Abstract
Mortalin is a stress chaperone belonging to the Hsp70 family of proteins. Frequently enriched in cancers, it is a multifunctional protein and regulates cell proliferation, apoptosis, mitochondrial functions, and the activity of tumor suppressor protein p53. In the present study, we investigated circulating mortalin and its autoantibody in normal, cirrhosis, and cancerous liver. We found that although mortalin is enriched in liver cancer cells and tumors, it is not detected in the serum of either the liver cirrhosis or cancer patients. In contrast, mortalin autoantibody was detected in patients’ sera and showed significant correlation with the occurrence of cirrhosis. It is suggested as a potential noninvasive marker for liver cirrhosis.
Keywords: Mortalin, Autoantibody, Liver cancer, Cirrhosis, Diagnostic marker
Introduction
Liver cirrhosis is an advanced and irreversible stage of liver dysfunction in which liver tissue is replaced by fibrosis (extracellular matrix) as a result of alcoholism, hepatitis, fatty liver, or other chronic etiologic factors. It is frequently associated with life-threatening pathologies including hepatic encephalopathy, hypertension, esophageal bleeding, hepatorenal syndrome, portal hypertensive gastropathy, chronic liver infections, and hepatocellular carcinoma (Lefton et al. 2009). Chronic viral hepatitis is the major cause of cirrhosis, especially HBV infections in Asia-Pacific region and sub-Saharan Africa, and has been tightly linked to HCC pathogenesis (El-Serag 2011). An early detection of cirrhosis may offer patients effective therapeutic options and lower the HCC incidence and mortality rate. Furthermore, liver biopsy, a common invasive method for definitive diagnosis and grading of liver fibrosis, is limited by factors including surgical complexity, high cost, and incompatibility with the disease follow-up regime. In this regard, noninvasive serum markers and imaging techniques are critical to address those high unmet medical needs, which have hence been under rapid development to serve as convenient tools for early diagnosis of the disease and to evaluate treatment outcomes.
Noninvasive circulating biomarkers of liver cirrhosis can be divided into two classes (Jarcuska et al. 2010). Class I biomarkers include serum proteins associated with the process of fibrogenesis signifying increased synthesis of extracellular matrix proteins. Class II biomarkers reflect status of liver dysfunction/structural damage. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are the two class II markers routinely used for staging fibrosis (Holmberg et al. 2013) that are usually combined to create a scoring system based on various statistical models. A panel of biochemical markers (also called FT-AT (FibroTest ActiTest) score) was found to have high diagnostic value for fibrosis and necroinflammatory histological activity in liver injury in patients with chronic hepatitis C (Poynard et al. 2004). It was reported that in addition to bilirubin, creatinine, and MELD (Model for End-Stage Liver Disease) score, serum cholesterol level is a significant and independent predictor of mortality of cirrhotic patients (Janicko et al. 2013). Addition of cholesterol to the MELD score improved prediction accuracy by 3 %. Similarly, addition of serum cholesterol level to a prognostic model considering bilirubin, creatinine, and INR (a measure of clotting tendency of blood) increased accuracy by 4 %. Very often, liver cirrhosis has been linked to inflammation and oxidative stress signaling (Brenner et al. 2013), suggesting that the stress proteins may serve as useful diagnostic markers as well.
Mortalin is a member of heat shock 70 family proteins that is enriched in human cancers (Dundas et al. 2005; Lu et al. 2011a, b; 2011a; Wadhwa et al. 2006). Several studies have shown significance of upregulated mortalin in hepatocellular carcinoma (HCC). The expression level of mortain showed significant correlation with cancer metastasis and disease recurrence even after curative therapies (Lu et al. 2011a; Wadhwa et al. 2006; Yi et al. 2008). On the other hand, hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin caused increase in heat shock proteins (Hsp27, alpha B-crystallin, Mortalin, Hsp105, and Hsp90s) in rat liver (Kim et al. 2012). Rozenberg et al. have demonstrated the presence of mortalin in the serum of colorectal cancer patients. A high level of serum mortalin (>60 ng/ml) was predicted as a risk factor for shorter survival (Rozenberg et al. 2011). In the present study, we investigated the presence and diagnostic value of circulating mortalin protein and mortalin autoantibody in serum samples from liver cirrhosis and HCC.
