Abstract
We investigated whether acetyl salicylic acid (ASA) protects chicken myocardial cells from heat stress-mediated damage in vivo and whether the induction of Hsp27 expression is connected with this function. Pathological changes, damage-related enzyme levels, and Hsp27 expression were studied in chickens following heat stress (40 ± 1 °C for 0, 1, 2, 3, 5, 7, 10, 15, or 24 h, respectively) with or without ASA administration (1 mg/kg BW, 2 h prior). Appearance of pathological lesions such as degenerations and karyopyknosis as well as the myocardial damage-related enzyme activation indicated that heat stress causes considerable injury to the myocardial cells in vivo. Myocardial cell injury was most serious in chickens exposed to heat stress without prior ASA administration; meanwhile, ASA pretreatment acted protective function against high temperature-induced injury. Hsp27 expression was induced under all experimental conditions but was one-fold higher in the ASA-pretreated animals (0.3138 ± 0.0340 ng/mL) than in untreated animals (0.1437 ± 0.0476 ng/mL) 1 h after heat stress exposure, and such an increase was sustained over the length of the experiment. Our findings indicate that pretreatment with ASA protects chicken myocardial cells from acute heat stress in vivo with almost no obvious side effects, and this protection may involve an enhancement of Hsp27 expression. However, the detailed mechanisms underlying this effect require further investigation.
Keywords: Hsp27, Aspirin, Heat stress, Chicken, Myocardial cell
Introduction
High environmental temperature and humidity, as well as global warming, increasingly affect the production of commercial animal agriculture in many countries (Fuquay 1981; Morrison 1983). Current models suggest that economic losses across several agricultural industries will be considerable if abatement techniques are not put in place. It was estimated that the annual loss could amount to $2.4 billion in the USA alone, including a loss of $128 million for the poultry industry (St-Pierre* et al. 2003). It is well known that modern poultry species such as the broiler chicken are highly sensitive to heat stress due to the feather cover, lack of sudoriferous glands, and fast growth (St-Pierre* et al. 2003; Piestun et al. 2013). Several investigations indicate that heat stress induces serious intracorporal damage even if the animal survives (Kamboh et al. 2013; Ma et al. 2013; Zhao et al. 2013). Acute heat stress may also contribute to sudden infant death in humans and animals, and myocardial cell damage-induced cardiac dysfunction is considered a possible cause (Tang et al. 2013). Indeed, many studies have demonstrated that lethal heart injury or damage results from high temperatures (Gathiram et al. 1987; Gathiram et al. 1988; Rai and Ambwany 1980). During heat stress, the membrane permeability of myocardial cells increases and enzymes are released from myocardial cells into the serum (Mitchell and Sandercock 1995; Saravanan et al. 2013; Tang et al. 2013). These enzymes, including isoenzyme creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH), are widely regarded as indicators of acute myocardial injury (Amani et al. 2013; Chen et al. 2013; Chon et al. 2013; Wu et al. 2013; Zeren et al. 2013). When exposed to elevated temperatures, animal cells synthesize a small number of highly conserved proteins called heat-shock proteins (Hsps) (Lindquist 1986). Induction of Hsp expression has been reported to protect cells against subsequent temperature changes as well as diminished oxygen and pressure, and loss of Hsp expression may cause damage or be lethal (Marber et al. 1995; Ryan et al. 1992; Rylander et al. 2005; Villar et al. 1994; Wischmeyer 2002). For example, suppressed Hsp90 and Hsp27 expression resulted in increased endoplasmic reticulum stress (ER stress) and apoptosis (Lamoureux et al. 2013). Hsp27, alpha β-crystallin, and Hsp22 are classified as sHSP whose expression is induced in response to a wide variety of unfavorable physiological and environmental conditions (Acunzo et al. 2012; Kargul and Laurent 2012). Hsp27 is also considered a potential diagnostic marker of cancer (Taba et al. 2010). Although the protective function of Hsp27 is still under intense investigation (Stetler et al. 2009), it is known to play an important role in oxidative stress and heat stress (Mearow et al. 2002). Interestingly, Hsp27 overexpression is able to attenuate cardiac dysfunction in transgenic mice (Liu et al. 2007). Collectively, based on these studies, we explored whether Hsp27 may play a role in protecting myocardial cells against stress injury.
A number of methods have been developed to induce Hsp expression to prevent injury from extreme stress. While the approaches may have been effective in the laboratory, the methods have lacked feasibility. For example, mineral salts such as sodium arsenite efficiently induce Hsp expression but are toxic with a 20 % mortality rate in animals (Lappas et al. 1994; Ribeiro et al. 1994). Organic reagents such as geranylgeranylacetone (GGA) and glutamine are also reported to induce Hsps (Endo et al. 2007; Wischmeyer 2002) but cannot be widely applied for veterinary use due to the high costs involved. Physical methods such as preheating were difficult to control and had detrimental effects on many cellular functions (Wong 1999). Acetyl salicylic acid (ASA), also known as aspirin, is widely used for its broadly therapeutic antiinflammatory and antipyretic properties. ASA has heart protective functions that were initially considered to act through platelet interactions (Patrono et al. 2005). Although non-aspirin non-steroidal antiinflammatory drugs (NSAIDs) have anti-inflammatory and anti-platelet properties similar to aspirin, few of them induce the same myocardial protective effects against acute myocardial infarction (AMI) (Solomon et al. 2002). It is possible that ASA confers heart protection by another mechanism. For instance, ASA upregulates expression of different HSP family members, including Hsp27 (Ebert et al. 2005), in different species (Amberger et al. 1999; Sandoval-Montiel et al. 2013). As Hsps (especially Hsp27) are widely believed to protect against cell injury caused by various stress factors, it is reasonable to consider that ASA may protect against myocardial injury from heat stress by modulating Hsp expression. Although previous studies indicate that ASA may reduce stress injury and that it is closely related to the heat shock response (Ghavami and Hardy 2002; Jurivich et al. 1992), this phenomenon still requires further examination. Therefore, we set out to determine the protective function of ASA. This study determines whether heat stress induces myocardial injury and whether ASA administration can reduce heat stress-induced injury in chicken myocardial cells in vivo, as it has the ability to induce Hsp27 expression.
