Abstract
Recent data indicate that serum Hsp70 (HSPA1A) levels are increased in type 1 and 2 diabetes mellitus. However, there is no report in the literature on circulating Hsp70 levels in gestational diabetes mellitus. In this pilot study, we measured serum Hsp70 levels in 11 pregnant women with pregestational diabetes, 38 women with gestational diabetes, and 40 healthy pregnant women with ELISA. Plasma glucose levels, serum insulin concentrations, HbA1c values, and the Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) index were also determined. According to our results, serum Hsp70 concentrations were significantly higher in women with pregestational and gestational diabetes mellitus than in healthy pregnant women. In addition, pregestational diabetic women had significantly higher Hsp70 levels than those with gestational diabetes. Furthermore, in the group of women with gestational diabetes mellitus, serum Hsp70 levels showed a significant positive correlation with HbA1c values. However, there was no other relationship between clinical features and metabolic parameters of the study subjects and their serum Hsp70 levels in either study group. In conclusion, we demonstrated for the first time in the literature that serum Hsp70 levels are increased and correlate with HbA1c values in women with gestational diabetes mellitus. Nevertheless, further studies are needed to determine whether circulating Hsp70 plays a causative role in the pathogenesis of gestational diabetes or elevated serum Hsp70 levels are only consequences of the disease.
Keywords: Gestational diabetes, Heat shock protein 70, Inflammation, Oxidative stress, Pregnancy
Introduction
Gestational diabetes mellitus (GDM) is a carbohydrate intolerance resulting in hyperglycemia of variable severity with an onset or first recognition during pregnancy (Alberti and Zimmet 1998). GDM affects 3–5 % of pregnant women and it is associated with an increased maternal and perinatal morbidity and mortality (Ben-Haroush et al. 2004). GDM mothers have an increased risk of preeclampsia, urinary tract infections, cesarean section, as well as type 2 diabetes mellitus later in life (Casey et al. 1997; Kim et al. 2002; Yogev et al. 2004). Perinatal complications include macrosomia, polyhydramnios, shoulder dystocia with birth trauma, neonatal metabolic disturbances, and perinatal death (Casey et al. 1997; Dudley 2007; Hod et al. 1991). To determine whether GDM is present in pregnant women, the World Health Organization recommends screening between 24 and 28 weeks of gestation by means of a 75-g oral glucose tolerance test (OGTT) (Alberti and Zimmet 1998). There is an increasing body of evidence that insulin resistance caused by high levels of several diabetogenic hormones produced by the placenta plays a central role in the pathogenesis of GDM (Dahlgren 2006). The development of GDM is influenced by both genetic and environmental risk factors, suggesting its multifactorial inheritance (Shaat and Groop 2007).
Heat shock proteins (Hsps) are ubiquitous and phylogenetically conserved molecules, which indicate their functional importance. They are usually considered to be intracellular proteins with molecular chaperone and cytoprotective functions (Hightower 1991). However, 70 kDa heat shock protein (Hsp70, HSPA1A) is present in the peripheral circulation of healthy non-pregnant individuals (Pockley et al. 1998). As HSPA1A is the most inducible form of the Hsp70 superfamily, we have chosen to study this protein. We have previously reported that serum Hsp70 levels are significantly lower in healthy pregnant than in healthy non-pregnant women (Molvarec et al. 2007b). In addition, we observed increased circulating Hsp70 concentrations in women with preeclampsia (Molvarec et al. 2006), as well as in pregnant asthmatics (Tamasi et al. 2010). Furthermore, we demonstrated that serum Hsp70 levels are significantly higher in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome) than in severely preeclamptic patients without HELLP syndrome (Molvarec et al. 2007a).
Recent data indicate that serum Hsp70 levels are increased in type 1 and 2 diabetes mellitus (Morteza et al. 2013; Nakhjavani et al. 2010, 2011, 2012; Oglesbee et al. 2005). However, there is no report in the literature on circulating Hsp70 levels in gestational diabetes mellitus. The aim of the present study was to determine serum Hsp70 (HSPA1A) levels in pregnant women with pregestational diabetes, women with gestational diabetes, and healthy pregnant women. We also examined whether parameters of carbohydrate metabolism are related to circulating Hsp70 levels in order to evaluate the involvement of this protein in the pathophysiology of gestational diabetes mellitus.