Material and methods
Patients and samples
Blood samples were obtained from 25 healthy liver donors, 16 cirrhosis patients, and 100 HCC patients (stage I–IV, each stage had 25 patients) by peripheral venous puncture. Blood was collected into the tubes (without any anticoagulant) and was immediately (within an hour of collection) centrifuged at 3000 rpm for 10 min, and the serum (the upper layer) was aliquoted and stored in −80 °C for future batch analysis. The study was performed according to guidelines approved by the Institute Ethical Committee of the University of Hong Kong, and all the samples were collected with signed consent from patients.
ELISA for mortalin and mortalin autoantibody
The levels of circulating mortalin and mortalin autoantibody in the serum were measured by sandwich ELISA, using anti-mortalin polyclonal antibody (Santa Cruz Biotechnology, Inc., CA) and monoclonal antibody (clone 37-6, generated in our laboratory). The levels were quantitated by plotting against the standard curve with purified antigen control of recombinant full-length mortalin protein. Anti-mortalin monoclonal antibody was coated on a 96-well ELISA plate (Corning Incorporated, NY) for overnight at 4 °C. Plates were washed with phosphate buffered saline (PBS) and blocked with 1 % BSA for 1 h. Serum (25 μl) or purified recombinant mortalin protein (100 ng) diluted in 100 μl PBS was incubated for 2 h followed by incubation with anti-mortalin rabbit polyclonal antibody (H-155, Santa Cruz, CA). After washing away any unbound proteins, secondary peroxidase-linked goat anti-rabbit IgG antibody was added and incubated at 37 °C for 2 h. The color was developed by incubation with ABST substrate (Invitrogen, CA) for 20 min. Optical density (415 nm) was measured by a microplate reader (Bio-Rad, CA). A standard curve showed that the detection sensitivity was 6.125 ng mortalin/ml.
For mortalin autoantibody detection, purified mortalin protein (50 ng/100 μl coating buffer) was coated on a 96-well ELISA plate (Corning Incorporated, NY) for incubation at 4 °C overnight. After PBS washings, the plates were blocked with 1 % bovine serum albumin. Serum samples (25 μl diluted in 100 μl of PBS) were incubated for 2 h followed by washing with PBS and incubation with secondary peroxidase-linked goat anti-rabbit IgG antibody at 37° for 2 h. The color was developed by incubation with ABST substrate (Invitrogena, CA) for 20 min, and the optical density (415 nm) was measured by an ELISA plate reader (Bio-Rad, CA). Each measurement was made in duplicates for both assays.
Statistical analysis
All statistical analyses were calculated by the Statistical Package for Social Science (SPSS for Windows, version 16; Chicago IL). The continuous data were presented as mean (±SEM). Categorical data were analyzed via Pearson’s exact test or Fisher’s exact test, whereas an independent t test was used for continuous data. One-way ANOVA analysis was applied for comparison of more than two groups. P < 0.05 was considered as a statistically significant. Receiver operating characteristic (ROC) curve were performed to determine the area under the curve (AUC).
