Abstract
In Leishmania donovani, the HSP90 chaperone complex plays an essential role in the control of the parasite’s life cycle, general viability and infectivity. Several of the associated co-chaperones were also shown to be essential for viability and/or infectivity to mammalian cells. Here, we identify and describe the co-chaperone P23 and distinguish its function from that of the structurally related small heat shock protein HSP23. P23 is expressed constitutively and associates itself with members of the HSP90 complex, i.e. HSP90 and Sti1. Viable P23 gene replacement mutants could be raised and confirmed as null mutants without deleterious effects on viability under a variety of physiological growth conditions. The null mutant also displays near-wild-type infectivity, arguing against a decisive role played by P23 in laboratory settings. However, the P23 null mutant displays a marked hypersensitivity against HSP90 inhibitors geldanamycin and radicicol. P23 also appears to affect the radicicol resistance of a HSP90 Leu33-Ile mutant described previously. Therefore, the annotation of L. donovani P23 as HSP90-interacting co-chaperone is confirmed.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0595-y) contains supplementary material, which is available to authorized users.
Keywords: Leishmania, HSP90, P23, Co-chaperone, Radicicol, Geldanamycin
Introduction
The protozoan parasites of the genus Leishmania cause a variety of human diseases, ranging from self-healing skin lesions (cutaneous leishmaniasis) to lethal, generalised infections of the reticulo-endothelial system that are difficult to control (visceral leishmaniasis). The infections are distributed over four continents with an estimated annual incidence between 900,000 and 1.8 million (Alvar et al. 2012).
Leishmania parasites exist in two morphologically distinct life cycle stages—the extracellular, flagellated, cigar-shaped promastigote stage in the insect vector and the aflagellated, ovoid amastigote stage found primarily in the phagosomes of infected mammalian macrophages. The transmission of Leishmania spp. by a poikilothermic insect vector to a homeothermic mammalian host is linked to an increase in the ambient temperature. This induces the parasite’s heat shock response which is directly linked to the pivotal conversion of the promastigote to the amastigote stage.
The heat shock response is a universal mechanism that protects cells and organisms against a variety of stresses such as elevated temperature, acidic milieu, heavy metal ions and oxidative stress (Morimoto et al. 1990; Vonlaufen et al. 2008). The cellular response against these stresses is characterised by an increased synthesis of heat shock proteins (HSPs). Under stressful conditions, HSPs interact with denatured proteins and inhibit the formation of cytotoxic protein aggregates (Bukau et al. 2006; Feder and Hofmann 1999; Mayer and Bukau 1999). Under physiological conditions, most HSPs have chaperone activities, thereby facilitating the activation, maturation, translocation or degradation of a diverse set of so-called client proteins.
The genomes of Leishmania spp. encode the full repertoire of HSP families ranging from large HSPs, such as HSP100, to small HSPs, such as HSP23 or HSP20 (Clos and Hombach 2015). The HSPs are either synthesised constitutively during both life cycle stages (Brandau et al. 1995; Rosenzweig et al. 2008) or their abundance increases during amastigote differentiation (Hombach et al. 2014; Hübel et al. 1995; Krobitsch et al. 1998; Schlüter et al. 2000; Zamora-Veyl et al. 2005).
HSP90 is an essential, conserved and constitutively expressed protein that is associated with the maturation, folding and activation of a variety of highly diverse client proteins such as kinases, transcription factors and growth hormone receptors (Buchner 1999; Nathan and Lindquist 1995; Picard 2002; Pratt and Toft 2003). HSP90 interacts with the chaperones HSP70, HSP40 and a various number of co-chaperones to form large complexes known as foldosomes. The interplay of chaperones and co-chaperones facilitates the proper folding of essential clients. The various co-chaperones are able to modulate HSP90 functions at different steps of the client maturation cycle. They can act as adaptor proteins between HSP70 and HSP90, inhibit or activate the ATPase activity of HSP90, deliver specific clients to the HSP90 dimer, stabilise the HSP90/client protein complex or influence the fate of clients (Johnson 2012; Johnson and Brown 2009; Li et al. 2012; Mayer and Bukau 1999).
The chaperone/client complex is disrupted when the HSP90 ATP-binding site is occupied by the specific inhibitors geldanamycin (GA) and/or radicicol (RAD) (Grenert et al. 1997; Prodromou et al. 1997; Schulte et al. 1998, 1999; Stebbins et al. 1997). These inhibitors block the ATP hydrolysis-dependent functions of the chaperone, which results in the release of the unfolded or unstable client from HSP90 and the subsequent proteolytic degradation of the client protein in the proteasome (Panaretou et al. 1998; Taipale et al. 2012).
One of the HSP90-interacting co-chaperones in eukaryotes is P23. It is a small acidic protein that acts late in the HSP90-mediated client maturation cycle and has affinity for the ATP-bound form of the HSP90 dimer (Grenert et al. 1999; Li et al. 2012). As a result, ATP hydrolysis, which is essential for the release of a client protein, is partially inhibited in the presence of P23 (Obermann et al. 1998; Panaretou et al. 1998). In addition to its HSP90 co-chaperone function, P23 has its own passive chaperoning activity in vitro and is able to suppress the aggregation of denatured proteins (Bose et al. 1996; Freeman et al. 1996).
P23 and the small HSPs share a region of approximately 80–100 amino acids, known as the α-crystallin domain (ACD). The ACD is located near the N terminus and forms a β-sheet structure which appears to be sufficient for the interaction with HSP90 (Forafonov et al. 2008; Garcia-Ranea et al. 2002; Martinez-Yamout et al. 2006; Weaver et al. 2000).
The crucial role of P23 in the HSP90 cycle can be proven using the HSP90 inhibitors GA and RAD, which prevent the interaction of P23 with HSP90. This disrupts the HSP90 cycle and the maturation of client proteins (Forafonov et al. 2008; Stebbins et al. 1997). SBA1, the P23 ortholog in yeast, is not essential for viability, but sba1 null mutants are more sensitive to the inhibitors GA and RAD. Thus, P23/SBA1 can protect HSP90 by blocking the interaction with these inhibitors (Forafonov et al. 2008).
In L. donovani, the function of HSP90 is crucial for the promastigote and amastigote stages. The pharmacological inhibition of HSP90 by GA and RAD arrests cell growth. It also induces the conversion from promastigotes to amastigote-like cells, an increased expression of HSPs and the synthesis of the amastigote-specific A2 protein family (Wiesgigl and Clos 2001). The pharmacological inhibition of HSP90 also impairs the proliferation of amastigotes in infected ex vivo macrophages (Hombach et al. 2013).
The genomes of Leishmania parasites encode a broad range of co-chaperones pointing towards the existence of the foldosome complex in these organisms (Clos and Hombach 2015; Johnson and Brown 2009; Ommen and Clos 2009). The leishmaniae are also well suited for reverse genetics, allowing the creation of null mutants and subsequent phenotype analysis. The functions and roles of several HSP90-associated co-chaperones in Leishmania spp. were analysed in this manner, including stress-inducible protein 1 (Sti1) (Hombach et al. 2013; Morales et al. 2010), heat shock protein organising protein 2 (HOP2) and heat-inducible protein (HIP) (Ommen et al. 2009), small glutamine-rich tetratricopeptide domain protein (SGT) (Ommen et al. 2010), and cyclophilin 40 (Cyp40) (Yau et al. 2014).