Materials and methods
Animal stress model
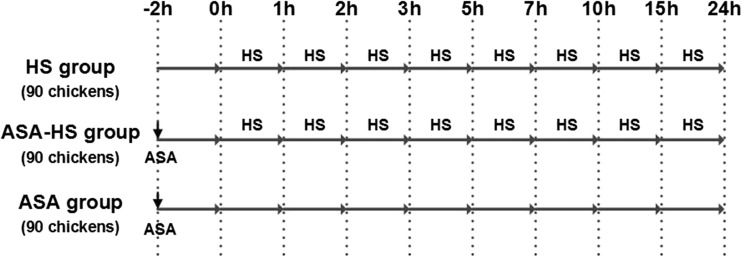
One-day-old specific pathogen free (SPF) chickens were purchased from Qian Yuan Hao Biotechnology Company, Nanjing, China. The entire chicken population was vaccinated against Newcastle disease (ND) and infectious bursal disease (IBD) on the 7th and the 14th days, respectively. The birds were given 30 days to acclimate to their new housing and to recover from environmental stress. Two hundred seventy chickens were randomly divided into three groups, designated as the HS (heat stress) group, the ASA-HS (aspirin administrated before heat stress) group, and the ASA (aspirin administration) group, respectively, with 90 chickens in each group and were not provided water for the 12 h leading up to the experiment. Chickens in the ASA-HS and ASA groups were administered aspirin orally at 1 mg/kg body weight (ASA powder >98 % purity was provided by Sigma, USA) 2 h before the heat stress phase of the experiment. During heat stress phase, chickens in ASA group were maintained under normal condition as a control group (not treated with heat), while the other two groups, HS and ASA-HS, were heat stressed by rapidly and gently shifting the animals from 25 ± 1 °C to a preheated air chamber (GJ-1, Suzhou Fengshi Laboratory Animal Equipment Co. Ltd, China) at 40 ± 1 °C with 60 ~ 70 % humidity over a series of time periods (0, 1, 2, 3, 5, 7, 10, 15, and 24 h). Birds were allowed free access to food and water ad libitum during heat stress exposure. After collecting 10-mL blood samples, chickens (10 birds from each group) were sacrificed humanely by decapitation at the following time points: 0, 1, 2, 3, 5, 7, 10, 15, and 24 h. Heart samples were collected and fixed in 10 % formalin for morphological studies or frozen in liquid nitrogen for biochemical analysis (Fig. 1).
Fig. 1.
Depiction of the heat-stressed chicken model. Chickens in the three groups were administrated aspirin or vehicle orally at 1 mg/kg BW 2 h before the heat stress exposure. To initiate heat stress, chickens in HS group and ASA-HS group were shifted from 25 ± 1 to 40 ± 1 °C with 60 ~ 70 % humidity. Ten birds from each group were sacrificed humanely by decapitation at the following time points: 0, 1, 2, 3, 5, 7, 10, 15, and 24 h, and the samples were collected. ASA aspirin administered orally at 1 mg/kg BW, HS heat stress exposure to 40 ± 1 °C with 60 ~ 70 % humidity
The heat stress experiment adhered to the guidelines of the regional Animal Ethics Committee and was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Detection of enzymes associate with heart damage
Serum (1.5 mL) was collected from chickens after exposure to the treatments described above. The LDH and CK-MB enzyme activities were measured according to the manufacturer’s instructions (Nanjing Jiancheng Biochemical Reagent Co. Ltd., Nanjing, China) with a clinical biochemical indicator auto-analyzer (Vital Scientific NV, The Netherlands). Each sample was analyzed five consecutive times.
Detection of Hsp27 expression
After washing in ice-cold saline, the chicken heart samples were homogenized on ice in 10 volumes of homogenization buffer [0.15 M NaCl, 20 mM Tris–HCl (pH 8.0), 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulphonyl fluoride, 0.1 μM E-46, 0.08 μM aprotinin, 0.1 μM leupeptin, and 0.1 % NP-40] using an Ultra-Turrax homogenizer. The homogenates were centrifuged at 12,000×g for 20 min at 4 °C to remove cellular debris. The supernatant was collected and stored at −20 °C for protein quantification. Hsp27 protein levels were measured using a commercially available ELISA Kit (MBS700383, MyBioSource, USA) according to the manufacturer’s instructions.
Histopathology
Heart tissue samples were obtained and preserved in 10 % formalin. The samples were embedded in paraffin and then cut into 5-μm-thick serial sections. Sections were stained with H&E, and images were obtained by light microscopy.
