Abstract
Long-term and high-dose glucocorticoids (GCs) supplementation has been linked to osteoporosis. In this study, we studied the protective role of plumbagin against GC-induced cell damage in MC3T3-E1 cells. The effect of dexamethasone (DEX) and plumbagin on cell viability was determined. DEX showed as IC-50 value of 95 μM. Further, 10 μM plumbagin treatment effectively ameliorated DEX-induced cell death by increasing the cell viability to 92 %. A further effect of plumbagin on DEX-induced oxidative stress was determined through reactive oxygen species (ROS) level, lipid peroxide content, and antioxidant status. Nrf-2 nuclear localization was analyzed through immunofluorescence. Protein expression of redox regulator Nrf-2 and their target genes HO-1 and NQO1 and osteogenic markers (OCN, OPN Runx-2) were determined by Western blot. Apoptotic effect was analyzed by mitochondrial membrane potential and caspase activities (3, 8, and 9). The results showed that DEX treatment showed a significant increase in oxidative stress through increased ROS levels and downregulation of cytoprotective antioxidant proteins and antioxidant enzyme activities. Further DEX treatment downregulated the osteogenic markers and upregulated apoptosis through decreased mitochondrial membrane potential and upregulation of caspase activities. Plumbagin treatment significantly reversed the levels of oxidative stress and apoptotic markers and protected against DEX-induced cell damage. Further, plumbagin treatment significantly improved the expression of osteogenic markers compared to DEX treatment. In conclusion, the present study shows that plumbagin offers significant protective role against DEX-induced cellular damage via regulating oxidative stress, apoptosis, and osteogenic markers.
Keywords: Osteogenesis, Glucocorticoids, Apoptosis, Oxidative stress, ROS
Introduction
A glucocorticoid (GC) is supplemented in treatment of various diseases, including autoimmune, pulmonary, gastrointestinal diseases, organ transplantation, and cancer. High dosage and long-term intake of dexamethasone (DEX), a synthetic GC hormone has been linked as an important mediator in osteoporosis (den Uyl et al. 2011; Weinstein 2011). Systemic effects of a glucocorticoid cause osteoporosis since the skeletal system is the major target. A GC-induced effect is often mentioned as secondary osteoporosis, which occurs in 30–50 % of patients with chronic glucocorticoid therapy. Bone remodeling occurs as a perfect functional balance between osteoblasts and osteocytes. Both these cells are the principle target for GC and mediate GC-induced osteoporosis through activating redox pathways and induction of apoptosis (Mazziotti et al. 2006). Osteoblasts are cells involved in bone formation; inhibition in bone formation by GC is regarded as the central role in pathogenesis of osteoporosis. GC is involved in inhibition of osteoblast cell proliferation, differentiation, and maturation (Canalis 1983, 1984). Osteoblast differentiation is regulated through signaling mechanisms, including PPARγ-2 activation and Wnt/β-catenin signaling. Inhibition of osteoblast-driven synthesis of type I collagen causes depletion of the bone extracellular matrix component, resulting in GC-induced osteoporosis (Canalis 2005; Delany et al. 1995). Antioxidant compounds that ameliorate osteoporosis should be explored as a therapeutic intervention for osteoporosis.
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is a naphthoquinone isolated from the roots of Plumbago zeylanica L. Recently, plumbagin has been reported to show various protective properties, and the plant has been hailed as a traditional Chinese medicine (Chen et al. 2009). Plumbagin has been reported in inhibition of azoxymethane-induced intestinal carcinogenesis (Sugie et al. 1998). Anti-inflammatory properties of plumbagin have been established (Sheeja et al. 2010). Inhibition of NF-kB expression by plumbagin acts as an important mediator of its anti-inflammatory effect (Luo et al. 2010). Plumbagin has also been identified as a neuroprotectant against cerebral ischemia by activating Nrf-2 pathway (Son et al. 2010). In this study, we have investigated the potential effect of plumbagin against GC-induced apoptosis and osteogenic differentiation.
Materials and methods
Chemicals
Plumbagin, Dulbecco’s modified Eagle’s medium, fetal bovine serum, trypsin, antibiotics (penicillin, streptomycin), DCF-DA, DiOC6 (3,3′-dihexyloxacarbocyanine iodide) were purchased from Sigma Aldrich Chemicals Private Limited, USA.