In L. infantum, as in other leishmaniae, a putative P23 (LinJ.35.4540) ortholog could be identified (Batista et al. 2012; Hombach et al. 2014; Johnson and Brown 2009). This protein possesses an ACD and shares other structural and functional properties with the human P23 or the yeast ortholog SBA1 (Batista et al. 2015; Hombach et al. 2014). Nevertheless, its in vivo functions have not been investigated to date.
Given our interest in HSP90 and the co-chaperone machinery, we characterised P23 by reverse genetics, overexpression analysis and subcellular localisation studies. Furthermore, we analysed the sensitivity of P23 null mutants (P23−/−) of L. donovani against various stresses, in particular the HSP90 inhibitors GA and RAD. We found the gene to be non-essential during the promastigote stage and for general stress tolerance in this life cycle stage. However, like its yeast ortholog SBA1, P23 protects the cells against HSP90 inhibitor-induced growth arrest and is therefore confirmed as P23 co-chaperone.
Materials and methods
L. donovani cell culture
L. donovani 1SR (Rosenzweig et al. 2007) promastigotes were grown at 25 °C in supplemented M199+ medium and cultivated as described before (Krobitsch et al. 1998). For routine cell culture, the promastigote cells were grown to late log phase in order to maintain exponential growth. Cell density was measured using the CASY Cell Counter (Roche, Penzberg, Germany). After electrotransfection, recombinant parasites were cultivated in supplemented M199+ with the appropriate selection antibiotics.
The IC50 were determined for absolut ethanol (Carl Roth, Karlsruhe, Germany), antimony tartrate (Sigma-Aldrich, München, Germany), geldanamycin (CAYLA-Invivogen, Toulouse, France) and radicicol (Sigma-Aldrich, München, Germany).
Electrotransfection and selection
Electrotransfection of L. donovani promastigotes was carried out as described before (Ommen et al. 2009). Leishmania parasites were taken from a late log phase culture, sedimented (720 g, 8 min, 4 °C), washed twice in ice-cold PBS, once in electroporation buffer and suspended at a density of 1 × 108 ml−1 in electroporation buffer (Kapler et al. 1990; Laban and Wirth 1989). Two micrograms of linear DNA or 50 μg of circular DNA were used for the transfection. The DNA was mixed on ice in a 4-mm electroporation cuvette with 0.4 ml of the cell suspension. Electroporation was carried out using a Gene Pulser apparatus (BioRad, München, Germany), with three pulses at 2.750 V/cm, 25 μF and 200 Ω. Following electroporation, cells were kept on ice for 10 min before they were transferred to 10-ml drug-free M199+ medium. The antibiotics, bleomycin (5 μg ml−1, Merck-Millipor, Germany), puromycin (25 μg ml−1, Sigma-Aldrich, München, Germany) and ClonNat (150 μg ml−1, Werner Bioreagents, Jena, Germany) were added after 24 h. Mock transfection was performed in identical fashion, without DNA, to obtain negative control cultures for antibiotic selection.
For cloning, promastigotes were seeded in supplemented M199+ with the appropriate antibiotics at 0.5 cells per well in microtitre plates. After 10–14 days, wells positive for promastigote growth were identified and the clonal cells were transferred to culture flasks.
In vitro stage differentiation
Axenic amastigotes of L. donovani 1SR were differentiated as described before (Krobitsch et al. 1998). For this purpose, the differentiation of promastigotes towards axenic amastigotes was induced by a combination of 37 °C cultivation temperature and a time-coordinated acidification of the culture medium to pH 5.5. Axenic amastigotes were cultivated at 37 °C with 5 % CO2. For amastigote quantification, the CASY Cell Counter was run in cumulative cell volume mode to include cell clusters. Equal cell volume aliquots were applied to sodium dodecyl sulfate polyacrylamide gel electrophoreses (SDS-PAGE) and Western blots or quantified via real-time RT-PCR.
In vitro infection experiments
Murine bone marrow macrophages (BMM) were isolated from the femurs and tibias of female C57BL/6 mice and differentiated in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10 % heat-inactivated FCS, 5 % horse serum and 30 % L929 supernatant (Hombach et al. 2014; Racoosin and Swanson 1989). For infection experiments, BMMs were harvested, washed and seeded into the wells of eight-well chamber slides (NUNC, Roskilde, Denmark) at a density of 2 × 105 cells per well and incubated for 48–72 h a 37 °C and 5 % CO2. Adherent BMMs were infected with stationary-phase promastigotes at a ratio of 1:10 (2 × 106). After 4 h of incubation at 37 °C in supplemented M199+, non-phagocytosed parasites were removed by multiple washing with PBS and incubation was continued for another 44 h in IMDM at 37 °C and 5 % CO2. The medium was removed, and the cells were washed twice with PBS. Finally, the infected BMMs were fixed with ice-cold methanol. Intracellular parasites were quantified by nuclear staining with DAPI (1.25 μm ml−1, Sigma-Aldrich, München, Germany). Infection rates and parasite loads were quantified. The stained cells and parasites were analysed by fluorescent microscopy.
Construction and preparation of recombinant DNA
Approximately 1000 bp of 5′ non-coding DNA (5′NC) and 3′ non-coding DNA (3′NC) of the P23 gene (LinJ.35.4540 ) were amplified enzymatically from genomic L. donovani DNA with primers that added EcoRI and KpnI sites (5′NC) or BamHI and HindIII sites (3′NC) to upstream and downstream ends. SwaI sites were introduced to flank the constructs. The 5′NC amplificates were digested with EcoRI and KpnI and ligated into pUC19 plasmid, cut with the same enzymes. The resulting plasmids were digested with BamHI and HindIII, and the 3′NC amplificates, cut with the same enzymes, were ligated between those sites. Subsequently, pUC19-P23-5′-3′NC was cut with KpnI and BamHI. Bleomycin resistance and puromycin resistance genes were amplified with primers that added KpnI and BamHI sites to their 5′ and 3′ ends. Cut with those enzymes, the resistance markers were ligated into pUC-P23-5′-3′NC to yield pUC-P23-5′bleo3′ and pUC-P23-5′puro3′ (Online resource Fig. 1a). After construction, the plasmids were amplified in E. coli, purified by caesium chloride density gradient ultracentrifugation (Sambrook and Russell 2001), and linearised with SwaI, to yield the constructs depicted in Online resource Fig. 1b. The fragments containing the recombination cassette were separated by agarose gel electrophoresis and purified using the Machery&Nagel NucleoSpin Extract II Kit.