Immunohistochemistry
The paraffin-embedded heart tissues were cut into 5-μm-thick serial sections. Serial sections of the heart tissue were immunostained with a standard avidin-biotin complex (ABC) immunoperoxidase detection system. The paraffin was removed by placing the sections in xylene twice for 5 min each. The slides were rehydrated in 100 % ethanol for 3 min twice, 95 and 85 % ethanol for 1 min each, then rinsed in distilled water. The endogenous peroxidase was then inactivated in 3 % H2O2 for 10 min at room temperature. Non-specific antigen binding was blocked with 5 % bovine serum albumin (BSA) for approximately 30 min at 37 °C. The sections were incubated with a 1:50 dilution of the Hsp27 primary antibody (ab49919, Abcam, USA) for 2 h at 37 °C. After washing with PBS, the sections were incubated with goat anti-mouse IgG-HRP(H+L) (Cat No. SN133, SunShine Bio, China) secondary antibody for 1 h at 37 °C. The sections were treated with two drops of diaminobenzidine (DAB) substrate chromogen solution (Boster Ar1022, Wuhan, China) for 10 min, and then, the reaction was stopped with the addition of water. The sections were counterstained with hematoxylin, and images were obtained by light microscopy. The corresponding negative controls were prepared by omitting the primary antibody.
The semi-quantitative analysis was performed by Service Bio Co., Ltd., (Wuhan, China). Staining intensities in the cytoplasm and nucleus were evaluated by determining the average optical density (AOD) using Image-Pro Plus 6.0 software.
Statistical analysis
The software Curve Expert 1.3 was used to make a standard curve for ELISA interpretation. Differences among the experimental groups were analyzed by the least significant difference (LSD) for both P < 0.05 and P < 0.01, using the Statistical Package for Social Sciences (SPSS version 20.0 for Windows). Differences were considered to be significant when P < 0.05 and highly significant when P < 0.01. All data are expressed as the mean ± standard deviation (SD). For statistical analysis, values in ASA-HS and ASA groups were compared with those obtained at the 0 h time point in the HS group, as this represented the baseline where the animals had not been exposed to heat stress or aspirin pretreatment.
Results
Clinical symptoms of heat-stressed chickens
All of the chickens became anxious immediately after exposure to heat stress, and most attempted to escape from the heat stress chambers. After 7 min of heat stress, the chickens began to pant constantly, and food intake stopped while water consumption increased significantly. After 30 min of heat stress, chickens lay down their wings with polypnea. After 1 h of heat stress, 90 % of chickens lay down on the ground with depressed emotion and responded slowly to external stimuli but continued to consume water. After 2 ~ 3 h of heat stress, 40 % of chickens were experiencing agonal respiration. However, clinical symptom did not get any worse with up to 4 h of heat stress in the remainder chickens. In fact, some animals gradually recovered from agonal stage over this time. There were no observable differences between HS group and ASA-HS group during the heat stress period.
Changes in the serum levels of damage-related enzymes in stressed chickens
The levels of cardiomyocyte damage-related enzymes in the serum of chickens were measured after exposure to various periods of heat (Fig. 2). In general, the CK-MB and LDH levels in the HS group chickens were elevated compared to that in the ASA-HS and ASA groups.
Fig. 2.
Changes in the serum levels of CK-MB and LDH in chickens. *P < 0.05, significant differences of least significant difference (LSD) with significance at the level. **P < 0.01, significant differences of LSD with significance at the level
Except for a brief return to normal levels at 3 h (1171.33 ± 88.89 IU/L) and 15 h (1722.50 ± 302.82 IU/L), the serum CK-MB levels in the heat-stressed chickens in the HS group remained significantly increased after heat exposure for 1 h (1993.67 ± 503.50 IU/L), 2 h (2009.67 ± 233.89 IU/L), 5 h (1810.00 ± 5.77 IU/L), 7 h (2589.00 ± 270.31 IU/L), 10 h (2499.50 ± 286.65 IU/L), and 24 h (2527.00 ± 219.40 IU/L), compared to that at 0 h (1026.00 ± 30.59956 IU/L). Compared to the control (HS group animals at 0 h, which were not exposed to heat stress or aspirin), the serum CK-MB levels in the chickens remained mostly unchanged in the ASA-HS group. The only increases were observed at 0 h (1429.00 ± 201.50 IU/L), which was 2 h after aspirin administration, but prior to heat exposure, as well as at 2 h (1383.00 ± 60.62 IU/L), 3 h (1339.00 ± 39.84 IU/L), and 15 h (2242.50 ± 53.40 IU/L) after heat stress. As expected, chickens in the ASA group presented with the least variation in serum CK-MB levels. Significant increases were observed at only 0 h (1429.00 ± 201.50 IU/L) and 2 h (1428.00 ± 80.25 IU/L). Statistical analysis revealed that the CK-MB activity in the HS group was significantly higher than that in the ASA-HS and ASA groups at 2, 5, 7, 10, and 24 h.
The changes in the serum LDH levels showed a similar trend as that observed for CK-MB, but this parameter appeared more sensitive to different changes. The LDH levels in the HS group increased from 730.50 ± 61.32 to 2261.33 ± 412.23 IU/L following a 2-h heat exposure. After a brief reduction, it rose again to 2029.33 ± 222.61 IU/L after a 5-h heat exposure. This significant increase (over the baseline) was maintained until the end of the experiment. Significant increases in LDH levels were also observed in ASA-HS group at multiple time points including 0 h (1199.00 ± 122.98), 1 h (1032.00 ± 56.15), 2 h (1015.00 ± 30.60), 5 h (1016.50 ± 97.28), 7 h (1058.50 ± 78.80 IU/L), 15 h (2406.50 ± 47.63 IU/L), and 24 h (975.50 ± 63.22 IU/L) and also in the ASA group at 0 h (1199.00 ± 122.98 IU/L), 1 h (1137.67 ± 90.14 IU/L), 2 h (1112.33 ± 62.89 IU/L), 5 h (1237.67 ± 75.45 IU/L), and 15 h (1116.00 ± 88.91 IU/L). However, what is worthy to know is that the LDH levels in the HS group were significantly higher than those in the ASA-HS and ASA groups at the corresponding time points (0, 1, 2, 5, 7, 10, and 24 h).
