 % |
| Specificitya | 100 % | 100 % | 100 % |
| Accuracy | 94.1 % | 52.9 % | 91.7 % |
| Per-lesion | |||
| Sensitivity | 91.5 % | 15.7 % | 51.3 % |
| Specificitya | 100 % | 100 % | 100 % |
| Accuracy | 92.6 % | 26.0 % | 57.8 % |
aSince any unequivocal lesion detected by either 68Ga-DOTATATE PET/CT, 131I-MIBG scintigraphy or 18F-FDG PET/CT was considered true positive, the specificities of all three modalities are thus, by definition, 100 %
Per-Lesion Analysis
In total (patient, n = 17), 472 true-positive lesions were detected; of which, 432 lesions were identified by 68Ga-DOTATATE PET/CT, whereas only 74 lesions were detected by 131I-MIBG scintigraphy. Out of the total of 300 true-positive lesions in the 18F-FDG PET/CT subgroup (patient, n = 12), 154 lesions were identified by 18F-FDG PET/CT (Fig. 1).
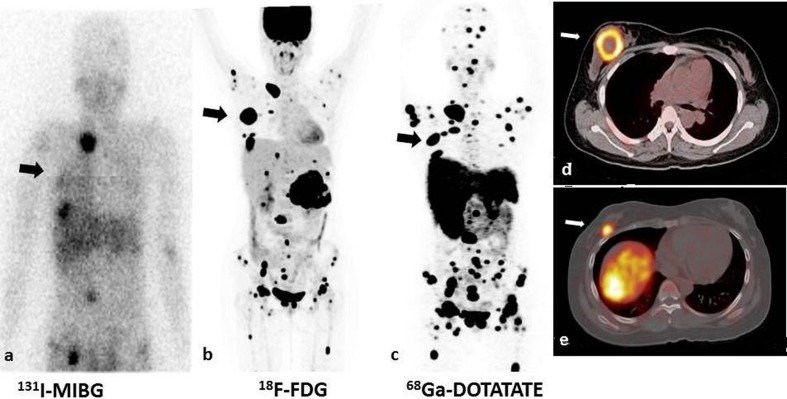
Fig. 1.
131I-MIBG, 18F-FDG, 68Ga-DOTATATE of a 17-year-old girl with malignant pheochromocytoma. c Maximum intensity projection (MIP) of 68Ga-DOTATATE image show multiple foci of somatostatin-receptor avid disease in a distribution consistent with extensive metastases. a, b The lesions demonstrated on DOTATATE images clearly outnumber planar 131I-MIBG images and MIP 18F-FDG images. d, e Rare pheochromocytoma metastasis to the breast was also seen in this patient (white arrow) on 18F-FDG PET/CT fusion image (d) and 68Ga-DOTATATE fusion image (e), which was later confirmed by biopsy
On lesion-based analysis, the sensitivity and accuracy of 68Ga-DOTATATE PET/CT, 131I-MIBG scintigraphy and 18F-FDG PET/CT were (91.5 %, 92.6 % p < 0.0001), (15.7 %, 26.0 % p < 0.0001) and (51.3 %, 57.8 % p < 0.0001) respectively (Table 2).
In 12 patients who underwent all three modalities, 6 patients were shown to have osseous metastases; of which a total of 230, 98 and 9 metastatic lesions were detected by 68Ga-DOTATATE PET/CT, 18F-FDG PET/CT and 131I-MIBG respectively. One patient demonstrated a rare PCC metastasis to the right breast on all three modalities (Fig. 1).
The range of measured SUVmax over 68Ga-DOTATATE uptake lesions was from 1.6 to 89.8; whereas the range of SUVmax over 18F-FDG uptake lesions was from 2.5 to 62.3.
Our data show no difference between functional and non-functional tumour on 68Ga-DOTATATE finding (p = 0.14), 131I-MIBG finding (p = 1.00) and 18F-FDG finding (p = 1.00).