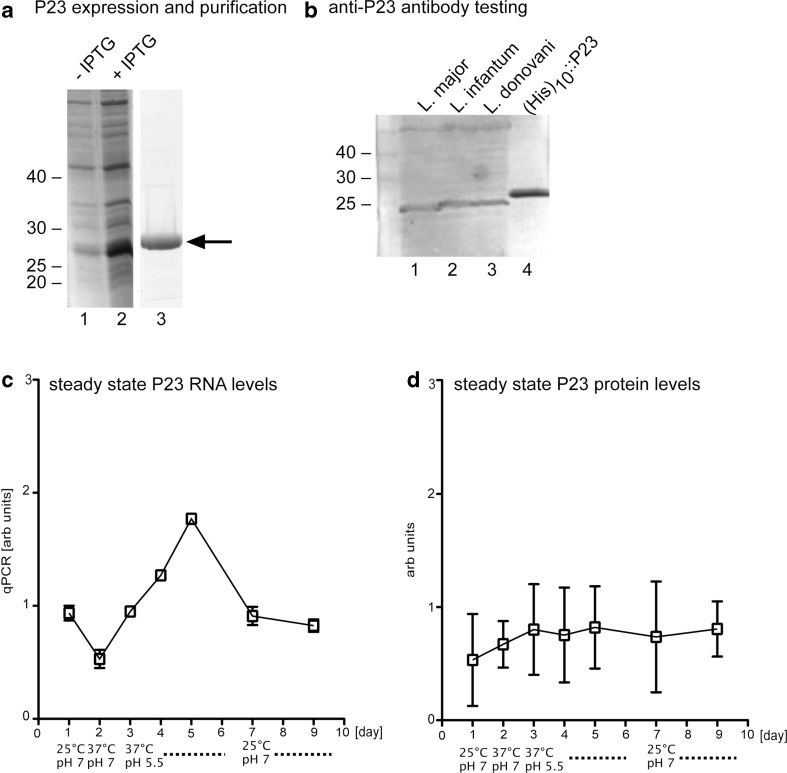
Fig. 1.
Expression of rLdP23. a Bacterial expression and purification of rLdP23. E. coli BL21 (DE3)[pAPlacIQ] transformed with pJC45-LdP23 before (lane 1) and after (lane 2) induction with IPTG. rLdP23 was purified using HisBind Resin (lane 3). The arrow points to the 25-kDa band representing rLdP23. b (His)10::P23 was used to raise antibodies in chicken. Western blot analysis showed that purified antibodies recognise the natural protein in the lysates of L. major, L. infantum, L. donovani (lanes 1, 2, and 3) and the recombinant protein (His)10::P23 (lane 4). c, d Expression kinetic of LdP23 during promastigote-to-amastigote conversion. L. donovani promastigotes grown at 25 °C and pH 7 (day 1) were exposed to 37 °C (day 2) and a subsequent acidification (pH 5.5) (days 3–6). To reconvert the axenic amastigotes back into promastigotes, the cells were subjected again to lower temperature and neutral pH (25 °C, pH 7) (days 7–9). The amounts of P23 RNA (arbitrary units) were measured by RT-qPCR (c). P23 protein abundance (arbitrary units) was quantified by Western blot analysis using the specific chicken anti-P23 antibody (d)
The plasmids pCLS, pCLS-P23, pCLS-HSP23, pTL.v6-HSP90 and pTL.v6-HSP90rr were described previously (Hombach et al. 2013, 2014).
Genomic DNA preparation and PCR
Mutant genotypes were verified by PCR analysis of genomic DNA. For the preparation of genomic DNA, the Gentra Systems Puregene Tissue Core Kit A (Qiagen, Hilden, Germany) was used, following the manufacturer’s instructions.
The quality of isolated gDNA, the gene replacement and the overexpression of P23 were verified by PCR using the primers listed below. PCR products were separated by agarose gel electrophoresis and ethidium bromide staining. Primers used are as follows: HSP23-specific-fwd (ATGTCCACCAGCGGCCCA), HSP23-specific-rev (CTCGAGGAGGACACGTGA), P23-specific-fwd (ATGTCTCACCTTCCGATC), P23-specific-rev (TTACGCGTTGAGATCGCTG).
Expression profiling
Semi-quantitative real-time RT-PCR was performed essentially as described (Choudhury et al. 2008). P23-gene-specific primers were P23-(F1) (CTGTGCCATAAAGAAAGAGCAGGG) and P23-(B1) (CGTCGTCCTCGTCCTTCCAAAG). P23 mRNA abundance was calculated relative to the Leishmania actin signal.
Recombinant protein expression in E. coli and protein purification
Preparation of the expression plasmid pJC45-LdP23 was performed as described before (Hombach et al. 2014). The expression plasmid was transformed into the bacterial strain BL21 (DE3)[pAPlacIQ], and the His-tagged proteins isolated from bacterial lysates were purified using HisBind Resin (Novagen, Madison, USA) as described previously (Clos and Brandau 1994).
The purified proteins were then used to immunise laying hens. The IgY were purified from the egg yolk as described previously (Hübel et al. 1995; Polson et al. 1985, 1980). The purified antibodies were tested against recombinant P23 and the lysates of different Leishmania species.
The immunisation of laying hens was performed in accordance with §8a, German Animal Protection Law, and registered with the Amt für Tierschutz, Freie und Hansestadt Hamburg.
Western blot analysis
Production of SDS cell lysates, discontinuous SDS-PAGE and Western blot were performed according to standard protocols. Membranes were treated with blocking solution (5 % milk powder and 0.1 % Tween 20 in Tris-buffered saline), before they were probed with anti-P23 (1:200 in blocking solution), anti-HSP23 (1:500 in blocking solution) and mouse anti-alpha-tubulin (1:4000 in blocking solution, Sigma-Aldrich, München, Deutschland), followed by incubation with anti-chicken IgG-alkaline phosphatase (1:2000 in blocking solution, Dianova, Hamburg, Germany) or anti-mouse IgG-alkaline phosphatase (1:2000 in blocking solution, Dianova, Hamburg, Germany) as secondary antibodies. Blots were developed using nitro blue tetrazolium chloride (Carl Roth, Karlsruhe, Germany) and 5-bromo-4-chloro-3-indolyl phosphate (Carl Roth, Karlsruhe, Germany). Protein bands were quantified using the software ImageJ v.1.42q (Wayne Rasband, National Institute for Health, USA).
Immunofluorescence and confocal microscopy
Log phase promastigotes (1 × 107 cells) were sedimented, washed twice with PBS and suspended in 1 ml of PBS. Aliquots of the suspension (2 × 105 cells) were applied on microscopic slides and air-dried. After fixing the cells for 2 min in ice-cold methanol, the slides were air-dried for 20 min. Non-adherent cells were removed by gentle washing (0.1 % Triton X-100 in PBS) followed by incubation in blocking solution (2 % BSA, 0.1 % Triton X-100 in PBS). Slides were then incubated for 1 h with mouse anti-P23 (1:250 in blocking solution) and with either chicken anti-HSP70 (1:500 in blocking solution), chicken anti-HSP90 (1:500 in blocking solution) or chicken anti-Sti1 (1:500 in blocking solution). Cell were washed thrice and then incubated for 1 h with anti-mouse-FITC (Dianova, Hamburg, Germany, 1:250), anti-chicken-Alexa594 (Dianova, Hamburg, Germany, 1: 500) and DAPI (Sigma-Aldrich, München, Germany, 1:25).
For the analysis of the cell body length, the fixed cells were incubated for 1 h with monoclonal mouse anti-tubulin (Sigma-Aldrich, München, Germany, 1:4000), washed thrice and then incubated for 1 h with mouse anti-Alexa 594 (Dianova, Hamburg, Germany, 1:250) and DAPI (Sigma-Aldrich, München, Germany, 1:25). After washing the slides thrice, Mowiol and coverslips were applied and the slides were left to dry for 24 h at 4 °C.
Fluorescence microscopy was carried out on an Olympus FluView1000 confocal microscope (SIM scanner and spectral detection) or for the cell body length analysis on an EVOS FL Auto epifluorescence microscope (Life Technologies, Darmstadt, Germany).