Cell culture and treatments
MC3T3-E1, osteoblastic cells were purchased from American Type Culture Collection (ATCC-CRL-2593). The cells were grown in alpha minimum essential medium with ribonucleosides, deoxyribonucleosides, 2 mM l-glutamine and 1 mM sodium pyruvate without ascorbic acid. The media were supplemented with 10 % FBS and antibiotics (penicillin/ streptomycin). DEX-induced cytotoxicity was determined at different concentration for 48 hrs. Cells were treated with different concentration plumbagin for 24 h, followed by a shortlisted dose of DEX for 24 h. Effective doses of plumbagin and DEX were used for further studies.
MTT assay
Cell viability was assessed by MTT assay. Briefly, cells (1 × 105) were seeded and treated with different concentrations of DEX (20, 40, 60, 80, 100, and 120 μM) for 48 h. From the shortlisted dose (IC-50), the protective role of plumbagin was assessed. Cells were treated with plumbagin at different concentrations (5, 10, 15, and 20 μM) for 24 h followed by DEX treatment. After the treatment, cells were incubated with MTT (4 mg/ml) for 5 h, and formozan crystals were dissolved in DMSO. The absorbance was read at 570 nm, and percentage cell viability was calculated compared to control (Mosmann 1983).
Estimation of intracellular ROS generation
The cells (1 × 105) were treated with plumbagin for 24 h, followed by DCF-DA (2′,7′-dichlorodihydrofluorescin diacetate) for 45 min. Cells were further treated with DEX for 24 h, and reactive oxygen species (ROS) levels were measured spectrophotometrically at excitation wavelength 480 nm and emission wavelength 520 nm. The results were expressed as % DCF fluorescence compared to control (Royall and Ischiropoulos 1993).
Lipid peroxidation
After treatment, cells were incubated in 8 % sodium dodecyl sulfate (SDS) and 0.8 % thiobarbituric acid (TBA) in 20 % acetic acid and heated for 1 h at 90 °C. Followed by which, butanol/pyridine mixture was added and shaken vigorously. The cells were centrifuged at 4000 rpm for 10 min, and the organic layer was read at 532 nm. The lipid peroxide content was expressed as nanomoles of TBA reactants per milligram of protein (Ohkawa et al. 1979).
Antioxidant enzyme activities
Superoxide dismutase (SOD) activity: the SOD activity was determined as described by Sun et al. (1988). The assay is based on reduction of nitroblue tetrazolium (NBT). The amount required for 50 % inhibition of NBT reduction is 1 U of SOD activity. The SOD activity is expressed as units per milligram of protein. Catalase (CAT) activity: the activity was determined according to the method described by Aebi (1984). The reaction mixture contained tissue homogenate and 30 mM H2O2 in a 50-mM phosphate buffer pH 7.0. The activity was estimated by decrease in absorbance of H2O2 at 240 nm. Glutathione-S-transferase (GST) activity: the reaction between 1-chloro-2,4-dinitro benzene (CDNB) and reduced glutathione results in formation of dinitrophenyl thioether which is measured at 340 nm (Habig et al. 1974). The amount of enzyme producing 1 mmol of CDNB-GSH conjugate/min is 1 U. Glutathione peroxidase (GPx) activity: the GPx was performed as described by Paglia and Valentine (1967). The oxidized glutathione (GSSG) is reduced by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ is measured by a decrease in absorbance at 340 nm. GPx activity is expressed as units per milligram of protein.
Mitochondrial membrane potential (Δψm)
After treatment as mentioned above, cells were treated with DiOC6 (3) (3,3′-dihexyloxacarbocyanine iodide) and incubated for 45 min, and fluorescence intensity was recorded at excitation wavelength of 488 nm and emission wavelength at 500 nm.
Caspase activity-ELISA
The cells (1 × 106) were treated with plumbagin and DEX as mentioned in the treatment schedule. The samples were analyzed for caspase 3, 8, and 9 activities (R and D systems) at 405 nm.