Discussion
Diagnostic Performance of Three Modalities
Similar to previous studies [14, 15], the observed diagnostic performance of 131I-MIBG scintigraphy in the detection of metastatic PCC/PGL on per-patient and per-lesion basis was rather disappointing. 131I-MIBG scintigraphy remarkably underestimates the disease extent, and implies that its use as a diagnostic agent should be confined to extra-adrenal screening in patient with initial presentation of benign PCC, or assessment of patient’s eligibility for 131I-MIBG therapy [28]. Although the use of SPECT/CT in 131I-MIBG would improve the diagnostic certainty in indeterminate lesions and contribute to better diagnostic performance than planar imaging [29, 30], it is arguable in our study because 58.8 % (10/17) of patients showed normal scintigraphic finding where application of SPECT/CT in these patients was not required. Furthermore, the number of lesions detected by 131I-MIBG planar images was so small that could be discretely correlated well to one of the PET/CT, CT or MRI images. However, we are aware that acquired SPECT/CT images as part of 131I-MIBG scan would make a better fair comparison with 68Ga-DOTATATE and 18F-FDG PET/CT images.
Although 68Ga-DOTATATE PET/CT demonstrates marginally better sensitivity and accuracy than 18F-FDG PET/CT on per-patient basis, it is significantly superior (p < 0.0001) on per-lesion basis (Table 2). Obviously, identification of metastatic disease is important in prognostication. However, accurate mapping of all metastatic lesions could have potential implication in assignment of cancer staging, and subsequent guidance in therapeutic approach. Underestimation of tumour extent could potentially lead to inappropriate management, in particular, when high risk curative metastasectomy, instead of palliative chemotherapy or targeted radiotherapy, is being carried out on a patient who is later found to have more extensive disease. Furthermore, assessment of therapeutic response requires more comprehensive evaluation of all target lesions [31, 32]. Change of any tumour volume and characteristics from the baseline may alter subsequent therapeutic approach. In sum, the significant higher diagnostic performance of 68Ga-DOTATATE PET/CT, on a per-lesion basis, in comparison with 131I-MIBG scintigraphy and 18F-FDG PET/CT may somehow have a potential impact on therapeutic decision making.
Surprisingly, our observed sensitivity of 131I-MIBG scintigraphy the in detecting metastatic PCC/ PGL is substantially lower than what was reported in other studies which used 123I-MIBG scintigraphy, being (46.7 % vs 65–77 %), and (15.7 % vs 57–66.6 %) on a per-patient and per-lesion basis respectively [14, 16, 26, 33]. Whereas, the sensitivity of 18F-FDG is rather similar on a per-patient basis (90.9 % vs 86–88 %) but is significantly lower on per-lesion basis (51.3 % vs 74 %) [14, 16]. These discrepancies may be related to the use of different reference standards in validating the disease. In previous studies, CT or MRI, which generally have low specificity and produce inconclusive results, particularly for small lesions, were served as the reference standard. This might possibly influence the sensitivity estimates. Therefore, to overcome such imperfect standards, we adopted a composite reference standard, which derived from anatomical and functional imaging findings, along with histopathological information.
Despite comparable results of 123I-MIBG and 131I-MIBG scintigraphy in PCC/PGL detection [34], the lower sensitivity of 131I-MIBG scintigraphy observed in this study could still be related to poorer inherent characteristics of 131I than 123I for imaging with current gamma cameras, which contribute to poorer target-to-background ratio, and therefore possibly lower detection rate. Other factors, such as different biological properties among patients or presence of MIBG-confounding therapy, may also contribute to these discrepancies.
Studies have demonstrated that de-differentiated neuroendocrine tumours (NET) exhibit high GLUT-1 glucose transporter expression with low expression of somatostatin receptor [35, 36]. This gives the notion that somatostatin receptor expression lesions are generally associated with differentiated tumours, and as these tumours transform to more aggressive behaviour, there is shift from 68Ga-DOTA-peptide uptake to 18F-FDG uptake—the so called ‘flip-flop’ phenomenon. However, in our data, significantly more metastatic lesions with somatostatin receptor expression than glucose metabolism were detected, particularly in osseous metastases. The finding of such high detectability in metastatic lesions clearly demonstrates that somatostatin receptor expression could be link to more aggressive behaviour of tumours [23–25]. Furthermore, a recently published study also showed that a more “complex relationship” exists, where matched somatostatin receptor expression and glucose metabolism lesions was seen in higher tumoural grade in NET [37].