In silico procedures
DNA and protein sequence analysis was performed using the MacVector® software suite, version 13.x (MacVector Inc, Cary NC, USA). Greyscale and colour images were optimised for contrast using Photoshop® CS3 (Adobe) or ImageJ (NIH). Numerical data were analysed using the Prism® software (version 5). Composite figures were assembled using the Intaglio® software, version 3.3 (Purgatory). Significance was calculated using the Mann-Whitney U test (Mann and Whitney 1947).
Results
Expression of LdP23
To detect P23 in Leishmania, we raised specific antibodies against recombinantly expressed P23 protein. The P23 coding sequence was inserted into the pJC45 expression vector (Schlüter et al. 2000) and expressed with an N-terminal His10 tag in E. coli (Fig. 1a, lanes 1 and 2). Total extracts were purified by metal chelate chromatography (Clos and Brandau 1994), resulting in a highly enriched (His)10::P23 fusion protein (Fig. 1a, lane 3). Laying hens were then immunised with the purified recombinantly expressed protein (rLdP23). Antibodies (IgY) were purified from egg yolk and tested in Western blot analysis. The antibodies recognise the rLdP23 faithfully (Fig. 1b, lane 4, (His)10::P23). More importantly, the antibodies recognise the natural protein in lysates from three Leishmania species (Fig. 1b, lanes 1, 2 and 3).
We also studied the expression kinetics of P23 during an in vitro life cycle (Fig. 1c, d). Promastigotes of L. donovani grown at 25 °C and neutral pH (day 1) were exposed to 37 °C (day 2) and then shifted to an acidic pH (days 3 to 7). On day 7, the axenic amastigotes were shifted back to 25 °C and neutral pH to facilitate amastigote-to-promastigote conversion of the parasites (days 7 to 9). At relevant time points, samples were taken and tested for P23 RNA abundance (Fig. 1c) and P23 protein levels (Fig. 1d, Online resource Fig. 3). The analysis of the results showed that P23 RNA abundance increases during in vitro amastigote conversion, doubling until day 6. The elevated RNA level drops after amastigote-to-promastigote conversion. By contrast, the P23 protein levels do not change significantly during axenic stage conversion. These data further illustrate the previously described lack of correlation between RNA and protein levels during Leishmania stage conversion (Lahav et al. 2011).
Subcellular localisation of LdP23 and its co-localisation with members of the foldosome complex
To investigate possible interactions of LdP23 with the foldosome complex in Leishmania, we used the P23-specific antibodies in confocal laser microscopy and analysed the localisation of P23 in promastigotes compared with HSP70, HSP90 and Sti1 (Fig. 2a–c). LdP23 showed a cytoplasmic localisation (Fig. 2a–c, panel 3) and general co-localisation with both HSP90 and Sti1 (Fig. 2b, c, panel 5). The co-localisation of LdP23 with HSP70 appears to be less pronounced (Fig. 2a, panel 5), suggesting that HSP70 and P23 do not interact specifically in Leishmania parasites. P23 belongs to the late-acting HSP90 co-chaperones and is not known to interact directly with HSP70. The co-localisation of the putative P23 co-chaperone with HSP90 and Sti1 is in agreement with P23 being a member of the foldosome complex in Leishmania.
Fig. 2.
Co-localisation studies by confocal microscopy. Log phase promastigotes at 25 °C were stained with mouse anti-P23 (1:250) and with either a chicken anti-HSP70 (1:500), b chicken anti-HSP90 (1:500) or c chicken anti-Sti1 (1:500). Images were taken by confocal laser microscopy and differential interference contrast (DIC, panels A1, B1, C1). Representative cells from each culture were visualised with DAPI (panels A2, B2, C2), anti-P23 antibody (panels A3, B3, C3), anti-Hsp70 antibody (panel A4), anti-Hsp90 antibody (panel B4), anti-Sti1 antibody (panel C4) and as overlays of DAPI, P23 with Hsp70 (panel A5) or with Hsp90 (panel B5) and/or with Sti1 (panel C5). k kinetoplast, n nucleus
Replacement of P23 in L. donovani
To unravel the in vivo functions of LdP23, we targeted the P23 alleles with specific replacement constructs (Online resource Fig. 1a), using a sequential homologous recombination strategy (Krobitsch and Clos 2000) (Online resource Fig. 1b). The replacement of both P23 alleles with resistance marker genes resulted in viable double-antibiotic-resistant clones. After preliminary verification, one P23 null mutant candidate clone, cl5, was selected and used as background to introduce episomal P23 and HSP23 transgenes. To verify the null mutant and add-back strains, the genotypes were tested by a P23-specific PCR (Fig. 3a) and by Western blot analysis using the P23-specific antibodies (Fig. 3b).
Fig. 3.
Verification of L. donovani P23 null mutants by PCR (a). The DNAs from L. donovani strains P23+/+, P23−/− cl5, P23−/−P23+, P23−/−HSP23+, P23−/− pCLS were used as templates for a P23-gene-specific PCR (upper panel). The qualities of the same DNAs were tested with a HSP23-specific PCR (lower panel). Position of size markers [bp] shown on the left. b Lysates from L. donovani-strains P23+/+, P23−/−cl5, P23−/−P23+, P23−/−HSP23+, P23−/−pCLS were separated by SDS-PAGE, subjected to Western blot and probed with chicken anti-P23 (1:200) (upper panel) or chicken anti-HSP23 (1:500) (middle panel). As loading control, a replica gel was stained with Coomassie Brilliant Blue (lower panel). The arrowheads (upper panel) point at the P23-specific bands
The 600-bp P23 gene could only be amplified from wild-type DNA (P23+/+) and from the P23−/−P23+ DNA (Fig. 3a, upper panel, lanes 1 and 3). The DNAs isolated from P23−/− cl5 (lane 2) and the control strains carrying the HSP23 transgene (lane 4) or empty pCLS vector (lane 5) were negative for P23-coding DNA. The quality of the isolated DNAs was verified by the amplification of the HSP23 coding sequence. All DNA samples were positive for HSP23 (Fig. 3a, lower panel). This confirms the loss of both P23 alleles in the P23−/− clone 5.
To confirm the null mutant, we analysed wild type cells, P23−/−, and P23−/−-derived transgenic strains using Western blot and P23-specific antibodies (Fig. 3b). The Western blot revealed that the protein is present in wild-type cells and in the add-back strain P23−/− P23+ (Fig. 3b, upper panel, lanes 1 and 3). Neither the null mutant nor its derivatives carrying the HSP23 transgene or the empty vector showed any detectable P23 signal (Fig. 3b, upper panel, lanes 2, 4 and 5). The quality of the isolated protein samples was verified with HSP23-specific antibodies (Fig. 3b, lower panel). In summary, we confirmed the P23 null mutant and its derivatives on the DNA and protein levels. We also conclude from this that LdP23 is not essential for viability of L. donovani promastigotes.
Tolerance of the P23 null mutant to various stresses
Having ascertained that P23 is not essential for viability, we next tested the effects of P23 gene replacement and transgenic complementation under various relevant in vitro growth conditions (Fig. 4).
Fig. 4.