Western blot
The samples (50 μg protein) were separated on 10 % SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes. After protein transfer, nonspecific sites were blocked with 5 % nonfat dried milk for 1 h at RT. The membrane was washed with TBST and incubated with primary mouse monoclonal antibodies against Nrf-2 (ab31163, Abcam, Cambridge, MA), HO-1 (ab13248, Abcam, Cambridge, MA), NQO1 (ab34173, Abcam, Cambridge, MA), osteocalcin (OCN) (ab13418, Abcam, Cambridge, MA), osteopontin (OPN) (ab8448, Abcam, Cambridge, MA), runt-related transcription factor-2 (Runx-2) (ab76956, Abcam, Cambridge, MA) (1:1000) at 4 °C overnight. Each membrane was further incubated for 1 h with secondary peroxidase-conjugated goat anti-mouse or anto-rabbit IgG (1:5000–1:10,000). The bands were visualized with an enhanced chemiluminescence (ECL) system according to the manufacturer’s instructions. Densitometric analyses of the Western blot bands were performed using ImageJ software (GE Healthcare Life Sciences).
Immuofluorescence
After the respective treatment period, the cells were fixed in formaldehyde. Permeabilized with Triton X-100 and incubated with primary (1:100) overnight at 4 °C followed by secondary antibody (1:5000) specific to Nrf-2. Images were captured using Leica DMR fluorescence microscope fitted with an ORCA digital camera. Images were quantified using ImageJ software.
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. All the experiments were performed thrice in triplicates to ensure reproducibility.
Results
Effect of DEX and plumbagin on cell viability
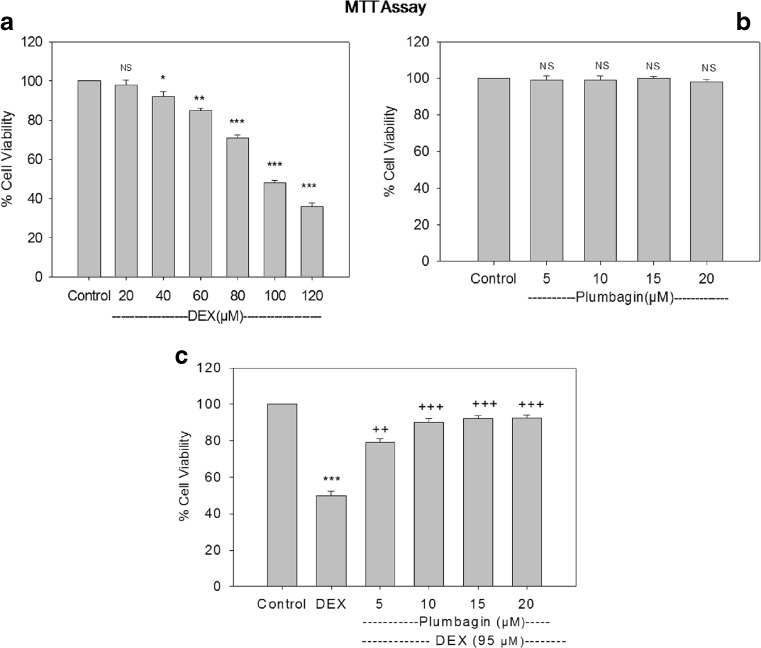
The present study showed that DEX treatment resulted in a dose-dependent decrease in cell viability compared to control. IC-50 value was found to be 95 μM. Further treatment with Plumbagin followed by DEX treatment significantly increased the cell viability compared to DEX treatment. Plumbagin at a concentration of 10 μM and above showed significant protection against DEX-mediated toxicity. Cell viability was found to be 79, 90, 92, and 92 %. So, further studies were carried out at 10 μM plumbagin and 95 μM DEX, whereas treatment with plumbagin alone did not show any cell death in the concentrations tested (Fig. 1 a, b).
Fig 1.
