Abstract
Febrile temperatures can induce stress responses which protect cells from damage and can reduce inflammation during infections and sepsis. However, the mechanisms behind the protective functions of heat in response to the bacterial endotoxin LPS are unclear. We have recently shown that Annexin-1 (ANXA1)-deficient macrophages exhibited higher TNFα levels after LPS stimulation. Moreover, we have previously reported that ANXA1 can function as a stress protein. Therefore in this study, we determined if ANXA1 is involved in the protective effects of heat on cytokine levels in macrophages after heat and LPS. Exposure of macrophages to 42 °C for 1 h prior to LPS results in an inhibition of TNFα production, which was not evident in ANXA1−/− macrophages. We show that this regulation involves primarily MYD88-independent pathways. ANXA1 regulates TNFα mRNA stability after heat and LPS, and this is dependent on endogenous ANXA1 expression and not exogenously secreted factors. Further mechanistic studies revealed the possible involvement of the heat shock protein HSP70 and JNK in the heat and inflammatory stress response regulated by ANXA1. This study shows that ANXA1, an immunomodulatory protein, is critical in the heat stress response induced after heat and endotoxin stimulation.
Keywords: Heat, Inflammation, TNF, HSP70, Annexin-A1
Introduction
The heat stress response is one of the body’s most important cellular defense mechanisms and is important for cellular function (Rattan et al. 2004). Heat is a form of stress which activates the heat stress response (Gabai et al. 1997) and involves the signaling of heat shock factors which translocate to the nucleus and bind to heat shock elements to induce the transcription and translation of heat shock proteins (HSP) (Morimoto 1998; Rattan et al. 2004). The main role of the heat stress response is to protect the cell during stressful conditions by promoting its cell survival. As a means of promoting cell survival, the heat stress response and HSPs are also known to play a role in inflammatory signaling by regulating the production of inflammatory cytokines (Asea et al. 2002; Shi et al. 2006).
HSPs act as molecular chaperones, enabling cells to cope with stress, misfolded proteins and their aggregation and to reduce cell damage (Cooper et al. 2010). Febrile temperatures can induce HSP70 expression and release by regulating signal transduction pathways, such as the mitogen-activated protein kinase (MAPK) (Gupta et al. 2013), extracellular regulated kinase 1/2 (ERK 1/2), p38, and c-Jun amino (N)-terminal kinases 1/2 (JNK 1/2) (Cargnello and Roux 2011).
We and others have demonstrated that the immunomodulatory protein Annexin-1 (ANXA1) may function as a stress protein (Nair et al. 2010; Rhee et al. 2000). ANXA1 belongs to the annexin superfamily of calcium and phospholipid-binding proteins and has been implicated in a myriad of functions, such as proliferation, apoptosis, and cell migration (Lim and Pervaiz 2007; Raynal and Pollard 1994). ANXA1 was demonstrated to have chaperone-like functions (Kim et al. 1997), and ANXA1 mRNA and protein levels are increased in response to stress (Nair et al. 2010; Rhee et al. 2000). ANXA1 was shown to protect cells against stress by preventing heat-induced growth arrest and DNA damage and translocates to the nucleus after heat (Nair et al. 2010). However, the role of ANXA1 in the regulation of the inflammatory stress response has not been studied.
Therefore, in this study, we determined the effect of heat stress on the inflammatory response of macrophages after LPS treatment and investigated the mechanisms involved in the inhibition of cytokine production by heat. We discovered that ANXA1 is involved in the regulation of the inflammatory response upon induction with heat in response to LPS, related to the modulation of JNK and HSP70.
Materials and methods
Bone marrow-derived macrophages and L929 cell conditioned media
Bone marrow-derived macrophages (BMMO) were isolated from the femurs and tibia of 8–10-week-old Balb/c wild-type (WT) and ANXA1−/− mice. All animal work was approved by the NUS Institutional Animal Care and Use Committee (IACUC) and in accordance with the National Advisory Committee for Laboratory Animal Research (NACLAR) guidelines. The bone marrow cells were flushed out from the bones and were then allowed to mature into macrophages by incubating them over a period of 7 days in DMEM media supplemented with L929 conditioned media, 10 % fetal bovine serum (FBS), and 1 % penicillin/streptomycin (P/S). The media was topped up at day 3 after the cells were seeded. Mouse fibroblast cell line L929 was cultured to obtain L929 conditioned medium for the growth of primary bone marrow-derived macrophages. Cells were grown in RPMI media supplemented with 10 % FBS and 1 % P/S. Cells were grown to 90 % confluence and then expanded over three passages. In some experiments, media was removed from the cells and filtered using a 0.2-μm filter (Thermo Scientific).
Heat stress
Heat stress was carried out at 42 °C, and control cells were incubated at 37 °C. Cells seeded in plates or dishes were sealed with parafilm and placed in a water bath set to 37 or 42 °C. Heat stress was carried out for 1 h, after which the cell culture dishes were taken out of the bath followed by treatment with LPS or returned to the incubator to be harvested at different time points.
Drugs and inhibitors
100 ng/ml LPS (Sigma Aldrich), 1 μM CPG 1826 ODN (Invivogen, CA, USA), and 10 μg/ml Poly (I:C) (Invivogen, CA, USA) were used as stimuli. U0126 (Selleck Chemicals, China), SP600125 (Selleck Chemicals, China), SB203580 (Cell Signaling, MA, USA), and VER155008 (Tocris Bioscience, UK) were added to cells 1 h prior to heat treatment.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed for cell supernatants harvested from the cells 24 h post treatment. Ready Set Go! ® ELISA kits (eBioscience) for mouse TNFα and ELISA MaxTM Deluxe Kits (Biolegend) for mouse IL-6 and mouse IL-12 (p40) were used. The assay was carried out according to the manufacturer’s protocol. The absorbance was measured using PerkinElmer Victor3 V Multilabel counter Model 1420 (MA, USA).