Results
Circulating mortalin in cirrhosis and HCC patients
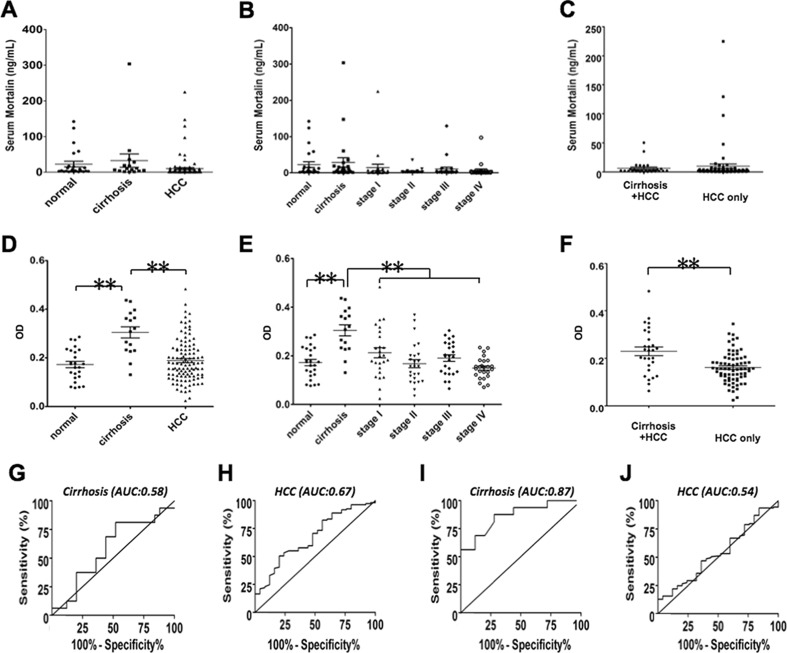
In order to detect circulating mortalin in serum samples of liver cirrhosis and cancer patients, we performed mortalin specific ELISA. Demographic and clinical characteristics of patients and control subjects are shown in Table 1. As shown in Fig. 1a, serum mortalin level showed no significant difference in normal, cirrhosis and HCC samples. We next examined the level of mortalin in the serum of patients with HCC, stages I to IV (Fig. 1b). No significant difference was observed across different disease stages. Likewise, there was no significant difference observed between the HCC patients, with or without cirrhosis (Fig. 1c). These data suggested that the circulating mortalin is not a sensitive biomarker for cirrhosis and/or HCC.
Table 1.
Demographic and clinicopathological characteristics of patients
| Cirrhosis (n = 16) | HCC (n = 100) | |
|---|---|---|
| Male/female | 11:5 | 83:17 |
| Age (year)a | 49.56 ± 9.1 | 57.59 ± 12.33 |
| HBsAg | 13:3 | 79:28 |
| AFP (ng/ml)a | 10.44 ± 18.1 | 7.64E4 ± 2.514E5 |
| Venous infiltrationb | ||
| Absent | 8 (8 %) | |
| Present | 20 (20 %) | |
| Tumor stage (AJCC) | ||
| Stage I | 25 (25 %) | |
| Stage II | 25 (25 %) | |
| Stage III | 25 (25 %) | |
| Stage IV | 25 (25 %) | |
| No. of tumor nodulesb | ||
| ≤4 | 68 (68 %) | |
| Multiple | 30 (30 %) | |
| Diffuse | 2 (2 %) |
aMean ± SD
bPartial data were not available, and statistics were based on available data
Fig. 1.
a–c Detection of circulating mortalin and mortalin autoantibody level in healthy, liver cirrhosis and HCC serum samples by sandwich ELISA. a Circulating mortalin protein levels in 150 serum samples of healthy, cirrhosis, and HCC samples. b The level of mortalin in HCC at each distinct tumor stage. c Serum mortalin level has no significant different in cirrhosis plus HCC and only HCC patients. d–f Detection of circulating mortalin autoantibody in healthy, cirrhosis, and HCC serum samples by sandwich ELISA. d The level of mortalin autoantibody significantly increased in cirrhosis patients in comparison to healthy individuals and HCC patients (P < 0.001). e There was no significant difference in mortalin autoantibody in each stage of HCC patients. f Serum mortalin autoantibody level was significantly increased in cirrhosis plus HCC patients compared to only HCC patients (P < 0.001). g–j Comparison of the diagnostic performance of serum mortalin and mortalin autoantibody in detection of cirrhosis and HCC patients by receiver operating characteristics (ROC), and the area under the ROC curve (AUC) is a measure of the overall performance of sensitivity and specificity. g Comparison of diagnostic performance of serum mortalin in detection of cirrhosis patients by receiver operating characteristics (ROC) (AUC = 0.58). h Comparison of diagnostic performance of serum mortalin in detection of HCC patients by receiver operating characteristics (ROC) (AUC = 0.67). i Comparison of diagnostic performance of serum mortalin autoantibody in detection of cirrhosis patients by receiver operating characteristics (ROC) (AUC = 0.87, P < 0.001). j Comparison of the diagnostic performance of serum mortalin autoantibody in detection of HCC patients by receiver operating characteristics (ROC) (AUC = 0.54)
Circulating mortalin autoantibody in cirrhosis and HCC patients
We next examined mortalin autoantibody in healthy, cirrhosis, and HCC patients. As shown in Fig. 1d, there was a significantly higher level of mortalin autoantibody in cirrhosis as compared to the healthy and HCC controls (P < 0.001). On the other hand, amongst the 100 HCC samples that were categorized in four HCC stages, mortalin autoantibody did not show any significant difference (Fig. 1e). We thus examined the level of mortalin autoantibody in patients with cirrhosis and HCC. We found that the cirrhosis patients possess higher level of mortalin autoantibody as compared to the patients with HCC only (P < 0.001) (Fig. 1f). These data suggested that mortalin autoantibody is a potential candidate marker to detect liver cirrhosis.