Cytotoxic effect of DEX and plumbagin on osteoblast cells. Cells were treated with different concentration of DEX and plumbagin. Cell viability was measured using MTT assay. Results were expressed as % cell viability compared to control. Results are given as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, when compared to control group. ++p < 0.01, +++p < 0.001 when compared to DEX treatment. NS nonsignificant compared to control group (one-way ANOVA followed by Tukey’s multiple comparison)
Effect of DEX and plumbagin on ROS generation and lipid peroxidation
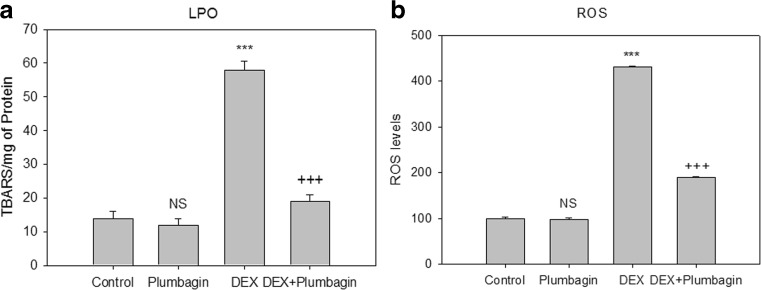
Treatment with DEX showed significant increase in ROS levels and lipid peroxide content compared to control group. Plumbagin treatment effectively reduced the oxidative stress through decreased levels of ROS and lipid peroxides (Fig. 2a, b).
Fig 2.
Effect of DEX and plumbagin on oxidative stress. Plumbagin inhibits lipid peroxide levels: the results are expressed as nanomoles of TBARS formed per milligram of protein. Plumbagin inhibits ROS levels: the results are expressed as ROS generated (%) when compared to control. Results are given as the mean ± SEM. ***p < 0.001, when compared to control group. +++ p < 0.001, when compared to DEX treatment. NS nonsignificant compared to control group (one-way ANOVA followed by Tukey’s multiple comparison)
Plumbagin increases Nrf-2 levels and antioxidant enzyme activities
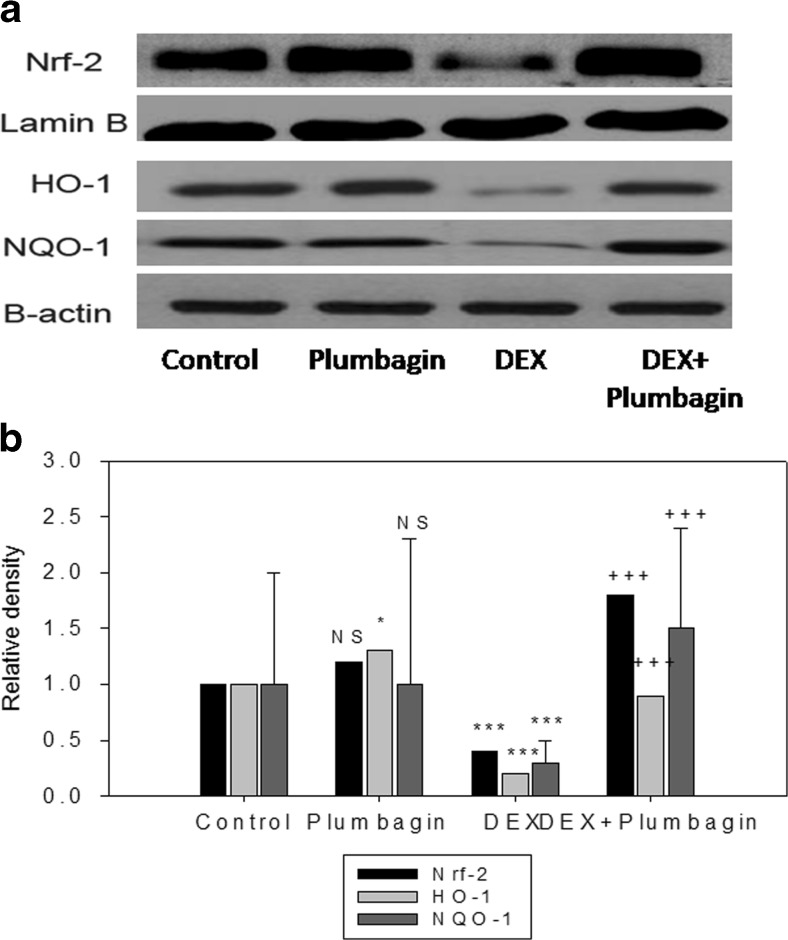
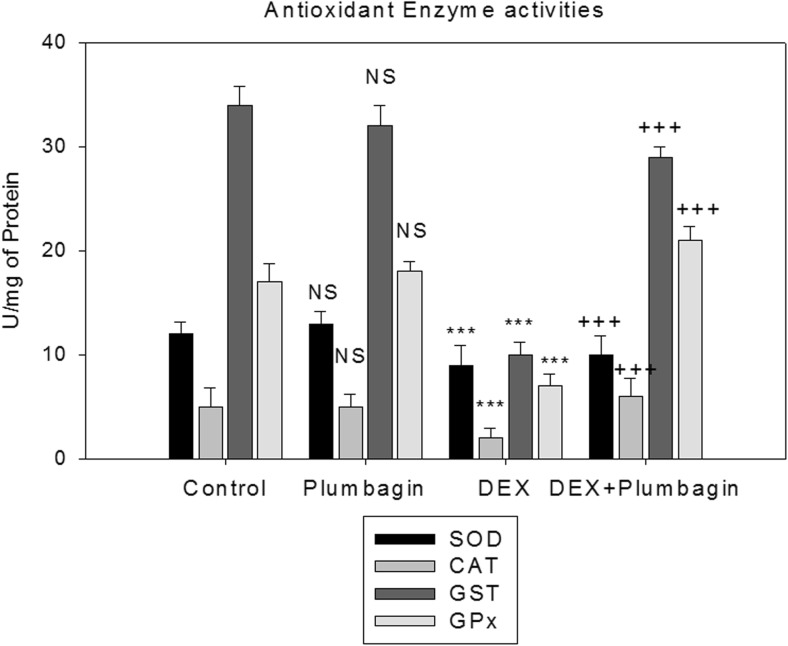
We next determined the effect of DEX and plumbagin on redox regulator Nrf-2 and antioxidant enzymes. Results show that DEX treatment significantly downregulated the expression of Nrf-2 and their downstream target genes (NQO1 and HO-1) and antioxidant enzyme activities. Further, treatment with plumbagin followed by DEX treatment upregulated Nrf-2 and their target genes and increased antioxidant status compared to DEX treatment (Figs. 3 and 4). DEX treatment showed cytoplasmic location for Nrf-2 while treatment with plumbagin during DEX treatment showed nuclear migration of Nrf-2 (Fig. 5).
Fig 3.