Quantitative real-time PCR
RNA extraction was performed using Omics Maestrozol™ RNA plus extraction reagent (Omics Biotechnology). RNA quality and quantity were determined using the nanodrop spectrophotometer (Biofrontier Technology). The RNA was first converted into complementary DNA (cDNA) in a two-step reaction, first, incubating 1 μg of RNA, Oligo (dT), and nuclease-free water at 65 °C for 5 min. Next, 10 μl of cDNA was added to a reaction mixture containing 5X M-MLV RT buffer, 10 mM dNTPs, and 40 U/μl RNAsin RNAse inhibitor, M-MLV Reverse Transcriptase (Promega), and nuclease-free water. cDNA was used to set up a quantitative real-time PCR (qPCR) reaction using the GoTaq® qPCR Master Mix (Promega) kit. The following primer sequences were used, Mouse HSP70.1 forward primer 5′ GAG ATC GAC TCT CTG TTC GAG G-3′, mouse HSP70 reverse primer 5′ GCC CGT TGA AGA AGT CCT G-3′ (Hunt and Calderwood 1990), mouse GAPDH forward primer 5′ AAC TTT GGC ATT GTG GAA GG-3′, and mouse GAPDH reverse primer 5′ ACA CAT TGG GGG TAG GAA CA-3′. GAPDH was used as the endogenous control for the mRNA markers evaluated in this study. qPCR data was analyzed based on the ΔΔCT approximation method (Livak and Schmittgen 2001). qPCR data was re-interpreted as fold change relative to untreated control samples.
mRNA stability assay
WT and ANXA1−/− BMMOs were treated with control or heat, as described above, followed by treatment with 100 ng/ml LPS for 0 min, 30 min, and 1 h. Actinomycin D was added to the cells to inhibit transcription, and the RNA was then isolated 0, 30 min, and 1 h after addition of actinomycin D. RNA was extracted and cDNA was harvested as described above and subject to qPCR. The relative quantity (RQ) values obtained from the qPCR analysis were expressed as fold change relative to mRNA levels at time zero. Log of the RQ values were plotted against time in minutes with an exponential trendline. Half-life was calculated by dividing the decay constant (ln2) by the exponent obtained from the equation of the graph.
Western blot
BMMOs were harvested at various time points after treatment with heat stress. Cells were washed with 1X PBS before protein lysis. The lysed proteins were stored at −80 °C till further use. Protein concentration was determined using 1X Bradford’s assay reagent (BioRad) and BSA protein standards (Thermo Scientific), and the absorbance was measured at a wavelength of 595 nm. Equal amounts of proteins were subjected to 12 % SDS-PAGE electrophoresis and transferred to nitrocellulose membranes using a wet transfer apparatus (BioRad). Protein expression was determined by Western blotting with specific antibodies. Expression signals were obtained by using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) or the WesternBright ECL HRP substrate (Advansta). Membranes were exposed to CL-Xposure (Thermo Scientific) films in an autoradiography cassette (Amersham Hypercassette) for various durations until optimal visualization of the bands was possible using the X-ray developer from Konica Minolta SRX-101A tabletop processor.
Statistical analysis
Data are represented as mean ± SEM. Individual groups were analyzed using the two-tailed student’s t test. F test was carried out to determine the equality of variance before selecting the appropriate t test for analysis. P value < 0.05 was considered to be statistically significant.
Results
ANXA1 promotes the inhibition of TNFα production after heat and LPS
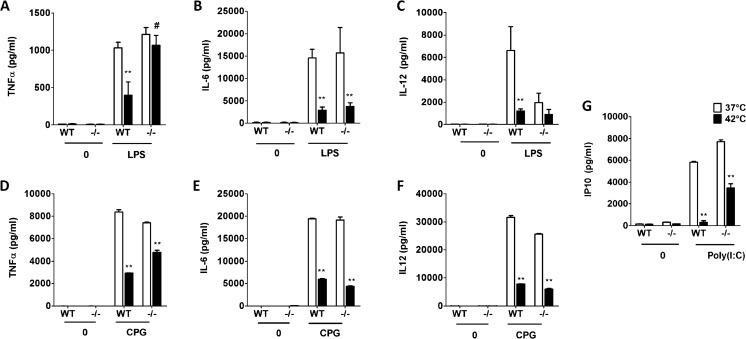
Previous studies have shown that heat pretreatment can inhibit cytokine production (Shi et al. 2006). To confirm this finding, BMMOs obtained from BALB/c mice were incubated at 37 or 42 °C for 1 h, after which cells were stimulated with 100 ng/ml LPS and the levels of TNFα, IL-6, and IL-12 were measured. As shown in Fig. 1a–c, all the cytokines were significantly inhibited by pretreatment with heat.
Fig. 1.
Heat pretreatment followed by TLR agonist stimulation inhibits cytokine production. WT- or ANXA1-deficient (−/−) macrophages were incubated at either 37 or 42 °C for 1 h prior to stimulation with (a, b, c) LPS (100 ng/ml) or (d, e, f) CPG ODN1826 (1 μM) or (g) Poly(I:C) (10 μg/ml) for 24 h, and the indicated cytokines were measured using ELISA. **p < 0.01 vs LPS 37 °C control. # p < 0.01 vs WT 42 ºC
We have recently reported that ANXA1 regulates TLR3- and TLR4-dependent cytokine production in macrophages (Bist et al. 2013). Therefore, to determine if ANXA1 could also be involved in heat-induced inhibition of cytokine levels after LPS, cytokine levels were measured in WT and ANXA1−/− BMMOs incubated at control temperatures or heat prior to LPS stimulation. In the absence of ANXA1, IL-12 was lower, while TNFα and IL-6 production was similar to WT macrophages stimulated with LPS (Fig. 1a–c). Interestingly, while WT macrophages stimulated with heat and LPS (H + L) exhibited lower TNFα production compared to LPS stimulation alone (Fig. 1a), this was not observed with ANXA1--/- macrophages stimulated with H + L (Fig. 2a). However, this observation was only seen with TNFα, as IL-6 and IL-12 production was reduced in both WT and ANXA1−/− macrophages treated with stimulated with H + L (Fig. 2b, c). Thus, although TNFα, IL-6, and IL-12 are regulated by heat stress, only TNFα production is regulated by ANXA1.