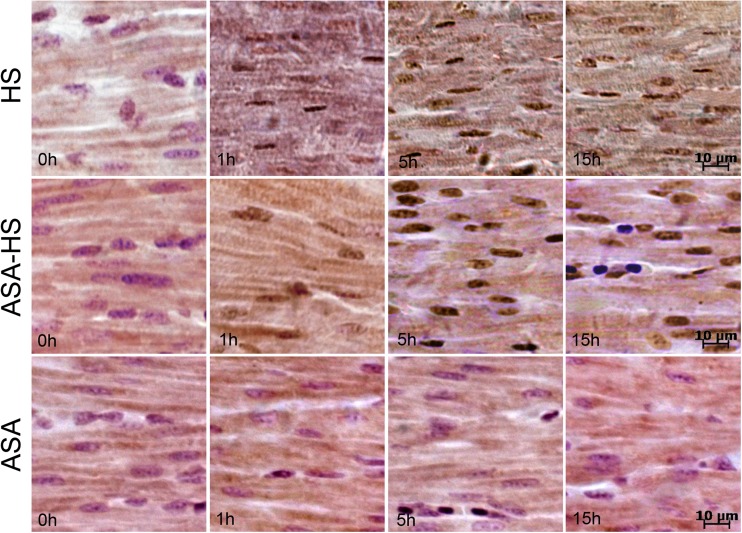
Histopathology
Cellular damage was revealed upon examination of the hearts from heat-stressed chickens (Fig. 3). In HS group, the myocardial cells became swollen in shape 1 h following heat exposure; uneven matrix staining suggests that acute alteration occurred in the cytoplasm as well (Fig. 3, HS group). By 5 h, some nuclei within the heart tissue appeared shrunken and dull-stained, characteristic of karyopyknosis (Fig. 3, HS group). At later time points, vacuolar degeneration was observed among the myocardial fibers of the heat-stressed chickens until 15 h (Fig. 3, HS group). Although the degeneration was not apparent 15 h post-heat stress, many nuclei had characteristics of karyopyknosis suggesting that acute injury still had occurred in myocardial cells by 15 h (Fig. 3, HS group). Moreover, this acute injury characterized by karyopyknosis could be observed for the remainder of the experiment (up to 24 h).
Fig. 3.
Representative histopathological images of chicken myocardial cells following heat exposure. Hematoxylin and eosin staining. Scale bar = 10 μm. HS group: By 1 h, the myocardial cells became swollen in shape with uneven matrix staining in the cytoplasm (↓). By 5 h, acute degeneration in numerous vacuoles (↑) was observed in the myocardial fibers. Some nuclei within myocardial cells appeared shrunken and dull-stained, characteristic of karyopyknosis (→). At later time points, several nuclei displayed the characteristics of karyopyknosis (Fig. 2, HS group, →), suggesting that acute injury remained in myocardial cells by 15 h. ASA-HS group: After 1 h of heat treatment in chickens that were pretreated with ASA, myocardial cells became swollen in size with acute degeneration of numerous vacuoles (↑) observed in the myocardial fibers (↑). By 5 h, there was no significant injury except for acute degeneration observed by myocardial cell morphology at later time points. ASA group: After ASA administration, there were no obvious pathological changes in myocardial fibers
Chickens that were administrated ASA prior to heat exposure also displayed swollen myocardial cells with vacuolar degeneration after 1 h of heat stress treatment (Fig. 3, ASA-HS group). Expect for the acute vacuolar degeneration at 1 h, there was no significant injury in myocardial cells at later time points in ASA-HS group. This reveals that ASA administration decreased myocardial cell injury in ASA-HS group with only a small amount of acute degeneration during the early stage of heat exposure.
ASA group was administered ASA but was not exposed to heat stress, and these chickens had no obvious pathological changes (Fig. 3, ASA group).
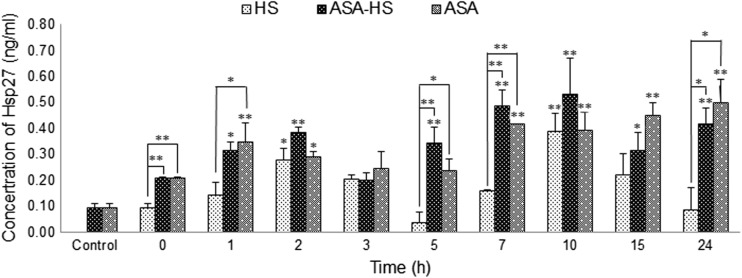
The expression of Hsp27 in heart tissues
The expression of Hsp27 in heart tissue from heat-treated chickens in HS group dramatically increased and decreased over time compared to animals in ASA-HS group and ASA group (Fig. 4). After exposure to heat stress for 2 h, the concentration of Hsp27 increased from 0.0938 ± 0.0177 to 0.2773 ± 0.0472 ng/mL. After a decreasing period, it reached 0.3899 ± 0.0699 ng/mL by the 10-h time point before decreasing again. The only significant increases (P < 0.05 comparing to the 0-h value) in Hsp27 expression in the HS group were observed at the 2 and 10-h time points.
Fig. 4.