Materials and methods
Study participants
Eleven pregnant women with pregestational diabetes, 38 women with gestational diabetes, and 40 healthy pregnant women with a normal oral glucose tolerance test were involved in our case–control study. The study participants were enrolled in the First Department of Obstetrics and Gynecology at the Semmelweis University, Budapest, Hungary, between January 2004 and January 2006. All women were Caucasian and resided in the same geographic area in Hungary. The women were fasting, none were in active labor, and none had rupture of amniotic membranes.
Gestational diabetes mellitus was diagnosed with 75-g oral glucose tolerance test (OGTT) using the World Health Organization criteria (Alberti and Zimmet 1998). Blood samples were taken before and 120 min after the glucose load (0 and 120 min). All women in the healthy pregnant control group had normal carbohydrate tolerance. Women in the pregestational diabetes group had diabetes mellitus before pregnancy and all of them were on insulin treatment.
The study protocol was approved by the Regional and Institutional Committee of Science and Research Ethics of the Semmelweis University, and written informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki.
Biological samples
Maternal blood samples were obtained from an antecubital vein into native tubes, as well as tubes containing sodium fluoride and disodium EDTA, and centrifuged at room temperature with a relative centrifugal force of 3000g for 10 min. The aliquots of serum were stored at −80 °C until the measurement of Hsp70 levels. For hemoglobin A1c (HbA1c) determination, EDTA-anticoagulated whole blood was used. In the healthy pregnant and GDM groups, blood samples were taken when OGTT was performed (at 0 min).
Laboratory methods
Serum Hsp70 levels were measured by using the ELISA Kit of R&D Systems (DYC1663E, Minneapolis, MN, USA). Ninety-six-well microtiter plates were coated with mouse anti-human Hsp70 capture antibodies (100 μl; 2 μg/ml) in carbonate buffer (pH 9.5) overnight at 4 °C. Plates were washed with phosphate-buffered saline (PBS) containing 0.1 % Tween 20 three times and non-specific binding sites blocked by incubation with 200 μl of PBS containing 0.5 % gelatine and Tween 20 for 1 h at room temperature. After washing, 100 μl of the reference preparation (recombinant human Hsp70, 0–10 ng/ml) or samples (1:1) were added and the plates were incubated for 2 h at room temperature. Plates were subsequently washed and Hsp70 binding was determined using biotinylated rabbit anti-human antibodies (100 μl; 0.5 μg/ml) in PBS gelatine. After 1.5 h at room temperature, plates were washed and incubated with streptavidin-horseradish-peroxidase (HRP, 1:200) in PBS gelatine for 20 min at room temperature. Plates were washed and 100 μl of o-phenylene-diamine (OPD, Sigma, St. Louis, MO, USA) in citrate buffer was added. The optical density was measured at λ = 490 nm (reference at λ = 620 nm). The detection range of the assay was 0.05–10 ng/ml, the intra/inter-assay variability <10/<16 %, respectively.
Plasma glucose levels were determined by the GOD-PAP method using Glucose LiquiColor reagent (Human Diagnostics, Wiesbaden, Germany, Cat. No. 10260) on a BioSystems A25 chemistry analyzer (BioSystems, Barcelona, Spain). Serum insulin concentrations were measured by electrochemiluminescence immunoassay (ECLIA) by an autoanalyzer (cobas 6000, Roche, Mannheim, Germany) using the manufacturer’s kits. HbA1c values were determined by high-performance liquid chromatography (HPLC) using the Variant™ II Turbo Hemoglobin Testing System (Bio-Rad Laboratories, Hercules, California, USA) according to the manufacturer’s instructions. The Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) index was calculated as follows: (fasting glucose (mmol/l) × fasting insulin (mU/l)) / 22.5.