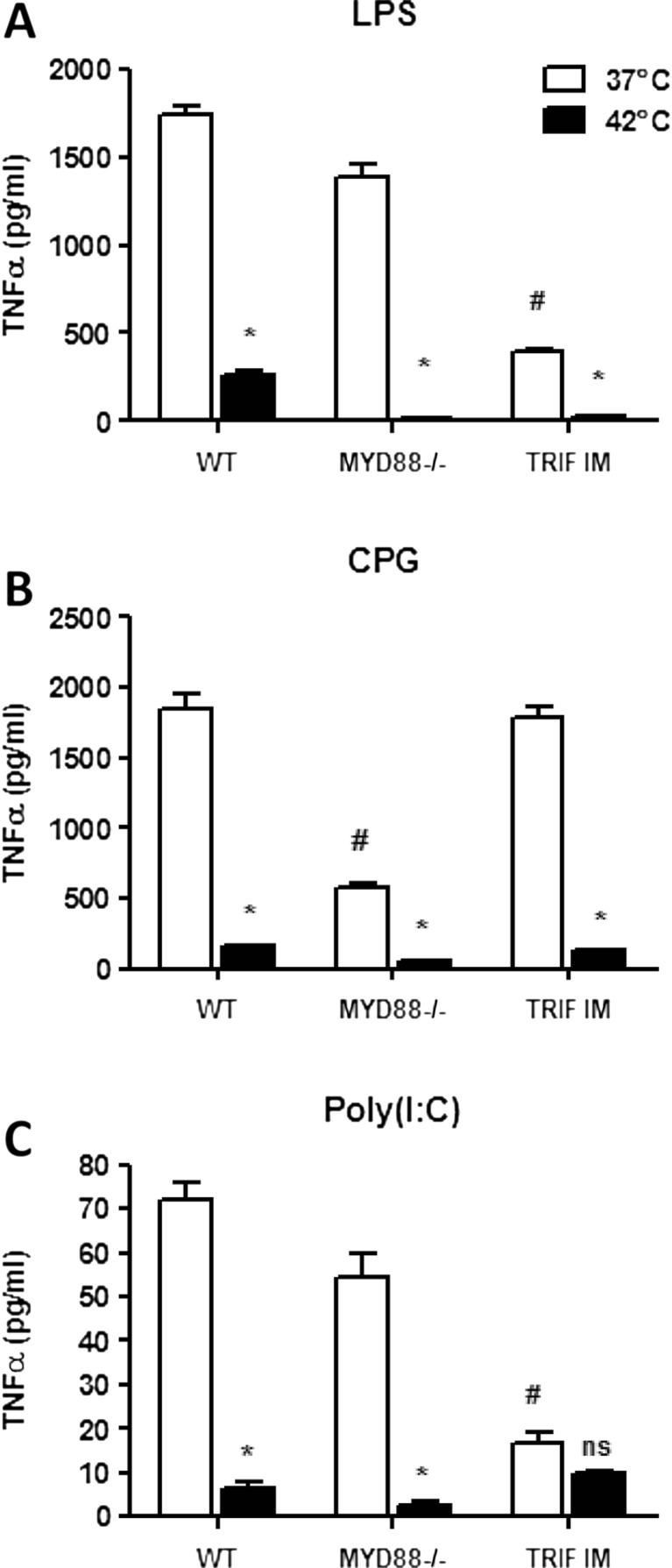
Fig. 2.
TRIF is involved in the regulation of TNFα production after heat and LPS. Macrophages from WT, MYD88-deficient mice, or TRIF inactive mutant mice were stimulated with heat and (a) LPS or (b) CPG ODN1826 or (c) Poly(I:C) as described in Figure 1. # p < 0.05 vs WT 37 ºC control; *p < 0.05 vs respective 37 °C controls; ns: not significant
ANXA1 regulates TLR9-induced TNFα and TLR3-induced IP10 production
Next, to assess if ANXA1 could regulate cytokine production induced by heat and other TLR agonists, cells were treated with CPG (ODN-1826), a TLR9 agonist for 24 h. In a similar manner to LPS, WT cells which were treated with heat prior to CPG produced significantly less TNFα, IL-6, and IL-12 when compared to cells treated with CPG alone (Fig. 1d–f). In the absence of ANXA1, a partial rescue of the inhibition of TNFα was observed when cells were stimulated with heat and CPG, and no difference was observed between WT and ANXA1−/− cells with IL-6 and IL-12 production.
LPS activates toll-like receptor 4 (TLR4), which stimulates both the MyD88 and TRIF pathways (Hoebe et al. 2003; Toshchakov et al. 2002), while the CPG activates TLR9, which only stimulates the MyD88-dependent pathway (Takeda and Akira 2004). As only a partial rescue was observed with CPG in ANXA1−/− macrophages, it is possible that the TRIF pathway could also be involved. Therefore, a TRIF-specific chemokine, IP10, was measured after heat and TLR3 stimulation with poly(I:C), which activates the TRIF pathway specifically. Figure 1g shows that IP10 production was also inhibited after heat and poly(I:C) treatment, indicating that TRIF pathway was also involved in the heat stress response. Furthermore, the absence of ANXA1 partially reverses the inhibition of IP10 production induced by heat and poly(I:C) treatment, suggesting that ANXA1 can regulate both the TRIF-dependent and the MYD88-dependent pathways.