Proliferation of P23 null mutants under stress. Cells (1 × 105 ml−1) were seeded into 10 ml of supplemented Medium199+ and grown for 4 days. Cell density on day 4 was then normalised as [%] of wild type (P23+/+) cell density. Parasites were grown at 25 °C and pH 7.0 (a), 37 °C and pH 7.0 (b), 25 °C and pH 5.5 (c). Additional cultures were grown at 25 °C and pH 7.0 with the addition of 2 % ethanol (d) or 112 μM antimonyl tartrate (Sb(III)) (e). *p < 0.05; **p < 0.01 (U test)
Under ideal in vitro growth conditions for promastigotes (25 °C, pH 7), P23−/− and its derivatives showed no growth defect compared to wild-type cells (Fig. 4a). The same was observed for parasite growth at mammalian tissue temperature (37 °C, Fig 4b) where we observed no growth defect of the null mutant compared to wild-type cells. The growth of the null mutant at pH 5.5 (Fig. 4c), at 2 % v/v (=IC50) of ethanol (Fig. 4d) or under trivalent antimony challenge (112 μM SbIII, Fig. 4e) also showed no difference compared to wild-type cells. In summary, the results show that P23 is not required for the protection of promastigotes against relevant chemical (pH, ethanol) and physical (37 °C) stresses or under therapeutic pressure (SbIII).
Infectivity and intracellular proliferation
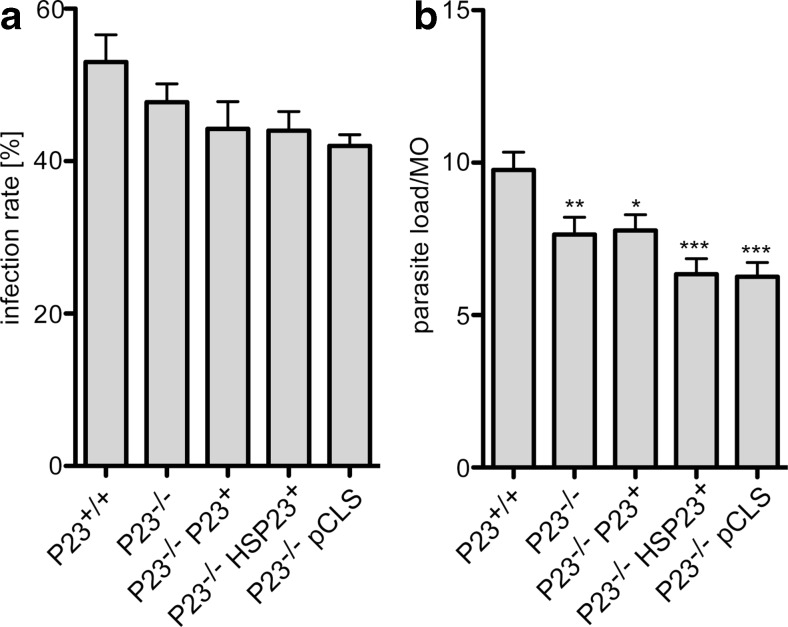
We also tested the ability of P23−/− and its derivatives to infect mouse bone marrow-derived macrophages in vitro and to amplify intracellularly. Late stationary phase promastigotes (2 × 106) of L. donovani wild type (P23+/+), P23−/−, P23−/− P23+, P23−/−Hsp23+, and P23−/−pCLS were added to plated macrophages (2 × 105) in a chamber slide. After 4 h, extracellular parasites were removed by washing and incubation was continued for 44 h. Infection rate (= % of infected macrophages) and parasite load (parasites per macrophage) were determined by DAPI staining and microscopical counting (n = 400). As shown in Fig. 5a, infection rates are unaffected by the mutation. All recombinant parasites show slightly but significantly reduced parasite loads (Fig. 5b). However, the effect of the P23−/− null mutant is not countermanded by the P23 transgene. Therefore, we conclude that the weak effect observed is not caused by the lack of P23.
Fig. 5.
Infectivity of the P23 null mutant in vitro. Bone marrow-derived macrophages (Mθ) were infected with P23+/+, P23−/− cl5, P23−/−P23+, P23−/−HSP23+, and P23−/− pCLS parasites at a 1:10 ratio. After 4 h, free parasites were removed and the infected macrophage cultures were further incubated at 37 °C and 5 % CO2 for 44 h. Cells were fixed and stained with DAPI. Four hundred macrophages per infection experiment were checked by fluorescence microscopy for infection rate (a, % infected Mθ) and parasite load (b, no. of parasites per Mθ). *p < 0.05; **p < 0.01; ***p < 0.001 (U test)
LdP23 and LdHSP23 protect against HSP90-inhibitor-induced growth arrest
Like LdP23, the ortholog in yeast cells, SBA1, is not essential for cell viability under physiological conditions. However, the lack of P23/SBA1 renders yeast cells hypersensitive to HSP90 inhibitors (Forafonov et al. 2008). To investigate whether LdP23 too protects HSP90 against inhibitors, we compared the proliferation of wild-type cells with the proliferation of the P23 null mutant and its derivatives in the presence of GA (Fig. 6a) and RAD (Fig. 6b).
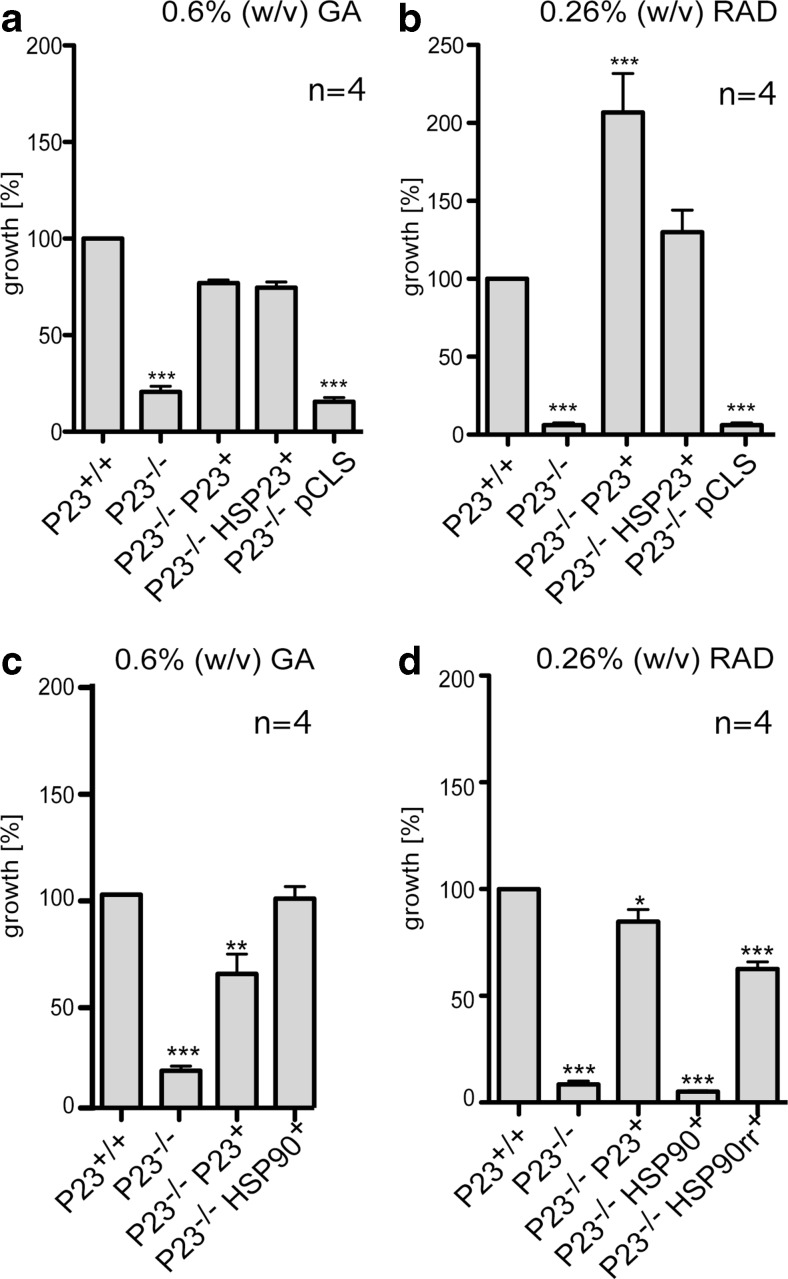
Fig. 6.