Statistical analysis
The normality of continuous variables was assessed using the Shapiro-Wilk’s W test. As the continuous variables were not normally distributed, nonparametric statistical methods were used. To compare continuous variables between two groups, the Mann-Whitney U test was applied, whereas to compare them among multiple groups, the Kruskal-Wallis analysis of variance by ranks test was performed. Multiple comparisons of mean ranks for all groups were carried out as post-hoc tests. As serum levels of Hsp70 showed skewed distributions, we performed analyses of covariance (ANCOVA) with logarithmically transformed data. The Spearman rank order correlation was carried out to calculate correlation coefficients. The scatterplot was created, as a nonparametric method, with logarithmically transformed values of the dependent variable.
Statistical analyses were carried out using the following software: STATISTICA (version 12; StatSoft, Inc., Tulsa, OK, USA) and Statistical Package for the Social Sciences (version 22 for Windows; SPSS, Inc., Chicago, IL, USA). For all statistical analyses, p < 0.05 was considered statistically significant. In the article, data are reported as median (interquartile range) for continuous variables.
Results
Patient characteristics and metabolic parameters
The clinical characteristics of the study participants are shown in Table 1. There were no statistically significant differences in pre-pregnancy body mass index (BMI), gestational age at delivery, and fetal birth weight among the three study groups. However, maternal age was significantly higher in women with gestational diabetes than in healthy pregnant women and women with pregestational diabetes. In addition, the gestational age at blood sampling was significantly lower in the group of women with gestational diabetes mellitus than in the control group. Birth trauma or congenital malformation was not observed in our study groups.
Table 1.
Clinical characteristics of the study participants
| Normal pregnancy (n = 40) | Gestational diabetes (n = 38) | Pregestational diabetes (n = 11) | |
|---|---|---|---|
| Age (years) | 30 (28–32) | 33 (29–36)a | 26 (24–33)b |
| Pre-pregnancy BMI (kg/m2) | 21.9 (19.0–26.2) | 23.3 (21.4–29.0) | 22.3 (19.9–30.0) |
| Gestational age at blood draw (weeks) | 26 (24–28) | 24 (23–25)a | 25 (24–27) |
| Gestational age at delivery (weeks) | 39 (38–40) | 38 (37–39) | 38 (37–39) |
| Fetal birth weight (grams) | 3350 (3050–3550) | 3150 (2650–3380) | 3400 (2700–3500) |
Data are presented as median (25–75 percentile)
BMI body mass index
a p < 0.05 versus normal pregnancy
b p < 0.05 pregestational diabetes versus gestational diabetes