Comparison of serum mortalin autoantibody level and liver fibrosis markers for detection of liver cirrhosis
In light of our above finding that the level of mortalin autoantibody in liver disease patients significantly correlated with the presence of cirrhosis disease stage, the receiver-operating characteristic curve was constructed to visualize the relationship between sensitivity and specificity of mortalin autoantibody for predicting the presence of cirrhosis (Fig. 1g–j). The area under the curve (AUC) reached 0.865 (P < 0.001). When using an optimal cutoff value of 0.225 (OD value), the sensitivity and specificity of mortalin autoantibody in determining the occurrence of cirrhosis were 87.5 and 72 %, respectively. To further validate the effectiveness of mortalin autoantibody in cirrhosis diagnosis, we compared mortalin autoantibody to certain current class I circulating liver fibrosis markers, such as hyaluronic acid etc. The AUC value, sensitivity, and specificity of commonly reported liver fibrosis class I markers are presented in Table 2. These markers were sequenced by the decrease of AUC value, which implies perfect forecast when the AUC = 1. Table 2 shows that mortalin autoantibody has the highest AUC value (AUC = 0.865) and the sensitivity and specificity were better than most of the known liver fibrosis candidate markers. This analysis indicated that mortalin autoantibody could be an attractive diagnostic marker of cirrhosis.
Table 2.
Class I biomarker of liver fibrogenesis (cirrhosis)
| AUC | Sensitivity (%) | Specificity (%) | Method | Reference | |
|---|---|---|---|---|---|
| Mortalin autoantibody | 0.865 | 87.5 | 72 | ELISA | |
| Hyaluronic acid (hyaluronan) | 0.854 | 80 | 80 | ELISA | Saitou et al. 2005 |
| Connective tissue growth factor (CTGF/CCN2) | 0.829 | 78 | 87.5 | ELISA | Kovalenko et al. 2009 |
| YKL-40 | 0.795 | 80 | 71 | ELISA | Saitou et al. 2005 |
| N-terminal propeptide (PIIINP) | 0.79 | 77 | 66 | ELISA | Saitou et al. 2005 |
| Tissue inhibitor of metalloproteinases (TIMP-1) | 0.765 | 83.3 | 61.5 | ELISA | Pereira et al. 2004 |
| (Pro)matrix metalloproteinase (MMP-2) | 0.598 | 62.9 | 59.7 | ELISA | Pereira et al. 2004 |
| Type IV-collagen | 0.596 | 60 | 61 | ELISA | Saitou et al. 2005 |
Discussion
Mortalin is a multifunctional stress chaperone and is present in wide subcellular localizations ranging from mitochondria, endoplasmic reticulum (ER), cytosol, cell surface, and nucleus (Ran et al. 2000; Wadhwa et al. 2002). It is enriched in a variety of cancers (Dundas et al. 2005; Lu et al. 2011a, b; 2011a; Wadhwa et al. 2006; Yi et al. 2008; Ando et al. 2014; Klaus et al. 2014; Ryu et al. 2014). Mortalin has also been detected in the serum of colorectal patients, suggesting it as a general cancer diagnostic marker (Rozenberg et al. 2011). In our earlier studies, we reported an upregulation of mortalin in a large variety of cancer cells and clinical samples of HCC (Dundas et al. 2005; Lu et al. 2011a, b; Yi et al. 2008; Klaus et al. 2014; Sane et al. 2014). Based on these data, we hypothesized that circulating mortalin and mortalin autoantibody might be elevated in serum of HCC patients. However, we found that in spite of highly expressed mortalin in HCC patients and its relation with cancer aggressiveness, serum mortalin and mortalin autoantibodies were not significantly increased in HCC as compared to the normal controls. These data suggested that serum mortalin could be a specific diagnostic marker for colorectal cancers.