Plumbagin upregulates Nrf-2, HO-1, and NQO1 expression. Protein expressions were analyzed through Western blot analysis. Results were expressed as mean ± SEM. Densitometric analysis show significant regulation of Nrf-2, HO-1, and NQO1 expression in DEX and plumbagin treatment. *p < 0.05, ***p < 0.001, when compared to control group. +++ p < 0.001, when compared to DEX treatment. NS nonsignificant compared to control group
Fig 4.
Plumbagin enhances antioxidant enzyme activities (SOD, CAT, GST, and GPx). Results are given as the mean ± SEM. SOD, CAT, GST, and GPx expressed in units per milligram protein. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to DEX treatment. NS nonsignificant compared to control group (one-way ANOVA followed by Tukey’s multiple comparison)
Fig 5.
Plumbagin mediates Nrf-2 nuclear localization. Control and plumbagin showed nuclear localization of Nrf-2, whereas DEX treatment (p < 0.001) showed cytoplasmic localization of Nrf-2 when compared to control cells. Plumbagin + DEX treatment augmented Nrf-2 localization (p < 0.001) into nucleus compared to DEX treatment (Nrf-2 conjugated with FITC; Nucleus-DAPI). Scale bar = 100 μm
Plumbagin restores osteogenic markers
We evaluated three important markers of osteogenesis (OCN, OPN, Runx-2) in MC3T3-E1 cells. Treatment with DEX significantly downregulated the protein expression compared to the control group. Plumbagin treatment upregulated the expression levels of these osteogenic markers, clearly showing its protective role against osteoporosis (Fig. 6).
Fig 6.
Plumbagin promotes osteoblast differentiation. Protein expression of OPN, OCN, and RUNX-2 were analyzed through Western blot analysis. Results were expressed as mean ± SEM. Densitometric analysis show significant regulation of OPN, OCN, and RUNX-2 expressions in DEX and plumbagin treatment. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to DEX treatment. NS nonsignificant compared to control group
Effect of plumbagin and DEX on caspase activation
In order to evaluate the effect of DEX and plumbagin on apoptosis, we first determined mitochondrial membrane potential. DEX treatment showed significant decline in the membrane potential compared to the control group. Plumbagin treatment maintained the membrane potential and prevented DEX-induced changes. Further, treatment with DEX upregulated caspase activities (3, 8 and 9) compared to the control. Plumbagin showed antiapoptotic effect by downregulating the caspase levels compared to DEX group (Figs. 7 and 8).