Interestingly, this high detectability of somatostatin receptor imaging in the detection of osseous metastases in PCC/PGL clearly contradicts a previously published study where 18F-FDG PET was shown to be superior to 111In-octreotide scintigraphy, a traditional somatostatin peptide imaging method [17]. It seems explicable that such a discrepancy in results is related to the use of hybrid PET/CT imaging in 68Ga-DOTATATE, which provides better spatial resolution and image quality, leading to higher lesional detectability compared to conventional gamma imaging with 111In-octreotide [38, 39].
In 12 patients who underwent all three modalities, discordant localisation of 68Ga-DOTATATE PET/CT, 131I-MIBG scintigraphy and 18F-FDG PET/CT was demonstrated (Fig. 2). Interestingly, all concordant lesions between 131I-MIBG and 18F-FDG (mixed differentiated and de-differentiated) showed positivity in 68Ga-DOTATATE. Also, a greater number of concordant lesions between 68Ga-DOTATATE and 18F-FDG was seen compared with concordant lesions between 131I-MIBG and 68Ga-DOTATATE. In lesions demonstrable by only one modality, 68Ga-DOTATATE expectably contributes the most (Fig. 3). All of these findings illustrate the important point that there is considerable inter-/intra-tumoural heterogeneity in malignant PCC/PGL, and the relationship among 68Ga-DOTATATE, 131I-MIBG and 18F-FDG is not as straightforward as previously thought.
Fig. 2.
Number of lesions detected in 12 patients who underwent all three modalities
Fig. 3.
A 47-year-old man with familial paraganglioma syndrome presented with primary site at the right neck (resected). a, b Static anterior and posterior planar 131I-MIBG images was negative. c, d 68Ga-DOTATATE PET/CT fusion image showed uptake in the aortocaval soft tissue mass (SUVmax 6.7) but 18F-FDG PET/CT fusion image showed no FDG avidity (arrow). The lesion was later confirmed to be metastasis on resection
The findings of somatostatin receptor expression in a large number of metastatic lesions, including mixed (differentiated and de-differentiated) lesions, suggest that somatostatin receptors may be an independent receptor expression in PCC/PGL cellular differentiation process, which is not solely confined to well-differentiated phenotype.
The limitation of this study is a relatively small sample size that hinders further analysis. However, it was justified by the rarity of the disease. Apart from that, the high number of lesions in a small number of patients could also introduce sampling bias and might subsequently influence the determination of sensitivity and accuracy estimate.
Another limitation was a considerably long study interval among the examinations in some patients. This was unavoidable considering the different locations of the individual examinations, the high workload and waiting time in public hospitals and the patients’ places of origin from different states.
Although every effort was made to recruit all eligible patients, the possibility of selection and ascertainment bias (such as known prior disease status or previous imaging results used as a basis for recruitment) cannot be excluded.
The verification of the diagnostic performance of an index test could be a strenuous undertaking when the gold standard is not available or imperfect. An attempt has been made to produce more meaningful results in clinical practice by adopting a composite reference standard. However, the possibility of reference-standard-related bias (such as misclassification of lesions, or error and variation in image interpretation) cannot be totally excluded.
Conclusions
Although a multimodality approach using different radiotracers could provide imaging of real disease extension in malignant PCC/PGL, it is time-consuming, impractical and not cost-effective. The high sensitivity and accuracy in 68Ga-DOTATATE PET/CT implies that somatostatin receptor PET/CT imaging may offer a better overview on tumour spread and extension than 131I-MIBG and 18F-FDG. 68Ga-DOTATATE PET/CT should be recommended into the standard algorithm of evaluation such tumours.
In addition, 68Ga-DOTATATE PET/CT provides valuable information on somatostatin receptor status in this group of patients when peptide receptor radionuclide therapy (PRRT) is being considered as part of the multimodal management in metastatic PCC/PGL [40, 41]. High detectability of somatostatin receptor expression lesions in metastatic PCC/ PGL provides the basis for consideration of PRRT as initial therapeutic strategy.