TRIF is involved in heat-induced inhibition of cytokine production
To confirm this observation, BMMOs from C57/BL/6, MyD88−/−, and TRIF inactive mutant (TRIF IM) mice were pretreated with control temperatures or 42 °C in the same way as above. Treatment of MyD88−/− BMMOs with LPS alone did not inhibit TNFα production, yet a significant inhibition was observed in TRIF IM cells, suggesting that TRIF is involved in LPS-induced TNFα production (Fig. 2a). Upon heat pretreatment, a complete inhibition of TNFα production was observed in both heat-treated MyD88−/− and TRIF IM cells. As CPG is a MYD88-specific stimulator, CPG treatment resulted in lower TNFα production in MyD88−/− compared to WT but not in the TRIF IM cells (Fig. 2b). Once again, heat pretreatment resulted in a further reduction in TNFα production in MyD88−/− but not TRIF IM BMMOs. As poly(I:C) is a TRIF-specific stimulator, no inhibition of TNFα production was observed in MyD88−/− BMMOs treated with poly(I:C), but a significant inhibition was observed in TRIF IM cells upon poly(I:C) treatment (Fig. 2c). Moreover, no further inhibition of TNFα production was observed in heat-treated poly(I:C) macrophages. These data suggest that TRIF is involved in the heat-induced inhibition of TNFα.
Cellular ANXA1 is responsible for the regulation of TNFα during heat stress
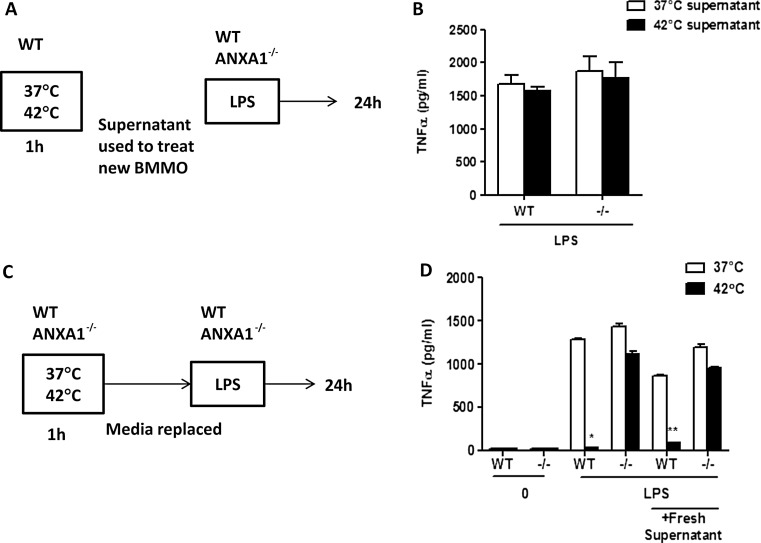
Previous reports have demonstrated that ANXA1 can also be secreted after cells are stimulated (Castro-Caldas et al. 2002). To determine if the regulation of TNFα production after H + L stimulation was due to exogenous ANXA1 or other factors regulated by ANXA1 which could be secreted into the media, supernatants were collected from WT BMMOs treated at 37 or 42 °C for 1 h. These conditioned supernatants were then used to treat WT and ANXA1−/− BMMOs, together with LPS for 24 h, and supernatants were collected for ELISA (Fig. 3a). Figure 3b shows that both WT and ANXA1−/− macrophages treated with WT control or heat supernatant produced similar levels of TNFα with no inhibition after heat suggesting that the effect of heat and ANXA1 on cytokine production is cellular. To confirm this, WT and ANXA1−/− macrophages were incubated at 37 or 42 °C for 1 h, after which media was removed and replaced with fresh media and cells were subsequently stimulated with 100 ng/ml LPS (Fig. 3c). Once again, WT macrophages produced less TNFα after heat pretreatment, which was not observed in the ANXA1−/− cells (Fig. 3d). These data demonstrate that endogenous ANXA1, and not exogenous or released ANXA1, is responsible for the regulation of TNFα during stress and also shows that no exogenous factors were involved in regulating the cytokine response during heat stress.
Fig. 3.
Endogenous ANXA1 is involved in TNFα production after heat and LPS. a Experimental set up for b where supernatants from WT macrophages incubated at either 37 or 42 °C for 1 h were used to treat WT or ANXA1 −/− macrophages. TNFα production was measured by ELISA after 24 h after LPS stimulation. c Experimental set up for d where WT or ANXA1 −/− macrophages were stimulated with heat for 1 h and media was replaced. LPS was added for 24 h and TNFα production was measured by ELISA. *p< 0.05, **p < 0.01 vs respective LPS 37 °C control
ANXA1 regulates TNFα mRNA stability
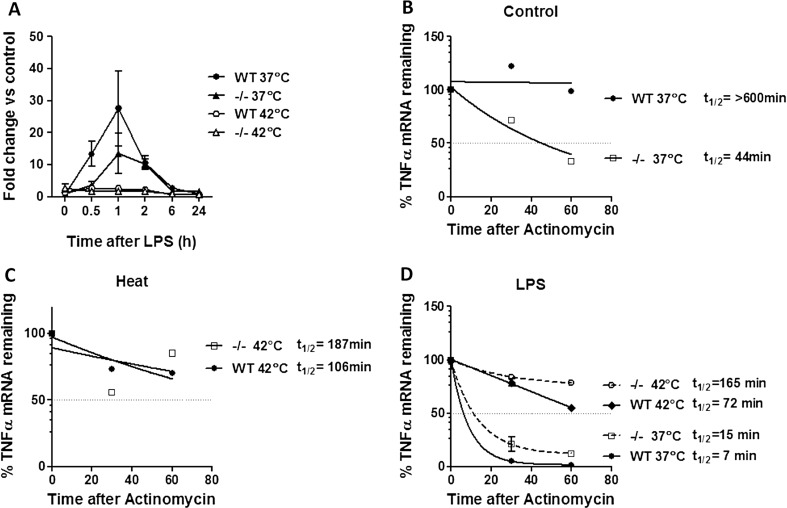
To investigate if the inhibition of TNFα during stress was due to transcriptional regulation (Meng and Harken 2002; Snyder et al. 1992), and if ANXA1 could modulate this, TNFα mRNA levels were assessed using real-time PCR. Treatment of cells with heat prior to LPS significantly inhibited TNFα mRNA expression in both WT and ANXA1−/− macrophages (Fig. 4a). These results indicate that ANXA1 may not affect TNFα expression at the transcriptional level. This result prompted further investigation into the posttranscriptional regulation of TNFα during heat and LPS treatment using mRNA stability assays with actinomycin D. Interestingly, ANXA1−/− cells exhibited a shorter TNFα half-life compared to WT cells (Fig. 4b), while heat alone reduced the TNFα half-life in WT but not ANXA1−/− cells (Fig. 4c). Furthermore, treatment of cells with LPS significantly reduced the TNFα half-life in both WT and ANXA1−/− cells, which was inhibited when cells were pretreated with heat (Fig. 4d). The consistently higher half-life of LPS-induced TNFα mRNA in both control and heat-treated ANXA1−/− cells correlate to the higher TNFα cytokine production when compared to the WT cells. Thus, we show here that ANXA1 can regulate the expression of TNFα mRNA transcript stability, thereby playing a role in the differential regulation of TNFα during heat stress.