Fig 7.
Plumbagin maintains mitochondrial membrane potential. Results are given as the mean ± SEM. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to DEX treatment. NS nonsignificant compared to control group (one-way ANOVA followed by Tukey’s multiple comparison)
Fig 8.
Effect of plumbagin and DEX on caspase (9, 8, and 3) activities. Caspase activities are expressed as relative units/b-actin. Results are given as the mean ± SEM. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to DEX treatment. NS nonsignificant compared to control group (one-way ANOVA followed by Tukey’s multiple comparison)
Discussion
GC-induced secondary osteoporosis is a highly prevalent health issue worldwide. The bone disorder slowly progresses due to the side effects of GC supplementation, which is often unnoticed. Reduced bone mineral density (BMD) with high bone fracture risk and low bone turnover is often associated with chronic glucocorticoid therapy and GC-induced osteoporosis is associated with decreased bone formation (Gohel et al. 1999; Sosa et al. 2008). To identify, effective protection against GC-induced osteoporosis, we have evaluated the protective role of plumbagin, a natural antioxidant compound against GC-induced toxic effects in vitro.
First, we evaluated the cytotoxic effect of DEX using different concentrations for 48 h and found that DEX caused dose-dependent cell death with IC-50 value 95 μM. Further, protective studies were carried out using various concentrations of plumbagin. Plumbagin at a concentration of 10 μM showed effective protection against DEX-induced cell death reaching up to 90 %. The concentration of plumbagin used in the present study was around 1 μg/ml. Oral bioavailability studies conducted in rats (100 mg/kg body weight) by Hsieh et al. (2006) reported that plumbagin could reach plasma levels up to 0.6 μg/ml maximum with stable levels and slow elimination rate. Further, the lipophilic nature of plumbagin has been reported to have extensive distribution in the body tissue owing to its slow elimination (Chandrasekaran and Nagarajan 1981). Thus, the dose used in the present study can achieve circulating levels (nearer to the in vivo data). Previous reports show that plumbagin exerts anticancer as well as cytoprotective properties in different cells with different ranges of concentrations. The IC-50 value of the plumbagin ranged from 3.87 to 14.6 μM in oral squamous cell carcinoma (Ono et al. 2015), whereas plumbagin (2.5–7.5 μM) offered significant protection against lipopolysaccharide (LPS)-induced inflammation through NF-kB and MAPK pathways in RAW 264.7 cells (Wang et al. 2014). In the present study, a significant cytoprotection was offered by plumbagin at s5–20 μM concentration. Thus, a low dose with maximum effect was chosen to analyze the effect of plumbagin on DEX-induced effects. Thus, a reactive property of a compound is dependent upon the cellular nature and concentration tested.
Osteoblast cell death, reduction in growth and differentiation has been implied, due to the higher ROS levels (Mody et al. 2001; Park et al. 2005). One of the important mediators of GC-induced osteoporosis is oxidative stress. Deregulation in redox balance causes activation of redox pathways with the ultimate result of oxidative stress with a decline in antioxidant defense mechanisms. The invariant short half-life of ROS formed is impacted in reduced osteoblast proliferation, differentiation, and induction of apoptosis (Lee et al. 2006). Recently, the impact of vitamins and antioxidants in bone healing has been reported to have a positive effect in preventing osteoporosis. Nrf-2 (nuclear factor (erythroid-derived 2)-like 2) is a transcriptional activator which binds to antioxidant responsive element (ARE) and enhances the expression of antioxidant enzymes. Nrf-2 is activated under a change in cellular oxidative stress conditions and offer cytoprotection through a wide range of phase II antioxidant enzymes: viz glutathione-S-reductase, NAD (P) H: quinone oxidoreductase 1, heme oxygenase-1 (HO-1), etc. Reactive oxygen species are involved in Nrf-2 activation through inhibition of Keap1-mediated Nrf2 proteasomal degradation resulting in its nuclear migration (Rushworth and Macewan 2011). The role of oxidative stress in osteoporosis is slowly gaining importance; the present study showed that DEX caused significant enhancement in oxidative stress markers with increased cellular ROS generation and lipid peroxide content. In addition, there was a significant decline in the Nrf-2 protein levels and their downstream target genes NQO1 and HO-1 during DEX treatment. Previous reports suggest that DEX-induced cellular injuries were mediated through oxidative stress mechanisms (Sato et al. 2010; You et al. 2009; Feng and Tang 2014). However, plumbagin treatment followed by DEX treatment significantly enhanced the Nrf-2 and their target protein expression with a decline in oxidative stress markers. Further, the levels of antioxidant enzymes were significantly declined during DEX treatment which was reversed by plumbagin treatment, clearing showing that plumbagin treatment has significant protection against DEX-induced oxidative stress. The protective role of plumbagin in the prevention of LPS-induced oxidative stress and inflammation is mediated through its antioxidant and anti-inflammatory property (Checker et al. 2014).