Variations of Hsp27 expression in myocardial cells after heat exposure. Significant changes in Hsp27 concentrations in the HS group are observable after 2 and 10 h of heat stress, while aspirin pretreatment results in a more continuous increase in the Hsp27 levels, both in ASA-HS and ASA group chickens. *P < 0.05 compared to control, **P < 0.01 compared to control
Compared to the control (0.0938 ± 0.0177 ng/mL), oral administration of ASA resulted in two continuous periods of increase in Hsp27 expression in the chicken heart tissues: from 1 h (0.3488 ± 0.0730 ng/mL) to 2 h (0.2910 ± 0.0205 ng/mL), and from 7 h (0.4157 ± 0.0020 ng/mL) to 24 h (0.5002 ± 0.0877 ng/mL). With double stimulation by heat stress and aspirin, the expression of Hsp27 in the heart of the heat-stressed chickens in ASA-HS group also continued to increase over time. Significant (P < 0.05) or very significant (P < 0.01) increases compared to the control were detected after exposure to high temperatures for 1 h (0.3138 ± 0.0340 ng/mL), 2 h (0.3830 ± 0.0222 ng/mL), 5 h (0.3460 ± 0.0590 ng/mL), 7 h (0.4857 ± 0.0614 ng/mL), 10 h (0.5311 ± 0.1406 ng/mL), 15 h (0.3138 ± 0.0705 ng/mL), and 24 h (0.4178 ± 0.0607 ng/mL). Comparison between different groups also indicated that the concentration of Hsp27 in the HS group was lower than that in the ASA group at a series of time points, including 0, 1, 5, 7, and 24 h.
Therefore, although Hsp27 expression did vary in all three groups, it was apparent that Hsp27 expression continuously increased in ASA-HS group and ASA group, compared to HS group. Therefore, ASA treatment must be responsible for the steady increase in Hsp27 expression over time.
Hsp27 localization
Semi-quantitative immunohistochemical detection was used to characterize the localization of Hsp27 in the myocardial cells of differentially treated chickens (Fig. 5). AOD variations at the same time points in the ASA-HS and ASA groups were compared with that in HS group, and the results are shown in Table 1.
Fig. 5.
Localization of Hsp27 determined by immunohistochemistry. Immunohistochemistry staining and Mayer’s hematoxylin counterstaining of heart tissues. Scale bar = 10 μm. HS group: Prior to heat exposure, Hsp27 (brown) was mainly located in the cytoplasm while weak signals were also observed in the nucleus of myocardial cells. Following heat exposure, there was a stronger density of Hsp27 localized in the nucleus and the cytoplasm with an especially strong signal in the nucleus. ASA-HS group: Hsp27 signals were located in the cytoplasm and the nucleus of myocardial cells. However, Hsp27 signals became stronger in the cytoplasm and the nucleus of myocardial cells at later time points following heat exposure. ASA group: Hsp27 was detected in the nucleus and cytoplasm of myocardial cells over the course of exposure.
Table 1.
Variations of average optical density (AOD) in each group
| Group | Localization | Average optical density (AOD) | |||
|---|---|---|---|---|---|
| 0 h | 1 h | 5 h | 15 h | ||
| HS | Cytoplasm | 0.1043 ± 0.0081 | 0.1255 ± 0.0225 | 0.1075 ± 0.0184 | 0.1258 ± 0.0105 |
| Nucleus | 0.0047 ± 0.0040## | 0.3514 ± 0.0319## | 0.6804 ± 0.0751## | 0.5157 ± 0.0504## | |
| ASA-HS | Cytoplasm | 0.1314 ± 0.0135** | 0.2556 ± 0.0215** | 0.2484 ± 0.0499** | 0.2698 ± 0.0434** |
| Nucleus | 0.0036 ± 0.0062## | 0.5722 ± 0.1780*# | 0.7168 ± 0.0621## | 0.6507 ± 0.0202**## | |
| ASA | Cytoplasm | 0.1846 ± 0.0114** | 0.2063 ± 0.0442* | 0.1596 ± 0.0177 | 0.1852 ± 0.0256 |
| Nucleus | 0.0373 ± 0.0301## | 0.0148 ± 0.0148**## | 0.0318 ± 0.0273**## | 0.0288 ± 0.0311**## | |
Comparison of the AOD in the cytoplasm and nucleus at corresponding time points in the ASA-HS and ASA groups to that in the HS group. *P < 0.05; **P < 0.01 compared to HS group values
AOD values of nucleus were compared to that of cytoplasm at every time points in all three groups. #P < 0.05; ##P < 0.01
Prior to heat exposure, Hsp27 was mainly localized in the cytoplasm of myocardial cells, while weak signals were also observed in the nucleus in the HS group. Then, following heat exposure, stronger signals of Hsp27 were detected in the nucleus, which were reflected by very significant (P < 0.01) increases in AOD (Table 1).
Compared to the 0-h time point in HS group (which was not exposed to ASA or heat), the 0-h time point in ASA-HS group (which has already administrated with ASA for 2 h) displayed a stronger Hsp27 signal in the cytoplasm (Table 1). The Hsp27 signals both in the cytoplasm and in the nucleus grew stronger at later time points following heat exposure in the ASA-HS group.
Following 2 h of ASA administration, the 0-h time point in ASA group displayed a stronger signal than HS group in cytoplasm (Table 1). Without heat exposure, a high level of Hsp27 expression in the nucleus was no longer observed (Table 1).