Metabolic parameters of women with gestational and pregestational diabetes mellitus are presented in Table 2. In pregestational diabetic women, fasting plasma glucose, HbA1c, fasting serum insulin, and HOMA-IR values were significantly higher than in women with gestational diabetes mellitus.
Table 2.
Metabolic parameters of women with gestational and pregestational diabetes mellitus at time of blood draw for serum Hsp70 measurement
| Gestational diabetes (n = 38) | Pregestational diabetes (n = 11) | Statistical significance (p value) | |
|---|---|---|---|
| Fasting plasma glucose (mmol/l) | 4.8 (4.5–5.2) | 5.6 (5.6–5.6) | <0.05 |
| OGTT 120 min (mmol/l) | 8.7 (8.3–9.5) | N.M. | |
| HbA1c (%) | 5.2 (5.1–5.6) | 6.2 (5.7–7.0) | <0.001 |
| Fasting serum insulin (mU/l) | 7.9 (4.8–13.5) | 22.7 (16.0–34.7) | <0.001 |
| HOMA-IR | 1.8 (0.9–3.3) | 5.6 (4.0–8.6) | <0.001 |
Data are presented as median (25–75 percentile)
N.M. not measured, OGTT 75-g oral glucose tolerance test, Hb hemoglobin, HOMA-IR Homeostatic Model Assessment-Insulin Resistance
Serum heat shock protein 70 (Hsp70, HSPA1A) levels
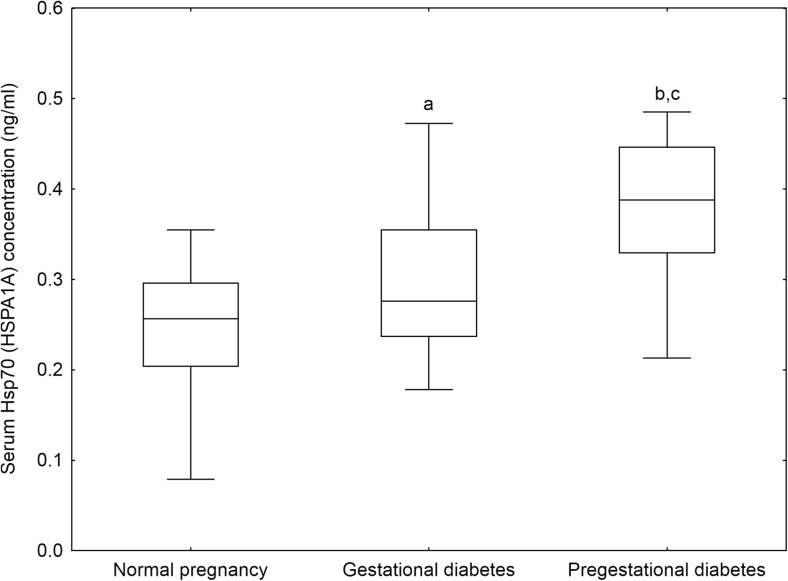
Serum levels of Hsp70 (HSPA1A) in our study groups are demonstrated in Fig. 1. Serum Hsp70 concentrations were significantly higher in women with pregestational (0.39 (0.33–0.45) ng/ml, p < 0.001) and gestational diabetes mellitus (0.28 (0.24–0.35) ng/ml, p < 0.05) than in healthy pregnant women (0.26 (0.20–0.30) ng/ml). Furthermore, pregestational diabetic women had significantly higher Hsp70 levels than those with gestational diabetes (p < 0.05). The differences in serum Hsp70 levels between the study groups remained significant even after adjustment for maternal age, pre-pregnancy BMI, and gestational age at blood draw in analysis of covariance (adjusted mean ± standard error of log (serum Hsp70 concentration (ng/ml)): −0.63 ± 0.03 in normal pregnancy, −0.51 ± 0.03 in GDM, and −0.42 ± 0.05 in pregestational diabetes; p < 0.05).
Fig. 1.
Serum heat shock protein 70 (Hsp70, HSPA1A) levels of the study participants. Middle line median, Box interquartile range (25–75 percentile), Whisker range (excluding outliers), a p < 0.05 versus normal pregnancy, b p < 0.001 versus normal pregnancy, c p < 0.05 pregestational diabetes versus gestational diabetes
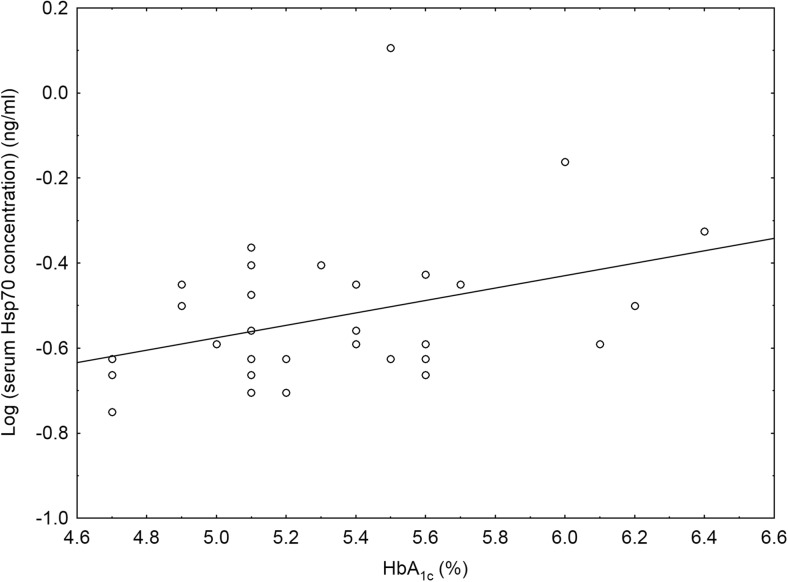
We also investigated whether the clinical characteristics and metabolic parameters of the study participants were related to their serum Hsp70 levels by calculating the Spearman rank order correlation coefficients. In the group of women with gestational diabetes mellitus, serum Hsp70 levels showed a significant positive correlation with HbA1c values (Spearman R = 0.37, p < 0.05; Fig. 2). However, there was no other relationship between clinical features and metabolic parameters of the study subjects and their serum Hsp70 levels in either study group.