Next, the osteoblastogenic markers of bone formation were evaluated in the presence of DEX and plumbagin. To determine the possible effect of DEX and plumbagin on osteoblast differentiation and maturation, osteogenic markers were determined. Osteoblast cells mature from the precursor mesenchymal stem cells (MSCs). Upon differentiation and maturation, they function in bone formation through activation of various osteogenic genes, including RUNX, OCN, and OPN. These proteins are regarded as important markers of bone formation. Of which, Runx2 is an important transcription factor activated and controls the expression of osteogenic markers through its regulatory activity (Ducy et al. 1997; Franceschi and Xiao 2003). We found that treatment with DEX resulted in significant downregulation of osteogenic markers, but treatment with plumbagin enhanced the protein expression compared to that of DEX-treated cells. Consistent with our results, previous studies show that oxidative stress contributes in inhibiting osteoblast differentiation and maturation (Bai et al. 2004; Arai et al. 2007). The present study reveals that plumbagin is an effective compound in promoting bone formation by improving the osteogenic protein expression.
Glucocorticoid-induced osteoporosis and their role in deregulation in bone formation are mediated through apoptotic signaling (Liu et al. 2004; O'Brien et al. 2004; Lin et al. 2014). Reports show that a glucocorticoid has a positive relation in mitochondrial ROS production (Bjelakovic et al. 2007). The present study showed that DEX induced significant decrease in mitochondrial membrane potential with the upregulation of caspases 9 and 3 showing that the intrinsic pathway of apoptosis is mediated by DEX treatment. Plumbagin treatment showed protection against DEX-induced apoptotic effect by enhancing the cell viability, preventing loss of membrane potential and downregulated caspases 3 and 9 expressions.
In conclusion, the present study shows that plumbagin acts as an effective cytoprotective compound against DEX-induced osteoporosis. Plumbagin reduced DEX-induced oxidative stress by reducing ROS levels and increased the antioxidant defense mechanisms through Nrf-2 nuclear translocation and expression. Plumbagin upregulated osteogenic markers and significantly inhibited DEX-induced apoptosis. Thus, the present study shows evidence that plumbagin could offer significant cytoprotection against DEX-induced osteoporosis by modulating oxidative stress and apoptosis, while promoting osteoblast differentiation.
Acknowledgments
Conflict of interest
The authors declare there is no conflict of interest.