Lastly, a prospective controlled multicentre study with a larger patient group would be beneficial in future. With the advent of genetic testing in PCC/PGL, potential relationship between 68Ga-DOTATATE PET/CT results and genetic abnormalities would provide a more comprehensive understanding of the correlation between germline mutation and tumour characteristics.
Acknowledgments
We are grateful to Dr. Nor Asiah Muhamad, Dr. Siti Zarina Amir Hassan, Dr. Nor Salita Ali, Dr. Jia Him Lau and all our staff for their assistance in this study. We also thank the Director-General of Health in Malaysia for permission to publish this paper.
Conflicts of Interest
This research was funded by the Advanced Medical and Dental Institute, University Sains Malaysia. Teik Hin Tan, Boon Nang Lee, Zanariah Hussein, Fathinul Fikri Ahmad Saad and Ibrahim Lutfi Shuaib declare that they have no conflicts of interest.
Ethics Statement
Informed consent was obtained from all patients for being included in the study. This study is approved by local ethics committee.
References
- 1.Harari A, Inabnet WB., 3rd Malignant pheochromocytoma: a review. Am J Surg. 2010;201:700–708. doi: 10.1016/j.amjsurg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Ilias I, Pacak K. Anatomical and functional imaging of metastatic pheochromocytoma. Ann N Y Acad Sci. 2004;1018:495–504. doi: 10.1196/annals.1296.061. [DOI] [PubMed] [Google Scholar]
- 3.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;10:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 4.Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31:41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Mehrotra PK, Jain M, Gupta SK, Mishra A, Chand G, et al. Size of the tumor and pheochromocytoma of the adrenal gland scaled score (PASS): can they predict malignancy? World J Surg. 2010;34:3022–3028. doi: 10.1007/s00268-010-0744-5. [DOI] [PubMed] [Google Scholar]
- 6.Feng F, Zhu Y, Wang X, Wu Y, Zhou W, Jin X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011;185:1583–1590. doi: 10.1016/j.juro.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2010;96:717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 8.Korevaar TI, Grossman AB. Pheochromocytomas and paragangliomas: assessment of malignant potential. Endocrine. 2011;40:354–365. doi: 10.1007/s12020-011-9545-3. [DOI] [PubMed] [Google Scholar]
- 9.Forssell-Aronsson E, Schuler E, Ahlman H. Advances in the diagnostic imaging of pheochromocytoma. Rep Med Imaging. 2011;4:19–37. doi: 10.2147/RMI.S5571. [DOI] [Google Scholar]
- 10.Taieb D, Rubello D, Al-Nahhas A, Calzada M, Marzola MC, Hindie E. Modern PET imaging for paragangliomas: relation to genetic mutations. Eur J Surg Oncol. 2011;37:662–668. doi: 10.1016/j.ejso.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman GA, Pacak K, O’Dorisio MS, Neumann DR, Waxman AD, Mankoff DA, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009;50:1448–1454. doi: 10.2967/jnumed.108.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilias I, Divgi C, Pacak K. Current role of metaiodobenzylguanidine in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin Nucl Med. 2011;41:364–368. doi: 10.1053/j.semnuclmed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonte JS, Robles JF, Chen CC, Reynolds J, Whatley M, Ling A, et al. False-negative 123I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer. 2012;19:83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taïeb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med. 2009;50:711–717. doi: 10.2967/jnumed.108.060731. [DOI] [PubMed] [Google Scholar]
- 16.Cantalamessa A, Caobelli F, Paghera B, Caobelli A, Vavassori F. Role of 18F-FDG PET/CT, 123I-MIBG SPECT, and CT in restaging patients affected by malignant pheochromocytoma. Nucl Med Mol Imaging. 2011;45:125–131. doi: 10.1007/s13139-011-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 18.Mundschenk J, Unger N, Schulz S, Hollt V, Steinke R, Lehnert H. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88:5150–5157. doi: 10.1210/jc.2003-030262. [DOI] [PubMed] [Google Scholar]