Effects of GA and RAD treatment on the cell proliferation of the P23 null mutant. Cells (1 × 105 ml−1) were seeded into 10 ml of supplemented Medium199+ with 0.6 % (w/v) GA (a, c) or 0.26 % (w/v) RAD (b, d) and grown for 4 days. Cell density on day 4 was then normalised as [%] of wild type (P23+/+) cell density. *p < 0.05; **p < 0.01 **p < 0.001 (U test)
Indeed, the P23 null mutant was hypersensitive to both GA and RAD, with its in vitro growth reduced by 80 and >90 %, respectively, compared to the wild type and at IC50 of either drug. Overexpression of P23 in the null mutant restored growth to near-wild-type levels under GA inhibition (Fig. 6a). Under RAD challenge too, the P23 over expressing null mutant shows restored in vitro growth (Fig. 6b).
HSP90 inhibition by GA or RAD causes a morphological shift towards an amastigote-like form, easily detectable by cell body length measurements (Hombach et al. 2013; Wiesgigl and Clos 2001). Therefore, we compared the cell body length and morphology of wild type, P23 null mutant and P23 gene add-back strain after 72 h of GA or RAD treatment (Online resource Fig. 2a, b). We observed that the inhibitor treatment of the null mutant caused a significant shortening of the cell body and the flagellum compared with inhibitor-treated wild-type cells or add-back parasites, corroborating the growth analyses.
The results convincingly show that LdP23 interferes with the effect of inhibitors on HSP90, thereby confirming that LdP23 is the Leishmania homolog of the P23 co-chaperones.
Interestingly, overexpression of the small heat shock protein HSP23 can also compensate for the lack of P23, both under GA or RAD challenge (Fig. 6a, b). Both proteins are structurally related and HSP23 is thought to be an offshoot of the P23 branch of ACD proteins. While P23 cannot replace HSP23 as stress tolerance factor, and HSP23 null mutants showed no hypersensitivity to GA (Hombach et al. 2014), HSP23 may still have residual P23 co-chaperone activity.
To test whether the effect of GA on the P23−/− growth is connected with inhibition of HSP90, we transfected the null mutant with an overexpression plasmid for HSP90. We then tested this strain against wild type, null mutant and P23 overexpressing null mutant under GA challenge. The results, shown in Fig. 6c indeed show that HSP90 over expression restores in vitro growth to the P23 null mutant. This HSP90 titration effect has been described before (Wiesgigl and Clos 2001) and demonstrates that the effect of GA on P23−/− depends on the drug-to-target ratio.
The simple amino acid exchange Leu33 to Ile33 in the ATP binding pocket of L. donovani HSP90 renders the mutated protein (HSP90rr) resistant to RAD. Over expression of this HSP90rr variant permits growth under RAD selection in vitro (Hombach et al. 2013). We therefore expressed HSP90rr in the P23 null mutant to observe whether a RAD resistant HSP90 can also compensate for the lack of P23 under RAD challenge. Figure 6d shows the results. As expected, overexpression of wild-type HSP90 does not compensate for the lack of P23 under RAD challenge (Fig. 6d), in keeping with previous observations (Hombach et al. 2013). By contrast, the overexpression of the RAD-resistant HSP90rr in the P23 null mutant can restore growth under RAD to 75 % of wild-type parasite proliferation. This result implies that HSP90rr expression alone cannot compensate fully for the loss of P23 under RAD challenge.
Discussion
Parasitic protozoa of the genus Leishmania are part of the Euglenozoa, a very early and divergent branch of the Eukaryotic tree, showing peculiarities such as unique gene expression and survival strategies (Clos and Hombach 2015; Leprohon et al. 2009; Ubeda et al. 2008). In spite of this, Leishmania spp. possess a rather complete and highly differentiated set of chaperone and co-chaperone proteins which have become part of their regulatory machinery to control life cycle progression.
Of the Leishmania chaperones, the HSP90 chaperone complex has seen much interest in recent years. For one, HSP90 is a pivotal regulator of the life cycle, stabilising the insect stage but also promoting intracellular parasite growth (Hombach et al. 2013; Wiesgigl and Clos 2001). Itself not accommodating to gene replacement, its co-chaperones nevertheless were subjected to reverse genetic studies. Two confirmed HSP90 complex members, Sti1 and SGT, are essential proteins for Leishmania viability (Hombach et al. 2013; Morales et al. 2010; Ommen et al. 2010). On the other hand, replacement of the genes coding for the putative HIP and for a second HOP ortholog were found to be dispensable for parasites in culture (Ommen et al. 2009). A fifth co-chaperone, cyclophilin 40 (Cyp40), was shown to be essential for late promastigote development and, more importantly, intracellular survival of the parasites (Yau et al. 2014). All these co-chaperones are distinguished by one or more tetratricopeptide repeat domains (TPR) that facilitate interaction with recognition sequences in the two major chaperones, HSP90 and HSP70 (Li et al. 2012).
Separate from this group of TPR-containing co-chaperones are the P23 proteins. These are related to small heat shock proteins and to the mammalian lens protein alpha-crystallin, sharing the common alpha crystallin domain (ACD) with both groups (Garcia-Ranea et al. 2002). Database mining revealed two P23-like genes in Leishmania of which one, HSP23, could be shown to possess important stress protection function in Leishmania (Hombach et al. 2014). The other, tentatively termed P23, was the subject of this study. By means of gene expression studies, subcellular localisation and reverse genetics, we have compiled evidence for P23 being a proper co-chaperone of HSP90 and the Leishmania homolog of the known P23 protein family.
We showed that P23 is not a stress-inducible protein in L. donovani. While an increase could be observed for the P23-specific RNA during axenic amastigote differentiation, this increase did not translate into an elevated P23 protein abundance. It is a common occurrence in Leishmania that RNA steady-state levels and changes thereof correlate poorly with the steady state levels of the corresponding proteins (Lahav et al. 2011), and P23 is yet another example. Our results are in agreement with an earlier quantitative analysis of P23 protein abundance (Rosenzweig et al. 2008). Moreover, the predicted interaction partner, HSP90, does not become more abundant during the axenic amastigote stage (Brandau et al. 1995; Rosenzweig et al. 2008), leaving no need for elevated expression of P23. By contrast, Plasmodium parasites, the causative agents of malaria, produce P23 as a relatively abundant cytoplasmic protein with a differential expression during the parasite life cycle stages (Wiser 2003).