Footnotes
Shuai Zhang and Dong Li contributed equally to this work.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:1–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Beninati S, Pavlovic D, Kocic G, Jevtovic T, Kamenov B, Saranac LJ, Bjelaković B, Stojanovic I. Glucocorticoids and oxidative stress. J Basic Clin Physiol Pharmacol. 2007;18:115–127. doi: 10.1515/JBCPP.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983;112:931–939. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- Canalis E. Effect of cortisol on periosteal and nonperiosteal collagen and DNA synthesis in cultured rat calvariae. Calcif Tissue Int. 1984;36:158–166. doi: 10.1007/BF02405312. [DOI] [PubMed] [Google Scholar]
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Nagarajan B. Metabolism of echitamine and plumbagin in rats. J Biosci. 1981;3:395. doi: 10.1007/BF02702627. [DOI] [Google Scholar]
- Checker R, Patwardhan RS, Sharma D, Menon J, Thoh M, Sandur SK, Sainis KB, Poduval TB. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-κB suppression. Inflammation. 2014;37:542–554. doi: 10.1007/s10753-013-9768-y. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Tan MX, Liu LM, Liu YC, Wang HS, Yang B, Peng Y, Liu HG, Liang H, Orvig C. Cytotoxicity of the traditional Chinese medicine (TCM) plumbagin in its copper chemistry. Dalton Trans. 2009;48:10824–10833. doi: 10.1039/b910133k. [DOI] [PubMed] [Google Scholar]
- Delany AM, Gabbitas BY, Canalis E. Cortisol down regulates osteoblast 1(I) procollagen mRNA by transcriptional and post-transcriptional mechanisms. J Cell Biochem. 1995;57:488–494. doi: 10.1002/jcb.240570314. [DOI] [PubMed] [Google Scholar]
- den Uyl D, Bultink IE, Lems WF. Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2011;13:233–240. doi: 10.1007/s11926-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffory V, Ridall AL, Darsenty G. Osf2/ Runx2: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Feng YL, Tang XL. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem Biol Interact. 2014;207:26–31. doi: 10.1016/j.cbi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88:446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. GlutathioneS-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hsieh YJ, Lin LC, Tsai TH. Measurement and pharmacokinetic study of plumbagin in a conscious freely moving rat using liquid chromatography/tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2006;844:1–5. doi: 10.1016/j.jchromb.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- Lin H, Wei B, Li G, Zheng J, Sun J, Chu J, Zeng R, Niu Y. Sulforaphane reverses glucocorticoid-induced apoptosis in osteoblastic cells through regulation of the Nrf2 pathway. Drug Des Devel Ther. 2014;8:973–982. doi: 10.2147/DDDT.S65410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Porta A, Peng X, Gengaro K, Cunningham EB, Li H, Dominguez LA, Bellido T, Christakos S. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 2004;19:479–490. doi: 10.1359/JBMR.0301242. [DOI] [PubMed] [Google Scholar]
- Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu L, Zhou H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-κB activation. J Pharmacol Exp Ther. 2010;335:735–742. doi: 10.1124/jpet.110.170852. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144–149. doi: 10.1016/j.tem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/S0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbitturic acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ono T, Ota A, Ito K, Nakaoka T, Karnan S, Konishi H, Furuhashi A, Hayashi T, Yamada Y, Hosokawa Y, Kazaoka Y. Plumbagin suppresses tumor cell growth in oral squamous cell carcinoma cell lines. Oral Dis. 2015 doi: 10.1111/odi.12310. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Park BG, Yoo CI, Kim HT, Kwon CH, Kim YK. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology. 2005;215:115–125. doi: 10.1016/j.tox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H. Evaluation of 2,7-dichlorofluorescein and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Macewan DJ. The role of nrf2 and cytoprotection in regulating chemotherapy resistance of human leukemia cells. Cancers (Basel) 2011;3:1605–1621. doi: 10.3390/cancers3021605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 2010;47:224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeja E, Joshi SB, Jain DC. Bioassay-guided isolation of anti-inflammatory and antinociceptive compound from Plumbago zeylanica leaf. Pharm Biol. 2010;48:381–387. doi: 10.3109/13880200903156424. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M, Jódar E, Saavedra P, Navarro MC, Gomez de Tejada MJ, Martin A, Pena P, Gomez J. Postmenopausal Canarian women receiving oral glucocorticoids have an increased prevalence of vertebral fractures and low values of bone mineral density measured by quantitative computer tomography and dual X-ray absorptiometry, without significant changes in parathyroid hormone. Eur J Intern Med. 2008;19:51–56. doi: 10.1016/j.ejim.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, Mori H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127:177–183. doi: 10.1016/S0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Oberley LW, Ying L. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. [PubMed] [Google Scholar]
- Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B, Yan W, Tang T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol. 2014;64:177–183. doi: 10.1016/j.fct.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- You JM, Yun SJ, Nam KN, Kang C, Won R, Lee EH. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol. 2009;87:440–447. doi: 10.1139/Y09-027. [DOI] [PubMed] [Google Scholar]