- 19.Tenenbaum F, Lumbroso J, Schlumberger M, Mure A, Plouin PF, Caillou B, et al. Comparison of radiolabeled octreotide and meta-iodobenzylguanidine (MIBG) scintigraphy in malignant pheochromocytoma. J Nucl Med. 1995;36:1–6. [PubMed] [Google Scholar]
- 20.Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP. 111In-octreotide is superior to 123Imetaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49:1232–1237. doi: 10.2967/jnumed.107.047738. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Li F, Zhuang H, Jing H, Du Y, Zeng Z. 99mTc-HYNIC-TOC scintigraphy is superior to 131I-MIBG imaging in the evaluation of extraadrenal pheochromocytoma. J Nucl Med. 2009;50:397–400. doi: 10.2967/jnumed.108.058693. [DOI] [PubMed] [Google Scholar]
- 22.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 23.Naji M, Zhao C, Welsh SJ, Meades R, Win Z, Ferrarese A, et al. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2010;13:769–775. doi: 10.1007/s11307-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 24.van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SWJ, et al. [123I]Metaiodobenzylguanidine and [111In]Octreotide uptake in benign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 25.Win Z, Al-Nahhas A, Towey D, Todd JF, Rubello D, Lewington V, et al. 68Ga-DOTATATE PET in neuroectodermal tumours: first experience. Nucl Med Commun. 2007;28:359–363. doi: 10.1097/MNM.0b013e32808ea0b0. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Dhull V, Arora S, Gupta P, Kumar R, Durgapal P, et al. Diagnostic accuracy of 68Ga-DOTANOC PET/CT imaging in pheochromocytoma. Eur J Nucl Med Mol Imaging. 2013;1–11. [DOI] [PubMed]
- 27.Taïeb D, Timmers H, Hindié E, Guillet B, Neumann H, Walz M, et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1977–1995. doi: 10.1007/s00259-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havekes B, King K, Lai EW, Romijn JA, Corssmit EPM, Pacak K. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol. 2010;72:9. doi: 10.1111/j.1365-2265.2009.03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P, Dhull VS, Jeph S, Reddy RM, Singh H, Naswa N, et al. Can hybrid SPECT-CT overcome the limitations associated with poor imaging properties of 131I-MIBG?: comparison with planar scintigraphy and SPECT in pheochromocytoma. Clin Nucl Med. 2013;38:e346–e353. doi: 10.1097/RLU.0b013e318279bcb2. [DOI] [PubMed] [Google Scholar]
- 30.Rozovsky K, Koplewitz BZ, Krausz Y, Revel-Vilk S, Weintraub M, Chisin R, et al. Added value of SPECT/CT for correlation of MIBG scintigraphy and diagnostic CT in neuroblastoma and pheochromocytoma. AJR Am J Roentgenol. 2008;190:1085–1090. doi: 10.2214/AJR.07.2107. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroiss A, Putzer D, Uprimny C, Decristoforo C, Gabriel M, Santner W, et al. Functional imaging in phaeochromocytoma and neuroblastoma with 68Ga-DOTA-Tyr 3-octreotide positron emission tomography and 123I-metaiodobenzylguanidine. Eur J Nucl Med Mol Imaging. 2010;38:865–873. doi: 10.1007/s00259-010-1720-x. [DOI] [PubMed] [Google Scholar]
- 34.Furuta N, Kiyota H, Yoshigoe F, Hasegawa N, Ohishi Y. Diagnosis of pheochromocytoma using [123I]-compared with [131I]-metaiodobenzylguanidine scintigraphy. Int J Urol. 1999;6:119–124. doi: 10.1046/j.1442-2042.1999.06310.x. [DOI] [PubMed] [Google Scholar]
- 35.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 36.Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927–1932. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 37.Oh S, Prasad V, Lee DS, Baum RP. Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. 2011; 2011:524130. [DOI] [PMC free article] [PubMed]
- 38.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 39.Krausz Y, Freedman N, Rubinstein R, Lavie E, Orevi M, Tshori S, et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan®) Mol Imaging Biol. 2010;13:583–593. doi: 10.1007/s11307-010-0374-1. [DOI] [PubMed] [Google Scholar]
- 40.Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J Nucl Med. 2011;52:841–844. doi: 10.2967/jnumed.110.084236. [DOI] [PubMed] [Google Scholar]
- 41.Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46:723–734. doi: 10.1080/02841860701441848. [DOI] [PubMed] [Google Scholar]