Discussion
The membrane permeability of myocardial cells increases under heat stress and releases a series of enzymes into the serum. These cardiomyocyte damage-related enzymes, such as CK-MB and LDH, are widely regarded as indicators of acute myocardial injury (Amani et al. 2013; Chen et al. 2013; Chon et al. 2013; Wu et al. 2013), especially enzyme CK-MB (Zeren et al. 2013). In the present study, we examined pathological lesions and cardiomyocyte damage-related enzyme concentrations to detect myocardial cell injury in chickens exposed to high temperatures. Although the CK-MB and LDH levels were slightly changed in response to aspirin, no morphological changes were observed. Overall, the results of these two cardiomyocyte damage-related assays coincided with each other. Changes in the level of damage-related enzymes accompanied acute degeneration and large amounts of karyopyknosis implying serious myocardial dysfunction and metabolic disorder, which can be lethal. Therefore, both cardiomyocyte damage-related enzymes and histopathological studies indicate that heat stress causes acute injury of myocardial cells in chickens in vivo.
High-dose aspirin has been shown to be protective by regulating Hsp expression (Ghavami and Hardy 2002). However, further investigation revealed that exposure to a higher dose of aspirin may lead to injury by causing cell apoptosis (Kim et al. 2009; Park et al. 2010; Singh and Rathaur 2010), which made it unacceptable for use in commercial farming, and shifted the focus to low-dose aspirin administration. In this study, based on previous studies and our series of preexperiment data (Mansoor et al. 2013; Sutcliffe et al. 2013), an oral administration dosage as low as 1 mg/kg was chosen and was found to be effective.
Myocardial cell injury was greatest in chickens exposed to heat stress without ASA treatment and was reduced with ASA pretreatment: Chickens treated with ASA and not exposed to heat stress showed almost no signs of myocardial cell injury. This reveals that heat stress causes considerable damage to chicken myocardial cells, and the responding CK-MB and LDH activity suggests that the extent of myocardial cell damage was serious and got worse over time. According to the clinical symptoms and histopathological lesions in the present study, the myocardial cell damage in chickens stressed after ASA oral administration was decreased compared with animals not treated with ASA. Furthermore, the levels of CK-MB and LDH in the serum of chickens given ASA were lower following heat stress. Together, this suggests that ASA may prevent myocardial cell damage caused by heat stress.
It has been reported that Hsps can regulate stress injury, protein synthesis (folding and unfolding) and degradation, the generation of an immune response, and apoptosis (Bukau and Horwich 1998; Mehlen et al. 1996). In the present study, ASA treatment reduced myocardial cell injury and efficiently induced Hsp27 expression. The expression of Hsp27 in the heart tissues of the heat-stressed chickens in HS group varied over time. However, the expression levels of Hsp27 in the hearts of the heat-stressed chickens in ASA-HS group and ASA group was continuously induced. The maintained expression levels of Hsp27 in both ASA-HS group and HS group suggest that the effect of ASA treatment lasts for a long period of time. However, previous studies indicate that ASA affects the heat shock response (HSR) reaction differently from heat stress (Jurivich et al. 1995). Unlike high temperatures, ASA alone was able to induce HSF1 binding to DNA but it was not sufficient to cause subsequent heat shock gene transcription. This was attributed to ASA-mediated HSF1 phosphorylation at threonine and from an inert HSF1-HSE complex, which was necessary but insufficient for transcriptional activation (Cotto et al. 1996). Meanwhile, heat stress induced the active HSF1-HSE complex containing a phosphotyroserine (Batulan et al. 2003; Jurivich et al. 1992). Previous studies reported that subsequent heat stress converts the ASA-induced inert HSF1-HSE complex into a full active complex (Cotto et al. 1996). Collectively, findings from the above studies indicate that ASA pretreatment alone may be insufficient to cause heat shock gene transcription. However, later studies revealed that the transcription of HSPs is regulated in multiple ways (Batulan et al. 2003). To date, four heat shock factors (1–4) have been discovered (Mathew et al. 1998). HSF2 plays an important role in heat shock gene transcription and is involved a novel pathway of HSP transcription (Jurivich et al. 1995; Mathew et al. 1998). In our studies, ASA alone was capable of inducing Hsp27 expression. This suggests that Hsp27 expression is at least partly HSF1-independent. Additionally, there may be other HSP regulation pathways, which may involve HSF2 or other factors. Other reports indicate that continuous heat or ASA exposure for 4 h will attenuate HSF-DNA binding (Abravaya et al. 1991; Jurivich et al. 1995). Similarly, in our study, Hsp27 expression was present at a low level by 3–5 h following heat stress, heat stress plus ASA, or ASA alone. This suggests that a potentially new regulation mechanism functions similarly to classic HSF regulation and that HSF may also function in this mechanism. Although the mechanism regulating Hsp27 expression remains unknown but may involve epigenetics or something beyond simple transcription, a number of studies have reported that ASA induces different HSP family members in several mammalian species (Amberger et al. 1999; Sandoval-Montiel et al. 2013; Wang et al. 2009). Our study extends this theory by showing that ASA induces Hsp27 expression in poultry. Furthermore, insufficient Hsp27 expression in heat-stressed myocardial cells was accompanied by myocardial damage suggesting that abundant Hsp27 expression may be required to protect cells from heat stress damage.
Following heat exposure, stronger Hsp27 signals were located in myocardial cells, especially in the nucleus, pretreated with ASA compared to control chickens. This suggests that heat stress induces the translocation of Hsp27 to the nucleus. The abundant translocation of this protein could possibly be related to the protective functions of Hsp27. Hsp27 is localized within the perinuclear region of cells at 37 °C and is translocated to the nucleus after heat stress (Arrigo et al. 1988). The chaperone function of Hsp27 only occurs when it forms large, unphosphorylated oligomers (Rogalla et al. 1999) by stabilizing cytoskeletal components (Hollander et al. 2004). The phosphorylated form of Hsp27 is a potent antiapoptotic molecule that may directly interfere with cell death signaling pathways (Benn et al. 2002; de Graauw et al. 2005). Phosphorylation of Hsp27 promotes dissociation of large HspB1 (Hsp27) oligomers (Kato et al. 1994; Lambert et al. 1999) and induces nuclear translocation (Geum et al. 2002). Generally speaking, the translocation of Hsp27 is closely related to its phosphorylation state and protective function.