Fig. 4.
ANXA1 regulates TNFα mRNA expression and stability. WT or ANXA1−/− macrophages were incubated at either 37 or 42 °C for 1 h prior to stimulation with LPS (100 ng/ml). a TNFα mRNA expression was measured using qPCR after the indicated time points. (b,c,d) TNFα mRNA stability was analyzed after actinomycin treatment. Half-life times were calculated using the equation –ln (2) / decay constant
Role of the MAP kinase pathway in the regulation of TNFα during heat stress
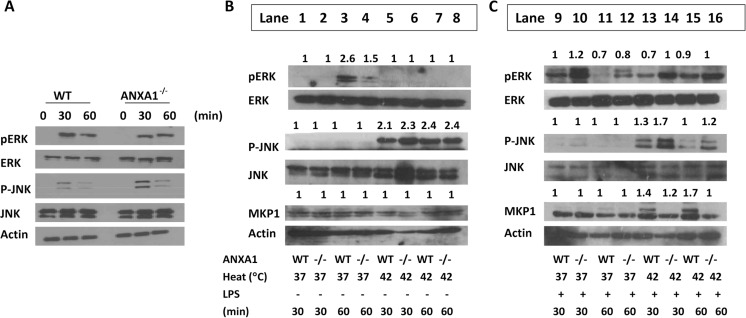
JNK is activated by stress stimuli, while ERK 1/2 is activated mainly by growth factors (Kyriakis and Avruch 2001; Kyriakis et al. 1994). Therefore, we investigated the contributing roles of ERK and JNK in the HSR following heat and LPS stimulation. First, we determine if ERK and JNK are activated following LPS stimulation. Indeed, ERK phosphorylation, and a slight activation of JNK, was induced at 30 min of LPS stimulation. Interestingly, ANXA1−/− macrophages exhibited higher JNK activation after LPS stimulation (Fig. 5a). Next, we treated WT and ANXA1 BMMO with heat alone (Fig. 5b, lanes 5–8) and observed that heat induces the phosphorylation of JNK, but not ERK. Once again, higher JNK activation was observed in ANXA1−/− cells after heat alone (Fig. 5b). Next, we subjected cells to heat pretreatment and LPS stimulation. Treatment of cells with LPS alone (Fig. 5c, lanes 9–12) once again induced the phosphorylation of ERK, but not JNK. However, upon treatment with LPS after heat, JNK was strongly phosphorylated, which was observed to be higher in ANXA1−/− macrophages (Fig. 5c, lanes 13–16).
Fig. 5.
JNK activation is enhanced, while MKP1 is inhibited in the absence of ANXA1. WT or ANXA1−/− macrophages were stimulated with LPS for 30 and 60 min or incubated at either 37 or 42 °C for 1 h prior to stimulation with a media or b LPS (100 ng/ml). Cells were lysed for Western blotting for the respective proteins after 30 and 60 min of LPS. Lane numbers are indicated. Fold change was determined using mean gray values of bands divided by their respective total proteins
MKP-1 is an endogenous inhibitor of the MAPK and is a dual-specificity phosphatase (Camps et al. 2000; English et al. 1999). As such, MKP-1 acts to inactivate the main members of the MAPK family such as ERK, P38, and JNK. To determine if MKP1 is involved in ANXA1-mediated TNFα signaling during heat stress, MKP1 levels were examined in WT and ANXA1−/− macrophages after heat and LPS treatment. Upon stimulation with heat and LPS, WT cells exhibited higher MKP-1 expression levels as compared to the heat-treated ANXA1−/− cells (Fig. 5a, b). Thus, ANXA1 may induce MKP1 expression which results in lower levels of phosphorylated JNK in heat- and LPS-treated cells, which in turn may inhibit TNFα production.