We found that mortalin autoantibody is present in significantly high level in serum of liver cirrhosis patients. Autoantibody is generated by the immune system in response to the foreign protein or substance within the body. Although the precise causes of autoantibody production varied and not very well understood, genetic predisposition combined with environmental factors has been implicated in this phenomenon. Cell surface mortalin has been shown to bind to the complement 9 and is involved in membrane attack complex (MAC) vesiculation, which contributes to cell resistance to MAC-induced cell membrane damage and cell death (Pilzer and Fishelson 2005). The membrane distribution of mortalin may facilitate its secretion from the cells and stimulation of the autoantibody production. It is likely that cirrhosis may have a unique configuration of cell surface of mortalin that induces the generation of autoantibody. Although the mechanism of autoantibody synthesis warrants further studies, we demonstrate, for the first time, a diagnostic correlation of serum mortalin autoantibody with cirrhosis in clinical samples. An earlier study on 2-dimensional electrophoresis-based proteomics and Western blot approaches on cystic fibrosis has reported that the mutated cystic fibrosis transmembrane conductance regulator (CFTR) protein leads to induced mortalin overexpression (Gomes-Alves et al. 2010). The mechanism of involvement of mortalin in fibrogenesis warrants further studies. Nevertheless, in view of the numerous shortages of liver biopsy, inclusion of the serum mortalin autoantibody to the already existing battery of markers may offer an improved and easy diagnosis for liver cirrhosis. Taken together, we demonstrated that the circulating mortalin autoantibody correlates with liver cirrhosis and hence could be a promising serological marker for its diagnosis. Mechanism of mortalin autoantibody and its clinicopathological relevance to liver cirrhosis warrant further studies.
Contributor Information
John M. Luk, Email: dr.johnluk@gmail.com
Sunil C. Kaul, Phone: 81-29-861-6713, Email: s-kaul@aist.go.jp
Renu Wadhwa, Phone: 81-29-861-9464, Email: renu-wadhwa@aist.go.jp.