In the present study, stronger Hsp27 signals were observed in the nuclei of chicken myocardial cells from HS group after exposure to heat stress compared to chickens from ASA group, which were not exposed to heat stress. Previous studies have reported that heat stress and other classic stress factors always lead to relocalization of Hsp27 into the nucleus (Acunzo et al. 2014; Arrigo et al. 1988; Arrigo and Landry 1994). Although, in our study, Hsp27 was induced more efficiently by ASA than heat stress, and relocalization of Hsp27 into the nucleus was not observed if ASA was the only inducing factor. This suggests that the mechanism of ASA-induced Hsp27 protection may differ from classic heat stress responses. Other than HSF2, it is possible that the arachidonic acid cascade pathway may be involved in this form of ASA-induced Hsp27 protection as reported previously (Ito et al. 1996). However, the detailed mechanism of this form of protection remains to be determined.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31372403), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Sino-German Agricultural Cooperation Project of the Federal Ministry of Food, the Agriculture and Consumer Production, Berlin, Germany.
References
- Abravaya K, Phillips B, Morimoto RI. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991;5:2117–2127. doi: 10.1101/gad.5.11.2117. [DOI] [PubMed] [Google Scholar]
- Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622–1631. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Acunzo J, Andrieu C, Baylot V, So A, Rocchi P. Hsp27 as a therapeutic target in cancers. Curr Drug Targets. 2014;15:423–431. doi: 10.2174/13894501113146660230. [DOI] [PubMed] [Google Scholar]
- Amani M, Jeddi S, Ahmadiasl N, Usefzade N, Zaman J. Effect of HEMADO on level of CK-MB and LDH enzymes after ischemia/reperfusion injury in isolated rat heart. Bioimpacts : BI. 2013;3:101–104. doi: 10.5681/bi.2013.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberger A, Hala M, Saurwein-Teissl M, Metzler B, Grubeck-Loebenstein B, Xu Q, Wick G. Suppressive effects of anti-inflammatory agents on human endothelial cell activation and induction of heat shock proteins. Mol Med. 1999;5:117–128. [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Landry J (1994) Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto RI, Tissieres A, Georgopoulos C (eds) The biology of heat shock proteins and molecular chaperones, p 335–373
- Arrigo AP, Suhan JP, Welch WJ. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci Off J Soc Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn SC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/S0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/S0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Chen TH, Yang YC, Wang JC, Wang JJ. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant Proc. 2013;45:3546–3549. doi: 10.1016/j.transproceed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Chon H, Lee S, Yoon SY, Lee EK, Chang SI, Choo J. SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction. Chem Commun (Camb) 2013 doi: 10.1039/c3cc47850e. [DOI] [PubMed] [Google Scholar]
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem. 2005;280:29885–29898. doi: 10.1074/jbc.M412708200. [DOI] [PubMed] [Google Scholar]
- Ebert MP, et al. Protective role of heat shock protein 27 in gastric mucosal injury. J Pathol. 2005;207:177–184. doi: 10.1002/path.1815. [DOI] [PubMed] [Google Scholar]
- Endo S, et al. Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol. 2007;72:1337–1348. doi: 10.1124/mol.107.039164. [DOI] [PubMed] [Google Scholar]
- Fuquay JW. Heat stress as it affects animal production. J Anim Sci. 1981;52:164–174. doi: 10.2527/jas1981.521164x. [DOI] [PubMed] [Google Scholar]
- Gathiram P, Gaffin SL, Brock-Utne JG, Wells MT. Time course of endotoxemia and cardiovascular changes in heat-stressed primates. Aviat Space Environ Med. 1987;58:1071–1074. [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Raidoo D, Brock-Utne JG, Gaffin SL. Portal and systemic plasma lipopolysaccharide concentrations in heat-stressed primates. Circ Shock. 1988;25:223–230. [PubMed] [Google Scholar]
- Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem. 2002;277:19913–19921. doi: 10.1074/jbc.M104396200. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Hardy SP. Heat shock protein and high-dose aspirin: effects on random skin flap survival in a rat model. Ann Plast Surg. 2002;48:60–67. doi: 10.1097/00000637-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Ito H, Hasegawa K, Inaguma Y, Kozawa O, Kato K. Enhancement of stress-induced synthesis of hsp27 and alpha B crystallin by modulators of the arachidonic acid cascade. J Cell Physiol. 1996;166:332–339. doi: 10.1002/(SICI)1097-4652(199602)166:2<332::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Pachetti C, Qiu L, Welk JF. Salicylate triggers heat shock factor differently than heat. J Biol Chem. 1995;270:24489–24495. doi: 10.1074/jbc.270.41.24489. [DOI] [PubMed] [Google Scholar]
- Kamboh AA, Hang SQ, Bakhetgul M, Zhu WY. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult Sci. 2013;92:2411–2418. doi: 10.3382/ps.2012-02960. [DOI] [PubMed] [Google Scholar]
- Kargul J, Laurent GJ. Small heat shock proteins: molecular protectors against the disease. Int J Biochem Cell Biol. 2012;44:1587. doi: 10.1016/j.biocel.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem. 1994;269:11274–11278. [PubMed] [Google Scholar]