P23 from Leishmania parasites share approximately 30 % sequence identity with human P23 as well as the so-called motif of P23 proteins (WPRLTKE). It was shown experimentally that this motif is crucial for the interaction of P23 with the N-terminal part of HSP90 (Batista et al. 2015; Garcia-Ranea et al. 2002; Martinez-Yamout et al. 2006). Comparing the subcellular localisation of LdP23 with HSP90 and with Sti1, another member of the foldosome complex, using confocal microscopy indeed revealed overlapping staining patterns. This view is supported by an in vitro analysis of recombinant P23 protein from L. braziliensis. That study showed that P23 is able to inhibit the ATPase activity of HSP90 and that one P23 molecule binds to an HSP90 dimer (Batista et al. 2015). The observed interaction of P23 with HSP90 and the resulting inhibition of the ATPase activity in Leishmania parasites are in agreement with previous data obtained for other organisms, indicating a similar function (Chua et al. 2010; Echeverria et al. 2010; Fang et al. 1998; Felts and Toft 2003; Richter et al. 2004).
Much like its ortholog SBA1 in the yeast Saccharomyces cerevisiae (Forafonov et al. 2008), Leishmania P23 is not an essential gene for general viability. We could establish and verify five independent clones (not shown) of the P23−/− null mutant which all showed unchanged vital parameters. This lack of a phenotype was observed under various physiological conditions signifying cellular stress (temperature, pH, ethanol exposure) and drug pressure (SbIII). More importantly, the P23−/− mutant was infective to macrophages and could proliferate as intracellular amastigote. We conclude that under physiological conditions, the contribution of P23 to the function of HSP90 foldosomes is not critical. We cannot exclude however that the unchanged viability and vitality of the P23 null mutant is a consequence of a spontaneous genetic rearrangement that masks the true phenotype. Such rearrangements have been described in the past, although usually as secondary events after prolonged selection (Reiling et al. 2006; Spath et al. 2004).
We also did not test the relative resistance of the P23−/− parasites against BrefeldinA. Work using Saccharomyces cerevisiae Sba1 null mutants showed an increased sensitivity to this inhibitor of Golgi-mediated protein transport (Echtenkamp et al. 2011). It is noteworthy that Leishmania spp export crucial immune modulatory proteins by using exosomes (Lambertz et al. 2012; Silverman et al. 2010). Therefore, the lack of P23 in the null mutant, given a homologous function in the parasite, cannot be expected to impact on the in vitro infectivity.
Another possible role for P23 in Leishmania spp is the demonstrated involvement with ribosome biogenesis (Echtenkamp et al. 2011). Again, this function only becomes apparent under drug challenge, namely under hygromycin B treatment, and we did not observe any reduction of the high in vitro proliferation rates observed for L. donovani. Therefore, a crucial role in ribosome integrity can be excluded.
The only phenotype we observed was the strong hypersensitivity to the benzoquinoid ansamycin, geldanamycin (GA) and even more so, to the antifungal macrolactone antibiotic, radicicol (RAD). Both compounds are well-characterised inhibitors of HSP90, competing with ATP for the nucleotide binding pocket located at the N terminus of HSP90 and blocking the ATP-driven reaction cycle of the HSP90 foldosome complex (Smith et al. 1998). For the yeast P23 homologue, SBA1, the same phenotypic effects were associated with a gene replacement (Bohen 1998; Forafonov et al. 2008), further supporting the notion that LdP23 is indeed the HSP90-specific co-chaperone regulating its ATP binding domain.
We could also show that not only the reintroduction of a P23 transgene into the null mutant but also the overexpression of the related small heat shock protein HSP23, could restore GA and RAD resistance to the mutant. Since the HSP23 null mutant did not show GA hypersensitivity by itself (Hombach et al. 2014), we suspect that HSP23 due to its structural similarity to P23 proteins has a residual affinity for HSP90 and, upon overexpression, can prevent binding of radicicol. This may be the cause for the lack of phenotype we observe under physiological conditions. In this context, it is interesting that our attempts to produce double null mutants for both P23 and HSP23 did not meet with success (G. Ommen, unpublished work).
The expression of a known, radicicol-resistant HSP90 variant (HSP90rr) in the P23 null mutant was also able to confer significant protection against RAD, but not completely so. It therefore seems as if the RAD resistant properties of HSP90rr depend partly on the presence of P23.
Electronic supplementary material
(PDF 2322 kb)
Acknowledgments
We thank technical Dorothea Zander and Nicole Rath for expert technical assistance and the members of the laboratory for stimulating discussions.
References
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista LF, Segatto M, Guedes CE, Sousa RS, Rodrigues CA, Brazuna JC, Silva JS, Santos SO, Larangeira D, Macedo AM, et al. An assessment of the genetic diversity of Leishmania infantum isolates from infected dogs in Brazil. AmJTrop Med Hyg. 2012;86:799–806. doi: 10.4269/ajtmh.2012.11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FA, Almeida GS, Seraphim TV, Silva KP, Murta SM, Barbosa LR, Borges JC. Identification of two p23 co-chaperone isoforms in Leishmania braziliensis exhibiting similar structures and Hsp90 interaction properties despite divergent stabilities. FEBS J. 2015;282:388–406. doi: 10.1111/febs.13141. [DOI] [PubMed] [Google Scholar]
- Bohen SP. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol Cell Biol. 1998;18:3330–3339. doi: 10.1128/mcb.18.6.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- Brandau S, Dresel A, Clos J. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem J. 1995;310:225–232. doi: 10.1042/bj3100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/S0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Choudhury K, Zander D, Kube M, Reinhardt R, Clos J. Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. Int J Parasitol. 2008;38:1411–1423. doi: 10.1016/j.ijpara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Chua CS, Low H, Goo KS, Sim TS. Characterization of Plasmodium falciparum co-chaperone p23: its intrinsic chaperone activity and interaction with Hsp90. Cell Mol Life Sci. 2010;67:1675–1686. doi: 10.1007/s00018-010-0275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos J, Brandau S. pJC20 and pJC40—two high-copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in Escherichia coli. Prot Expression Purif. 1994;5:133–137. doi: 10.1006/prep.1994.1020. [DOI] [PubMed] [Google Scholar]
- Clos J, Hombach A (2015) Heat shock proteins of Leishmania: chaperones in the driver’s seat. Caister Academic Press17-36
- Echeverria PC, Figueras MJ, Vogler M, Kriehuber T, de Miguel N, Deng B, Dalmasso MC, Matthews DE, Matrajt M, Haslbeck M, et al. The Hsp90 co-chaperone p23 of Toxoplasma gondii: Identification, functional analysis and dynamic interactome determination. Mol Biochem Parasitol. 2010;172:129–140. doi: 10.1016/j.molbiopara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, Garabedian M, Freeman BC. Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol Cell. 2011;43:229–241. doi: 10.1016/j.molcel.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Rao J, Caplan AJ. SBA1 encodes a yeast hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol Cell Biol. 1998;18:3727–3734. doi: 10.1128/mcb.18.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Felts SJ, Toft DO. p23, a simple protein with complex activities. Cell Stress Chaperones. 2003;8:108–113. doi: 10.1379/1466-1268(2003)008<0108:PASPWC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forafonov F, Toogun OA, Grad I, Suslova E, Freeman BC, Picard D. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28:3446–3456. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- Garcia-Ranea JA, Mirey G, Camonis J, Valencia A. p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. 2002;529:162–167. doi: 10.1016/S0014-5793(02)03321-5. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, Fadden P, Haystead TAJ, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Johnson BD, Toft DO. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- Hombach A, Ommen G, Chrobak M, Clos J. The Hsp90-Sti1 interaction is critical for Leishmania donovani proliferation in both life cycle stages. Cell Microbiol. 2013;15:585–600. doi: 10.1111/cmi.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach A, Ommen G, MacDonald A, Clos J. A small heat shock protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J Cell Sci. 2014 doi: 10.1242/jcs.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübel A, Brandau S, Dresel A, Clos J. A member of the ClpB family of stress proteins is expressed during heat shock in Leishmania spp. Mol Biochem Parasitol. 1995;70:107–118. doi: 10.1016/0166-6851(95)00012-P. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823:607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Brown C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones. 2009;14:83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobitsch S, Clos J. Cross-species homologous recombination in Leishmania donovani reveals the sites of integration. Mol Biochem Parasitol. 2000;107:123–128. doi: 10.1016/S0166-6851(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Krobitsch S, Brandau S, Hoyer C, Schmetz C, Hübel A, Clos J. Leishmania donovani heat shock protein 100: characterization and function in amastigote stage differentiation. J Biol Chem. 1998;273:6488–6494. doi: 10.1074/jbc.273.11.6488. [DOI] [PubMed] [Google Scholar]
- Laban A, Wirth DF. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989;86:9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav T, Sivam D, Volpin H, Ronen M, Tsigankov P, Green A, Holland N, Kuzyk M, Borchers C, Zilberstein D, et al. Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. FASEB J: Off Publ Fed Am Soc Exp Biol. 2011;25:515–525. doi: 10.1096/fj.10-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz U, Silverman JM, Nandan D, McMaster WR, Clos J, Foster LJ, Reiner NE. Secreted virulence factors and immune evasion in visceral leishmaniasis. J Leukoc Biol. 2012;91:887–899. doi: 10.1189/jlb.0611326. [DOI] [PubMed] [Google Scholar]
- Leprohon P, Legare D, Raymond F, Madore E, Hardiman G, Corbeil J, Ouellette M. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 2009;37:1387–1399. doi: 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta. 2012;1823:624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- Martinez-Yamout MA, Venkitakrishnan RP, Preece NE, Kroon G, Wright PE, Dyson HJ. Localization of sites of interaction between p23 and Hsp90 in solution. J Biol Chem. 2006;281:14457–14464. doi: 10.1074/jbc.M601759200. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Molecular chaperones: the busy life of Hsp90. Curr Biol. 1999;9:R322–325. doi: 10.1016/S0960-9822(99)80203-6. [DOI] [PubMed] [Google Scholar]
- Morales MA, Watanabe R, Dacher M, Chafey P, Osorio y Fortea J, Scott DA, Beverley SM, Ommen G, Clos J, Hem S, et al. Phosphoproteome dynamics reveal heat-shock protein complexes specific to the Leishmania donovani infectious stage. Proc Natl Acad Sci U S A. 2010;107:8381–8386. doi: 10.1073/pnas.0914768107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, Georgopoulos C. The stress response, function of the proteins, and perspectives. In: Morimoto RI, Tissières A, Georgopoulos C, editors. Stress proteins in biology and medicine. Plainview: Cold Spring Harbor Laboratory Press; 1990. pp. 1–36. [Google Scholar]
- Nathan DF, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommen G, Clos J. Heat shock proteins in protozoan parasites—Leishmania spp. In: Calderwood S, Santoro G, Pockley G, editors. Prokaryotic and eukaryotic heat shock proteins in infectious disease. Berlin: Springer; 2009. pp. 135–151. [Google Scholar]
- Ommen G, Lorenz S, Clos J. One-step generation of double-allele gene replacement mutants in Leishmania donovani. Int J Parasitol. 2009;39:541–546. doi: 10.1016/j.ijpara.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Ommen G, Chrobak M, Clos J. The co-chaperone SGT of Leishmania donovani is essential for the parasite's viability. Cell Stress Chaperones. 2010;39:541–546. doi: 10.1007/s12192-009-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. Embo J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci: CMLS. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson A, von Wechmar B, van Regenmortel MHV. Immunol Commun. 1980;9:475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Polson A, Coetzer T, Kruger J, von Maltzahn E, van der Merwe KJ. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol Investig. 1985;14:323–327. doi: 10.3109/08820138509022667. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med(Maywood NJ) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling L, Jacobs T, Kroemer M, Gaworski I, Graefe S, Clos J. Spontaneous recovery of pathogenicity by Leishmania major hsp100-/- alters the immune response in mice. Infect Immun. 2006;74:6027–6036. doi: 10.1128/IAI.00773-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Walter S, Buchner J. The Co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J Mol Biol. 2004;342:1403–1413. doi: 10.1016/j.jmb.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, Zilberstein D. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J. 2007 doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, Zilberstein D. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J. 2008;22:590–602. doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schlüter A, Wiesgigl M, Hoyer C, Fleischer S, Klaholz L, Schmetz C, Clos J. Expression and subcellular localization of cpn60 protein family members in Leishmania donovani. Biochim Biophys Acta. 2000;1491:65–74. doi: 10.1016/S0167-4781(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:ARBTTN>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Murakata T, Agatsuma T, Sugimoto S, Nakano H, Lee YS, Simen BB, Argon Y, Felts S, et al. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. 2010;185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Katsanis E. Molecular chaperones: biology and prospects for pharmacological intervention. Pharmacol Rev. 1998;50:493–514. [PubMed] [Google Scholar]
- Spath GF, Lye LF, Segawa H, Turco SJ, Beverley SM. Identification of a compensatory mutant (lpg2-REV) of Leishmania major able to survive as amastigotes within macrophages without LPG2-dependent glycoconjugates and its significance to virulence and immunization strategies. Infect Immun. 2004;72:3622–3627. doi: 10.1128/IAI.72.6.3622-3627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/S0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda JM, Legare D, Raymond F, Ouameur AA, Boisvert S, Rigault P, Corbeil J, Tremblay MJ, Olivier M, Papadopoulou B, et al. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol. 2008;9:R115. doi: 10.1186/gb-2008-9-7-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen N, Kanzok SM, Wek RC, Sullivan WJ., Jr Stress response pathways in protozoan parasites. Cell Microbiol. 2008;10:2387–2399. doi: 10.1111/j.1462-5822.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Weaver AJ, Sullivan WP, Felts SJ, Owen BA, Toft DO. Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone. J Biol Chem. 2000;275:23045–23052. doi: 10.1074/jbc.M003410200. [DOI] [PubMed] [Google Scholar]
- Wiesgigl M, Clos J. Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol Biol Cell. 2001;12:3307–3316. doi: 10.1091/mbc.12.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser MF. A Plasmodium homologue of cochaperone p23 and its differential expression during the replicative cycle of the malaria parasite. Parasitol Res. 2003;90:166–170. doi: 10.1007/s00436-003-0929-z. [DOI] [PubMed] [Google Scholar]
- Yau WL, Pescher P, MacDonald A, Hem S, Zander D, Retzlaff S, Blisnick T, Rotureau B, Rosenqvist H, Wiese M, et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol Microbiol. 2014;93:80–97. doi: 10.1111/mmi.12639. [DOI] [PubMed] [Google Scholar]
- Zamora-Veyl FB, Kroemer M, Zander D, Clos J. Stage-specific expression of the mitochondrial co-chaperonin of Leishmania donovani, CPN10. Kinetoplastid Biol Dis. 2005;4:3. doi: 10.1186/1475-9292-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2322 kb)