Fig. 2.
Scatterplot with linear fit and the regression line of logarithmically transformed values of serum heat shock protein 70 (Hsp70, HSPA1A) levels versus hemoglobin A1c (HbA1c) values in patients with gestational diabetes mellitus
Discussion
In this pilot study, we determined serum Hsp70 (HSPA1A) levels in pregnant women with pregestational diabetes, women with gestational diabetes, and healthy pregnant women. According to our results, serum Hsp70 concentrations were significantly higher in women with pregestational and gestational diabetes mellitus than in healthy pregnant women. In addition, pregestational diabetic women had significantly higher Hsp70 levels than those with gestational diabetes. Furthermore, in the group of women with gestational diabetes mellitus, serum Hsp70 levels showed a significant positive correlation with HbA1c values.
A growing number of studies demonstrate that circulating Hsp70 levels are elevated in type 1 and 2 diabetes mellitus (Morteza et al. 2013; Nakhjavani et al. 2010, 2011, 2012; Oglesbee et al. 2005). Inflammation and oxidative stress, characteristic features of diabetes mellitus, have been suggested to account for the increase in serum Hsp70 concentrations in these disorders. Indeed, oxidative stress has been implicated in the exercise-induced circulating Hsp70 response and the supplementation with vitamin C and the vitamin E isoform γ-tocopherol completely blunted this response (Fischer et al. 2006). Interestingly, Hsp70 levels in peripheral blood mononuclear cells were found to be elevated reflecting increased oxidative stress in patients with type 2 diabetes (Yabunaka et al. 1995). Additionally, a significant up-regulation of Hsp70 has been detected in peripheral blood lymphocytes from type 2 diabetic patients with and without nephropathy, representing a maintained response in counteracting the systemic pro-oxidant status (Calabrese et al. 2007, 2012). It is noteworthy that peripheral blood mononuclear cells are capable of releasing Hsp70 into the extracellular space (Hunter-Lavin et al. 2004a). Furthermore, the antioxidant folic acid, which reduces oxidative stress in vivo, significantly decreased serum Hsp70 levels in non–insulin-treated type 2 diabetes patients (Hunter-Lavin et al. 2004b).
Inflammatory mediators and oxidative stress have also been implicated in the pathogenesis of gestational diabetes mellitus (Ategbo et al. 2006; Lappas et al. 2011; Winkler et al. 2002). Therefore, it is tempting to speculate that increased serum Hsp70 levels in gestational diabetes observed in this study reflect—at least in part—systemic inflammation and oxidative stress. Indeed, we have previously demonstrated that increased serum Hsp70 concentrations in women with preeclampsia are related to pro-inflammatory changes in circulating cytokine profile, as well as to serum C-reactive protein and plasma malondialdehyde levels (Molvarec et al. 2009, 2011). Further studies are required to determine the relationship between serum Hsp70 levels and markers of inflammation and oxidative stress in women with GDM.
Hyperglycemia is another candidate to be responsible for the increase in serum Hsp70 concentrations in pregnant women with pregestational diabetes and GDM. Certainly, circulating Hsp70 levels paralleled changes in serum glucose levels in diabetic ketoacidosis, suggesting that hyperglycemia might induce the release of Hsp70 from hepato-splanchnic viscera (Oglesbee et al. 2005). In addition, serum levels of Hsp70 markedly increased after glucose ingestion in non-obese Black pregnant women (Jaffe et al. 2013). In an experimental model, serum Hsp70 levels were significantly higher in pregnant rats with severe than in those with mild diabetes possessing lower blood glucose levels (Saito et al. 2013). Interestingly, we observed a significant positive correlation between serum Hsp70 concentrations and HbA1c values in women with GDM, suggesting that chronic hyperglycemia might contribute to the elevation in serum Hsp70 levels observed in GDM. Our finding that circulating Hsp70 concentrations are significantly higher in pregnant women with pregestational diabetes than in those with GDM is consistent with previous data demonstrating that serum Hsp70 levels are associated with the duration of diabetes, especially in women (Nakhjavani et al. 2010, 2011).