References
- Ando K, Oki E, Zhao Y, Ikawa-Yoshida A, Kitao H, Saeki H, Kimura Y, Ida S, Morita M, Kusumoto T, Maehara Y. Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastric Cancer. 2014;17:255–262. doi: 10.1007/s10120-013-0279-1. [DOI] [PubMed] [Google Scholar]
- Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Dundas SR, Lawrie LC, Rooney PH, Murray GI. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205:74–81. doi: 10.1002/path.1672. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- Gomes-Alves P, Couto F, Pesquita C, Coelho AV, Penque D. Rescue of F508del-CFTR by RXR motif inactivation triggers proteome modulation associated with the unfolded protein response. Biochim Biophys Acta. 2010;1804:856–865. doi: 10.1016/j.bbapap.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijayadeva V, Boscarino JA, Henkle EM, Gordon SC. Chronic hepatitis cohort study (CHeCS) investigators. Non-invasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicko M, Veseliny E, Lesko D, Jarcuska P. Serum cholesterol is a significant and independent mortality predictor in liver cirrhosis patients. Ann Hepatol. 2013;12:581–587. [PubMed] [Google Scholar]
- Jarcuska P, Janicko M, Veseliny E, Skladany L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009–1017. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park SY, Yoo KY, Lee SK, Jung WW. Induction of heat shock proteins and antioxidant enzymes in 2,3,7,8-TCDD-induced hepatotoxicity in rats. Korean J Physiol Pharmacol. 2012;16:469–476. doi: 10.4196/kjpp.2012.16.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus C, Kaemmerer E, Reinartz A, Schneider U, Plum P, Jeon MK, Hose J, Hartmann F, Schnolzer M, Wagner N, Kopitz J, Gassler N. TP53 status regulates ACSL5-induced expression of mitochondrial mortalin in enterocytes and colorectal adenocarcinomas. Cell Tissue Res. 2014;357:267–278. doi: 10.1007/s00441-014-1826-8. [DOI] [PubMed] [Google Scholar]
- Kovalenko E, Tacke F, Gressner OA, Zimmermann HW, Lahme B, Janetzko A, Wiederholt T, Berg T, Muller T, Trautwein C, Gressner AM, Weiskirchen R. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat. 2009;16:612–620. doi: 10.1111/j.1365-2893.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787–799. doi: 10.1016/j.mcna.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM. Induction of mutant p53-dependent apoptosis in human hepatocellular carcinoma by targeting stress protein mortalin. Int J Cancer. 2011;129:1806–1814. doi: 10.1002/ijc.25857. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM. Mortalin-p53 interaction in cancer cells is stress dependent and constitutes a selective target for cancer therapy. Cell Death Differ. 2011;18:1046–1056. doi: 10.1038/cdd.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TN, Lewindon PJ, Smith JL, Murphy TL, Lincoln DJ, Shepherd RW, Ramm GA. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol. 2004;2004(41):576–583. doi: 10.1016/j.jhep.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Pilzer D, Fishelson Z. Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int Immunol. 2005;17:1239–1248. doi: 10.1093/intimm/dxh300. [DOI] [PubMed] [Google Scholar]
- Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, Ratziu V, Mercadier A, Benhamou Y, Hainque B. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8–19. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers RN, Bick RJ, Smith JR, Pereira-Smith OM. Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun. 2000;275:174–179. doi: 10.1006/bbrc.2000.3237. [DOI] [PubMed] [Google Scholar]
- Rozenberg P, Kocsis J, Saar M, Prohaszka Z, Fust G, Fishelson Z. Elevated levels of mitochondrial mortalin and cytosolic HSP70 in blood as risk factors in patients with colorectal cancer. Int J Cancer. 2011;133:514–518. doi: 10.1002/ijc.28029. [DOI] [PubMed] [Google Scholar]
- Ryu J, Kaul Z, Yoon AR, Liu Y, Yaguchi T, Na Y, Ahn HM, Gao R, Choi IK, Yun CO, Kaul SC, Wadhwa R. Identification and functional characterization of nuclear mortalin in human carcinogenesis. J Biol Chem. 2014;289:24832–24844. doi: 10.1074/jbc.M114.565929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, Sugimoto K, Murata K, Nakano T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane S, Abdullah A, Boudreau DA, Autenried RK, Gupta BK, Wang X, Wang H, Schlenker EH, Zhang D, Telleria C, Huang L, Chauhan SC, et al. Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells. Cell Death Dis. 2014;5:e1118. doi: 10.1038/cddis.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002;7:309–316. doi: 10.1379/1466-1268(2002)007<0309:AHFCMM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR, Kaul SC. Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer. 2006;118:2973–2980. doi: 10.1002/ijc.21773. [DOI] [PubMed] [Google Scholar]
- Yi X, Luk JM, Lee NP, Peng J, Leng X, Guan XY, Lau GK, Beretta L, Fan ST. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics. 2008;7:315–325. doi: 10.1074/mcp.M700116-MCP200. [DOI] [PubMed] [Google Scholar]