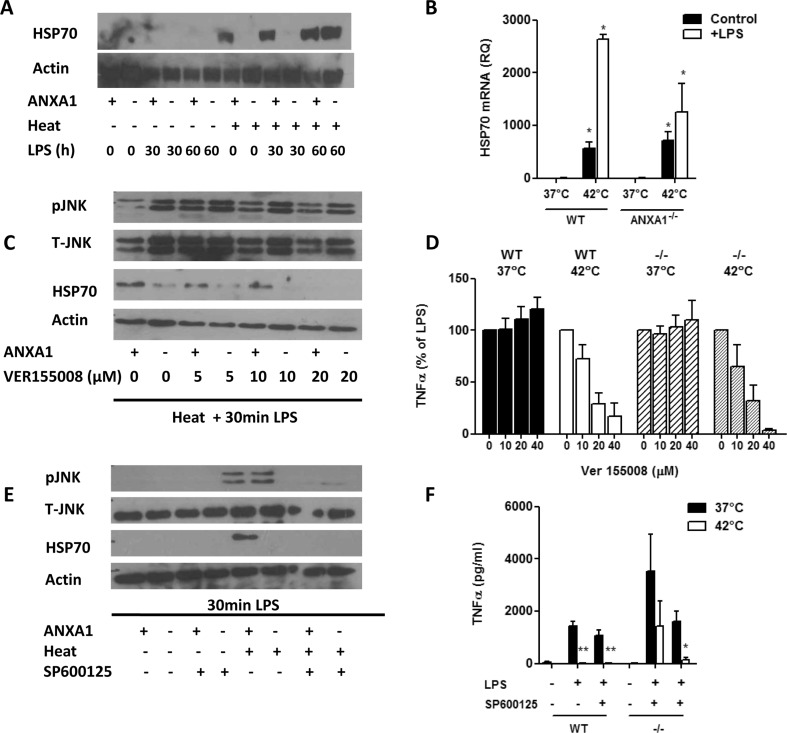
We next determined if HSP70 levels were different in WT and ANXA1−/− macrophages. Figure 6a shows that HSP70 is strongly expressed in cells treated with heat alone. Interestingly, while HSP70 is expressed in WT heat-treated cells, its expression is low or absent in the ANXA1−/− cells (Fig. 6a). Similarly, ANXA1−/− cells express less HSP70 mRNA as compared to WT cells after heat and LPS (Fig. 6b). Thus, ANXA1 may mediate the heat stress response through the modulation of HSP70. To prove this, HSP70 expression was inhibited using VER155008, a novel small molecule adenosine inhibitor of HSP/HSC70 (Lee et al. 2005). Once again, ANXA1−/− cells expressed less HSP70 after treatment with heat + LPS and HSP70 decreased in a dose-dependent manner after inhibition with VER155008 in WT cells (Fig. 6c). Interestingly, inhibition of HSP70 with VER155008 in control (37 °C) cells increased TNFα production. However, this inhibition was not sufficient to reverse the inhibition of TNFα in heat + LPS cells (Fig. 6d). Therefore, while HSP70 may be regulated by ANXA1 in the heat stress response, and HSP70 activation may modulate TNFα production, it may not play a role in the protective effects of ANXA1 on TNFα production after heat and LPS.
Fig. 6.
JNK functions upstream of HSP70 in regulation of TNFα production after heat and LPS. WT or ANXA1−/− macrophages were incubated at either 37 or 42 °C for 1 h prior to stimulation with LPS (100 ng/ml). Cells were lysed for a Western blotting for HSP70 after 30 and 60 min of LPS or b real-time PCR for HSP70 mRNA after 30 min of LPS. c Cells were pretreated for 1 h with the HSP70 inhibitor VER155008 prior to heat and LPS treatment. Cells were lysed for Western blotting for the indicated proteins after 30 min of LPS, and d TNFα production was measured by ELISA after 24 h. e Cells were pretreated for 1 h with the JNK inhibitor SP600125 (10 μM) prior to heat and LPS treatment. Cells were lysed for Western blotting for the indicated proteins after 30 min of LPS, and d TNFα production was measured by ELISA after 24 h. *p < 0.05, **p < 0.01 vs respective LPS controls
Since HSP70 and JNK are specifically activated by heat stress, and are both differentially expressed in WT and ANXA1−/− cells, we next examined if HSP70 and JNK regulate each other to inhibit TNFα levels during stress. Furthermore, HSP70 is known to prevent the activation of JNK during heat stress (Gabai et al. 1997) and can inhibit the MAPK by the upregulation of MKP-1 (Lee et al. 2005). Inhibition of HSP70 with VER155008 resulted in a slight decrease in pJNK levels in WT macrophages, which was not seen in ANXA1−/− cells, possibly due to the low levels of HSP70 present (Fig. 6c). More evidently, inhibition of JNK with SP600125 resulted in the complete inhibition of HSP70 in WT macrophages after heat stress (Fig. 6e), indicating that JNK is upstream of HSP70. This data indicates that JNK could be functioning upstream of HSP70 to mediate TNFα regulation by ANXA1. To confirm this, TNFα production was measured in macrophages pretreated with SP600125 prior to heat and LPS stimulation. Figure 6f illustrates that firstly, JNK inhibition is not able to reverse the inhibition of TNFα in WT cells treated with heat + LPS. Furthermore, treatment of ANXA1−/− cells with SP600125 results in a complete inhibition of TNFα production. This indicates that active JNK is required for the heat tolerance observed in ANXA1−/− cells.
Discussion
The stress response is one of the body’s most important cellular defense mechanisms (Rattan et al. 2004). Heat stress is one of the inducers of the stress response pathway and is used in this study to activate the HSR. As part of promoting the cell survival process, HSR plays a role in inflammation and regulates the production of pro- and anti-inflammatory cytokines. Hence, the cells were treated with LPS to determine the impact of HSR on cytokine production.