Adipose tissue expansion has also been linked to elevated circulating Hsp70 levels in diabetes. In a previous study, plasma Hsp70 levels were observed to be increased only in obese but not in non-obese type 2 diabetic patients as compared to obese controls (Rodrigues-Krause et al. 2012). However, the authors did not involve non-obese controls in their study. There were significant positive correlations between logarithmic-transformed serum Hsp70 concentrations and BMI values in patients with long-standing diabetes, in those with newly diagnosed disease, as well as in healthy controls in another study (Nakhjavani et al. 2010). These data are at variance with our results, as we did not find any association between BMI values and serum Hsp70 levels in pregnant women with pregestational or gestational diabetes, or in healthy pregnant women. Nevertheless, the majority of our study participants were of normal weight.
Elevated circulating Hsp70 level may not only be a marker of gestational diabetes mellitus, but might also play a role in its pathogenesis. Extracellular Hsp70 derived from stressed and damaged, necrotic cells can act as an intercellular stress signaling molecule, representing an ancestral danger signal of a non-physiological condition, such as cellular stress or damage, to elicit innate and adaptive pro-inflammatory immune responses (Pockley 2003). Extracellular Hsp70 acts through binding to surface receptors (CD14, CD36, CD40, CD91, LOX-1, and Toll-like receptors 2 and 4) on antigen-presenting cells, stimulating their pro-inflammatory cytokine (tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6) and chemokine production, as well as costimulatory molecule expression (Asea 2005; Asea et al. 2000, 2002; Basu et al. 2001). Therefore, it is possible that circulating Hsp70 may contribute to the development of systemic inflammation observed in gestational diabetes mellitus. Indeed, increased circulating Hsp70 levels have already been revealed to be associated with inflammatory responses in several pathological conditions, such as in acute infections, after liver resection and coronary artery bypass grafting, as well as following myocardial infarction (Dybdahl et al. 2002, 2005; Kimura et al. 2004; Njemini et al. 2003). Furthermore, although intracellular Hsp70 may combat insulin resistance (Bruce et al. 2003; Chung et al. 2008; Kavanagh et al. 2011; Kurucz et al. 2002; McCarty 2006; Simar et al. 2012), extracellular Hsp70 has recently been shown to correlate with insulin resistance in vivo and to cause pancreatic β cell dysfunction and death in vitro (Krause et al. 2014). However, we did not observe any relationship between circulating Hsp70 concentrations and serum insulin levels or HOMA-IR values in our study groups.
Nevertheless, the lack of association between increased serum Hsp70 levels and clinical features and metabolic parameters of pregnant women with pregestational or gestational diabetes may also be due to genetic variations in Hsp70 production, release into the extracellular space or stability. Additionally, Hsp70, with its intrinsic ability to act as a chaperone, can bind to other macromolecules, which might mask its detection by ELISA. Moreover, as all women in the pregestational diabetes group were on insulin treatment, serum insulin levels, and HOMA-IR values are not useful to determine insulin resistance in this group.
In conclusion, we demonstrated for the first time in the literature that serum Hsp70 levels are increased and correlate with HbA1c values in women with gestational diabetes mellitus. However, additional studies are needed to determine whether circulating Hsp70 plays a causative role in the pathogenesis of gestational diabetes or elevated serum Hsp70 levels are only consequences of the disease.
Acknowledgments
The skillful technical assistance of Szigeti Antalné is acknowledged with many thanks. This work was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, as well as by a research grant from the Hungarian Scientific Research Fund (PD 109094).