- Kim SR, Bae MK, Kim JY, Wee HJ, Yoo MA, Bae SK. Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem Biophys Res Commun. 2009;387:342–347. doi: 10.1016/j.bbrc.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Lamoureux F, Thomas C, Yin MJ, Fazli L, Zoubeidi A, Gleave ME. Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas GD, Karl IE, Hotchkiss RS. Effect of ethanol and sodium arsenite on HSP-72 formation and on survival in a murine endotoxin model. Shock. 1994;2:34–39. doi: 10.1097/00024382-199407000-00007. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:762–769. doi: 10.1016/j.ejheart.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Ma X, et al. Heat shock protein 27 attenuates neointima formation and accelerates reendothelialization after arterial injury and stent implantation: importance of vascular endothelial growth factor up-regulation. FASEB J. 2013 doi: 10.1096/fj.13-230417. [DOI] [PubMed] [Google Scholar]
- Mansoor AH, Mujtaba MT, Silver B. Antiplatelet therapy to prevent recurrent stroke: Three good options. CleveClin J Med. 2013;80:787–795. doi: 10.3949/ccjm.80a.12149. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–5098. doi: 10.1128/mcb.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mearow KM, Dodge ME, Rahimtula M, Yegappan C. Stress-mediated signaling in PC12 cells - the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem. 2002;83:452–462. doi: 10.1046/j.1471-4159.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mitchell MA, Sandercock DA. Creatine kinase isoenzyme profiles in the plasma of the domestic fowl (Gallus domesticus): effects of acute heat stress. Res Vet Sci. 1995;59:30–34. doi: 10.1016/0034-5288(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Morrison S. Ruminant heat stress: effect on production and means of alleviation. J Anim Sci. 1983;57:1594–1600. doi: 10.2527/jas1983.5761594x. [DOI] [PubMed] [Google Scholar]
- Park IS, et al. Aspirin induces apoptosis in YD-8 human oral squamous carcinoma cells through activation of caspases, down-regulation of Mcl-1, and inactivation of ERK-1/2 and AKT. Toxicol in Vitro. 2010;24:713–720. doi: 10.1016/j.tiv.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- Piestun Y, Druyan S, Brake J, Yahav S. Thermal manipulations during broiler incubation alter performance of broilers to 70 days of age. Poult Sci. 2013;92:1155–1163. doi: 10.3382/ps.2012-02609. [DOI] [PubMed] [Google Scholar]
- Rai UC, Ambwany P. Cardiovascular changes during varied thermal stress. Indian J Physiol Pharmacol. 1980;24:119–125. [PubMed] [Google Scholar]
- Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22:922–929. doi: 10.1097/00003246-199406000-00008. [DOI] [PubMed] [Google Scholar]
- Rogalla T, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol (1985) 1992;73:1517–1522. doi: 10.1152/jappl.1992.73.4.1517. [DOI] [PubMed] [Google Scholar]
- Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann N Y Acad Sci. 2005;1066:222–242. doi: 10.1196/annals.1363.009. [DOI] [PubMed] [Google Scholar]
- Sandoval-Montiel AA, Zentella-de-Pina M, Ventura-Gallegos JL, Frias-Gonzalez S, Lopez-Macay A, Zentella-Dehesa A. HSP-72 accelerated expression in mononuclear cells induced in vivo by acetyl salicylic acid can be reproduced in vitro when combined with H2O2. PLoS One. 2013;8:e65449. doi: 10.1371/journal.pone.0065449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S. Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol. 2013;165:494–498. doi: 10.1016/j.ijcard.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Singh A, Rathaur S. Combination of DEC plus aspirin induced mitochondrial mediated apoptosis in filarial parasite Setaria cervi. Biochimie. 2010;92:894–900. doi: 10.1016/j.biochi.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. 2002;162:1099–1104. doi: 10.1001/archinte.162.10.1099. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9:863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre* NR, Cobanov B, Schnitkey G† (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86
- Sutcliffe P, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taba K, et al. Heat-shock protein 27 is phosphorylated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2010;30:2539–2543. [PubMed] [Google Scholar]
- Tang S, et al. Localization and expression of Hsp27 and alphaB-crystallin in rat primary myocardial cells during heat stress in vitro. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–921. doi: 10.1097/00003246-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gao R, Huang Y, Tian B, Zhou Y. Effects of mitogen-activated protein kinase signal pathway on heat shock protein 27 expression in human lens epithelial cells exposed to sodium salicylate in vitro. J Huazhong Univ Sci TechnologMed Sci. 2009;29:377–382. doi: 10.1007/s11596-009-0323-x. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18:225–228. doi: 10.1016/S0899-9007(01)00796-1. [DOI] [PubMed] [Google Scholar]
- Wong HR. Heat shock proteins. Facts, thoughts, and dreams. A. De Maio. Shock 11:1-12, 1999. Shock. 1999;12:323–325. doi: 10.1097/00024382-199910000-00012. [DOI] [PubMed] [Google Scholar]
- Wu NC, Chen TH, Yang YC, Liao FT, Wang JJ. N-acetylcysteine improves cardiac contractility and ameliorates myocardial injury in a rat model of lung ischemia and reperfusion injury. Transplant Proc. 2013;45:3550–3554. doi: 10.1016/j.transproceed.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Zeren G, et al. Relation of heart-type fatty acid-binding protein with the degree and extent of atherosclerosis in patients with non-ST elevation acute coronary syndrome. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2013;41:610–616. doi: 10.5543/tkda.2013.26974. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wisniewski M, Wang W, Liu J, Liu Y. Heat-induced oxidative injury contributes to inhibition of Botrytis cinerea spore germination and growth. World J Microbiol Biotechnol. 2013 doi: 10.1007/s11274-013-1513-z. [DOI] [PubMed] [Google Scholar]