In the current study, we show that potent pro-inflammatory cytokines TNFα and IL-6 were downregulated upon heat and LPS treatment as compared to LPS treatment. This is similar to other studies on TNFα (Shi et al. 2006), IL-12 (Li et al. 2001), and IL-6 (Ensor et al. 1994), but opposite to other reported cytokines such as MIP2 (Garekar et al. 2006). This regulation of cytokine production by heat can be related to another protective phenomenon known as endotoxin tolerance (Biswas and Lopez-Collazo 2009). The two main pathways of the TLR signaling are the MyD88-dependent and MyD88-independent or TRIF-dependent pathways (Akira et al. 2001; Takeda and Akira 2004). Our results show that both the MyD88-dependent and MyD88-independent pathways could be involved in the heat-induced reduction in cytokine production, as heat was able to reduce the cytokine production induced by CPG, a TLR9 agonist that activates the MyD88-dependent pathway specifically, as well as polyI:C, a TLR3 agonists which activates the MyD88-independent pathway specifically. To further prove this, MyD88−/− (MKO) and TRIF inactive mutants (TIM) were used. Upon treatment with LPS, TNFα was inhibited to a greater extent in TIM cells as compared to MyD88−/− and WT cells, clearly indicating the signaling via the TRIF pathway resulted in most of the TNFα produced. Upon heat stress, TNFα levels were downregulated in the WT cells and inhibited in both MyD88−/− and TIM cells, hence demonstrating that both MyD88 and TRIF signaling are important and necessary for TNFα production after heat + LPS. This is similar to studies on endotoxin tolerance where both MyD88 and TRIF (Biswas and Lopez-Collazo 2009) are involved.
Here, we show that the presence of ANXA1 is necessary for the downregulation of TNFα during heat stress. The absence of ANXA1 resulted in less inhibition of TNFα upon heat stress, which is a novel finding. We recently showed that ANXA1−/− macrophages exhibited a higher production of TNFα (Bist et al. 2013), which we also show in this study. ANXA1 has also been related to the stress response in its possible role as a stress protein (Kim et al. 1997; Nair et al. 2010; Rhee et al. 2000). Its mRNA and protein expression levels were upregulated upon exposure to stress and played a protective in preventing heat-induced growth arrest and DNA damage in stressed cells. While ANXA1 has been reported to translocate to the nuclear or peri-nuclear region upon induction of stress (Nair et al. 2010), it can also be secreted out of the cell upon activation (Castro-Caldas et al. 2002; Perretti et al. 1996). However, our current study shows that ANXA1 acts more as an endogenous protein rather than a secreted protein as cells treated with supernatant from heat-treated cells did not exhibit suppressed TNFα levels. These results strongly indicate the absence of exogenous factors including secreted ANXA1 in regulating TNFα.
ANXA1 has also been shown to regulate MAPK and thus controls inflammation by playing a negative role in the regulation of the MAPK (Alldridge et al. 1999; Yang et al. 2006). In the present study, indeed, absence of ANXA1 induces a sustained activation of ERK after heat + LPS stimulation, which may be important for the protection against the heat stress response. Unlike ERK, pJNK was expressed only upon heat stress, and once again, ANXA1 deficiency resulted in a higher expression after heat and LPS. Given its specific response to stress stimuli and in this case, its higher expression in stressed ANXA1−/− cells, the inhibition of JNK using SP600125 resulted in significant inhibition of TNFα in both heat-stressed WT and ANXA1−/− cells as compared to the control cells. This indicates that in response to heat and LPS, ANXA1 downregulates JNK activity, which leads to low TNFα production. This could be through the higher expression of MKP1, which is an endogenous dual-specificity phosphatase inhibitor of the MAPK (Chi et al. 2006). This is in line with other studies showing the involvement of MKP-1 in stress response, with high levels during heat stress response, mediated by HSP70 (Lee et al. 2005). MKP1 has also been shown to regulate cytokine mRNA stability, which we also show in this study to be altered (Yu et al. 2011). Indeed, ANXA1 has been shown to negatively regulate IL-6 expression through the modulation of MKP1 (Yang et al. 2006), and HSP70 levels were also higher in WT cells expressing ANXA1 in our study, indicating that ANXA1 is required for HSP70 induction after heat. The role of HSP70 in reducing TNFα levels has previously been shown (Shi et al. 2006; van der Bruggen et al. 1999) and supports our postulation that in response to heat and LPS, ANXA1 increases HSP70 expression (mRNA and protein), which can increase MKP1 levels, which in turn inhibit ERK, p38, and more likely JNK activation, leading to low TNFα levels (Fig. 7).
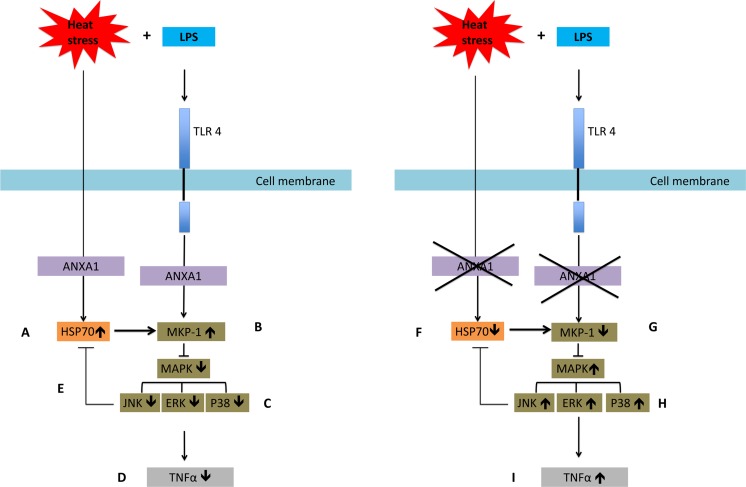
Fig. 7.
Summary of pathways involved. Upon heat treatment and LPS stimulation, a ANXA1 promotes HSP70 expression transcriptionally and translationally. b This leads to increased MKP1 expression, c which in turn inhibits the activation of the MAP kinases JNK, ERK, and p38, d which leads to a reduction in TNFα production. e JNK can also inhibit HSP70 to control its expression. In the absence of ANXA1, f HSP70 expression is reduced, g which leads to lower MKP1 expression, h which in turn increases the activation of the MAP kinases JNK, ERK, and p38. i This results in an increase in TNFα production
Our results also show that HSP70 and JNK regulate each other, which may be affecting TNFα production after heat. Inhibition of HSP70 led to an increase in pJNK levels, confirming that HSP70 was upstream of JNK. This is in line with other studies where HSP70 inhibits JNK activation during stress inducing the expression of MKP-1 (Gabai et al. 1997; Lee et al. 2005). However, when JNK was inhibited, HSP70 expression was also inhibited. This is the first report that JNK can also be regulating HSP70 in a feedback loop. In response to heat and LPS, HSP70 expression is increased, which leads to JNK inhibition, which in turn inhibits HSP70 expression. This may be the mechanism through which HSP70 returns back to original levels.