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med : J Br Diabet Assoc. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Asea A, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Ategbo JM, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med : J Br Diabet Assoc. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Calabrese V, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822:729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869–873. doi: 10.1016/S0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- Chung J, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J. Pregnancy and insulin resistance. Metab Syndr Relat Disord. 2006;4:149–152. doi: 10.1089/met.2006.4.149. [DOI] [PubMed] [Google Scholar]
- Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Obstet Gynecol Clin N Am. 2007;34:293–307. doi: 10.1016/j.ogc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CP, et al. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol. 2006;100:1679–1687. doi: 10.1152/japplphysiol.00421.2005. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hod M, Merlob P, Friedman S, Schoenfeld A, Ovadia J. Gestational diabetes mellitus. A survey of perinatal complications in the 1980s. Diabetes. 1991;40(Suppl 2):74–78. doi: 10.2337/diab.40.2.S74. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, et al. Folate supplementation reduces serum hsp70 levels in patients with type 2 diabetes. Cell Stress Chaperones. 2004;9:344–349. doi: 10.1379/CSC-28R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe S, et al. Induction of the 72 kDa heat shock protein by glucose ingestion in black pregnant women. Cell Stress Chaperones. 2013;18:527–530. doi: 10.1007/s12192-013-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys American journal of physiology. Endocrinol Metab. 2011;300:E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- Kimura F, et al. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg. 2004;187:777–784. doi: 10.1016/j.amjsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Krause M, et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci. 2014;126:739–752. doi: 10.1042/CS20130678. [DOI] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011;15:3061–3100. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66:527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case–control study. J Hum Hypertens. 2006;20:780–786. doi: 10.1038/sj.jhh.1002060. [DOI] [PubMed] [Google Scholar]
- Molvarec A, et al. Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2007;73:172–179. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, et al. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74:163–169. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, et al. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14:151–159. doi: 10.1007/s12192-008-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Szarka A, Walentin S, Beko G, Karadi I, Prohaszka Z, Rigo J., Jr Serum heat shock protein 70 levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in women with preeclampsia. Clin Chim Acta; Int J Clin Chem. 2011;412:1957–1962. doi: 10.1016/j.cca.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Morteza A, Nakhjavani M, Larry M, Nargesi AA, Esteghamati A. Heat shock protein 70 and albuminuria in patients with type 2 diabetes: a matched case control study. Cell Stress Chaperones. 2013;18:815–819. doi: 10.1007/s12192-013-0435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, et al. Serum heat shock protein 70 and oxidized LDL in patients with type 2 diabetes: does sex matter? Cell Stress Chaperones. 2011;16:195–201. doi: 10.1007/s12192-010-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Asgarani F, Khalilzadeh O, Ghazizadeh Z, Bathaie SZ, Esteghamati A. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498:107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58:664–669. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. Clin Biochem. 2005;38:900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito FH, Damasceno DC, Dallaqua B, Linhares IM, Rudge MV, De Mattos Paranhos Calderon I, Witkin SS. Heat shock protein production and immunity and altered fetal development in diabetic pregnant rats. Cell Stress Chaperones. 2013;18:25–33. doi: 10.1007/s12192-012-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaat N, Groop L. Genetics of gestational diabetes mellitus. Curr Med Chem. 2007;14:569–583. doi: 10.2174/092986707780059643. [DOI] [PubMed] [Google Scholar]
- Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signalling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17:615–621. doi: 10.1007/s12192-012-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamasi L, et al. Increased circulating heat shock protein 70 levels in pregnant asthmatics. Cell Stress Chaperones. 2010;15:295–300. doi: 10.1007/s12192-009-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G, et al. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Res Clin Pract. 2002;56:93–99. doi: 10.1016/S0168-8227(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Yabunaka N, Ohtsuka Y, Watanabe I, Noro H, Fujisawa H, Agishi Y. Elevated levels of heat-shock protein 70 (HSP70) in the mononuclear cells of patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;30:143–147. doi: 10.1016/0168-8227(95)01151-X. [DOI] [PubMed] [Google Scholar]
- Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655–1660. doi: 10.1016/j.ajog.2004.03.074. [DOI] [PubMed] [Google Scholar]