In conclusion, our study shows that ANXA1 played a protective role in inflammatory stress response to heat and thus protects cells from potential inflammatory insult during stress. We show that this could be through the regulation of MKP1, which results in lower MAPK activation and, thus, lower cytokine production after heat and LPS. Furthermore, ANXA1 promotes HSP70 activation, which could be important in the heat stress response (Fig. 7).
Acknowledgments
This work was supported by grants from Biomedical Research Council (BMRC) of Singapore (07/1/21/19/507) and NUHS (R-185-000-239-750) to LL.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
SN and LL conceived and designed the experiments; SN, SA, and LHL performed the experiments; SN and LL analyzed the data; JYL contributed reagents/tools; and SN and LL wrote the manuscript.
Abbreviations
- LPS
Lipopolysaccharide
- TNF
Tumor necrosis factor
- IL
Interleukin
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- ANXA1
Annexin-A1
- MYD88
Myeloid differentiation primary response 88
- HSP
Heat shock protein
- JNK
Jun kinase
- ERK
Extracellular signal-regulated kinase
- MAPK
Mitogen-activated protein kinase
- MKP1
Mitogen-activated protein kinase phosphatase-1
References
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Alldridge LC, Harris HJ, Plevin R, Hannon R, Bryant CE. The annexin protein lipocortin 1 regulates the MAPK/ERK pathway. J Biol Chem. 1999;274:37620–37628. doi: 10.1074/jbc.274.53.37620. [DOI] [PubMed] [Google Scholar]
- Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bist P, et al. Annexin-A1 regulates TLR-mediated IFN-beta production through an interaction with TANK-binding kinase 1. J Immunol. 2013;191:4375–4382. doi: 10.4049/jimmunol.1301504. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Caldas M, Duarte CB, Carvalho AP, Lopes MC. Dexamethasone induces the secretion of annexin I in immature lymphoblastic cells by a calcium-dependent mechanism. Mol Cell Biochem. 2002;237:31–38. doi: 10.1023/A:1016502120139. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZA, Singh IS, Hasday JD. Febrile range temperature represses TNF-alpha gene expression in LPS-stimulated macrophages by selectively blocking recruitment of Sp1 to the TNF-alpha promoter. Cell Stress Chaperones. 2010;15:665–673. doi: 10.1007/s12192-010-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-alpha expression. Am J Physiol. 1994;266:C967–974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Garekar S, Heidemann SM, Glibetic M. Heat stress response results in increased macrophage inflammatory protein-2 concentration in a lipopolysaccharide-exposed macrophage cell line. J Endotoxin Res. 2006;12:87–92. doi: 10.1179/096805106X89071. [DOI] [PubMed] [Google Scholar]
- Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, Singh IS. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem. 2013;288:2756–2766. doi: 10.1074/jbc.M112.427336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Hunt C, Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Genetics. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Kim GY, Lee HB, Lee SO, Rhee HJ, Na DS. Chaperone-like function of lipocortin 1. Biochem Mol Biol Int. 1997;43:521–528. doi: 10.1080/15216549700204321. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Preheating accelerates mitogen-activated protein (MAP) kinase inactivation post-heat shock via a heat shock protein 70-mediated increase in phosphorylated MAP kinase phosphatase-1. J Biol Chem. 2005;280:13179–13186. doi: 10.1074/jbc.M410059200. [DOI] [PubMed] [Google Scholar]
- Li CL, Wang XY, Shao J, Zhang JS, Feng WG, Wang YB, Chang ZL. Heat shock inhibits IL-12 p40 expression through NF-kappa B signalling pathway in murine macrophages. Cytokine. 2001;16:153–159. doi: 10.1006/cyto.2001.0971. [DOI] [PubMed] [Google Scholar]
- Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meng X, Harken AH. The interaction between Hsp70 and TNF-alpha expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17:345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes & Development. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Nair S, Hande MP, Lim LH. Annexin-1 protects MCF7 breast cancer cells against heat-induced growth arrest and DNA damage. Cancer Lett. 2010;294:111–117. doi: 10.1016/j.canlet.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Eskildsen-Helmond YE, Beedholm R. Molecular mechanisms of anti-aging hormetic effects of mild heat stress on human cells. Nonlinearity Biol Toxicol Med. 2004;2:105–116. doi: 10.1080/15401420490464376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rhee HJ, Kim GY, Huh JW, Kim SW, Na DS. Annexin I is a stress protein induced by heat, oxidative stress and a sulfhydryl-reactive agent. Eur J Biochem. 2000;267:3220–3225. doi: 10.1046/j.1432-1327.2000.01345.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, et al. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock. 2006;26:277–284. doi: 10.1097/01.shk.0000223134.17877.ad. [DOI] [PubMed] [Google Scholar]
- Snyder YM, Guthrie L, Evans GF, Zuckerman SH. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukoc Biol. 1992;51:181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Toshchakov V, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck BS. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 1999;67:3824–3829. doi: 10.1128/iai.67.8.3824-3829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, et al. Annexin 1 negatively regulates IL-6 expression via effects on p38 MAPK and MAPK phosphatase-1. J Immunol. 2006;177:8148–8153. doi: 10.4049/jimmunol.177.11.8148. [DOI] [PubMed] [Google Scholar]
- Yu H, Sun Y, Haycraft C, Palanisamy V, Kirkwood KL. MKP-1 regulates cytokine mRNA stability through selectively modulation subcellular translocation of AUF1. Cytokine. 2011;56:245–255. doi: 10.1016/j.cyto.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]