Abstract
Butyric acid (BA) is a common secondary metabolite by-product produced by oral pathogenic bacteria and is detected in high amounts in the gingival tissue of patients with periodontal disease. Previous works have demonstrated that BA can cause oxidative stress in various cell types; however, this was never explored using neuronal cells. Here, we exposed nerve growth factor (NGF)-treated PC12 cells to varying BA concentrations (0.5, 1.0, 5.0 mM). We measured total heme, H2O2, catalase, and calcium levels through biochemical assays and visualized the neurite outgrowth after BA treatment. Similarly, we determined the effects of other common periodontal short-chain fatty acids (SCFAs) on neurite outgrowth for comparison. We found that high (1.0 and 5.0 mM) BA concentrations induced oxidative stress and altered calcium homeostasis, whereas low (0.5 mM) BA concentration had no significant effect. Moreover, compared to other SCFAs, we established that only BA was able to induce neurite retraction.
Keywords: Butyric acid, Neurite retraction, Nerve growth factor, Oxidative stress, PC12 cells
Introduction
Reactive oxygen species (ROS) homeostasis maintains a balance between ROS amounts and the anti-oxidant activity (Mittler et al. 2004; Takada et al. 2002). However, if ROS homeostasis is disrupted, then oxidative stress is induced as a result of an imbalance between excessive pro-oxidant amounts and low anti-oxidant activity (Arora et al. 2002; Scandalios 2002; Torres et al. 2006). Periodontopathic bacteria produce secondary metabolites, like butyric acid (BA), which contribute to periodontal disease development (Kurita-Ochiai and Ochiai 2010). BA has both beneficial and detrimental effects on various cell types (Hu et al. 2011; Kurita-Ochiai and Ochiai 2010; Kurita-Ochiai et al. 2008). In previous works related to our group, we have shown that in a simulated BA-based periodontal infection using rats, BA has prolonged retention in the gingival tissue and gradually enters the blood stream which consequentially induces both blood mitochondrial and cytosolic heme-related oxidative stresses (Cueno et al. 2013, 2014).
In neuron cells, oxidative stress generation occurs concurrently with caspase activation leading to apoptosis induction (Annunziato et al. 2003). Moreover, the nerve growth factor (NGF) protein is suggested to have neuroprotective activity that involves stimulating heme oxygenase-1 (HO-1) (Liu et al. 2003) which in turn regulates heme (Ponka 1999). Free heme and heme proteins have been associated with ROS formation leading to oxidative stress (Balla et al. 2000; Hasan and Schafer 2008). In an earlier work, it was previously shown that BA treatment of nerve growth factor (NGF)-untreated PC12 cells affected cell viability and lead to apoptosis induction (Cayo et al. 2009). It is, however, unclear whether BA can induce heme-related oxidative stress in NGF-treated PC12 cells. A better understanding of the effects of BA-induced oxidative stress on neuronal cells may help determine the impact of periodontal diseases on neuronal cells.
Here, we showed that only high BA levels induced oxidative stress and alter calcium homeostasis in NGF-treated PC12 cells. In addition, we observed that NGF-treated cells exposed to high BA amounts resulted in neurite retraction (suppression of NGF-mediated neurite outgrowth) while cells treated with low BA amounts were unaffected. Moreover, compared to other periodontal short-chain fatty acids (SCFAs), we found that only cells exposed to periodontal disease level-BA (PDL-BA) amounts resulted to neurite retraction.
Results and Discussion
BA contributes to oxidative stress induction and calcium homeostasis alteration in PC12 cells
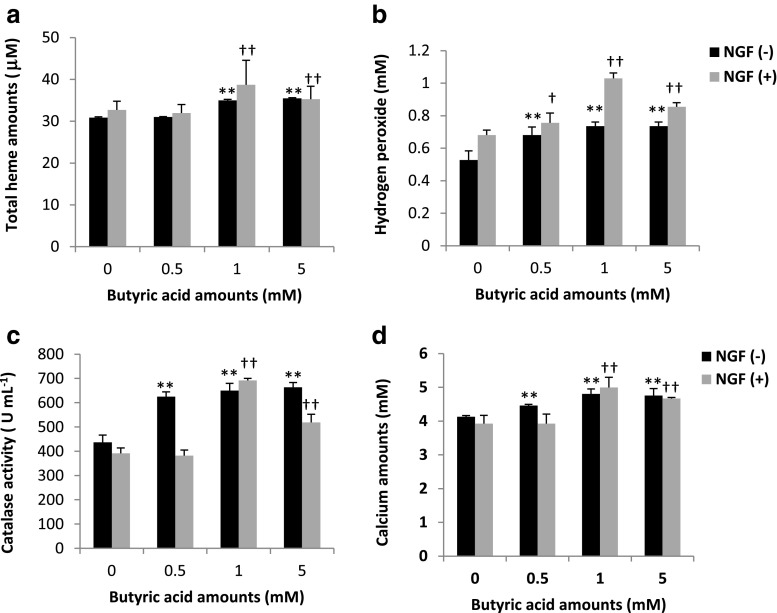
To establish whether BA induces oxidative stress in a model neuronal cell line (PC12), we measured total heme, H2O2, CAT, and calcium levels in NGF(+)- and NGF(−)-treated PC12 cells incubated with varying BA concentrations [0 (used as control), 0.5, 1.0, and 5.0 mM]. PDL-BA amount is 5.0 mM (Kurita-Ochiai et al. 2008). PC12 cells were used throughout this study since this cell line is the most commonly used cell line to study neuronal cells (Darios and Davletov 2006; Marszalek et al. 2004). Our results show that total heme levels were increased in both NGF(+)- and NGF(−)-treated cells only at high BA concentrations (Fig. 1a). This would insinuate that low BA concentration (0.5 mM) does not affect heme levels while high BA concentrations (1 and 5 mM) contribute to increased heme amounts. Heme is a biomolecule that gives rise to functional heme-proteins essential in various biological reactions, including events associated to toxicity (Balla et al. 2000; Hasan and Schafer 2008). In our earlier works, we reported that BA-induced heme increase is associated with oxidative stress (Cueno et al. 2013, 2014). We suspected that BA-induced heme increase in PC12 cells is also associated with oxidative stress.
Fig. 1.
Oxidative stress induction and calcium homeostasis alteration in PC12 cells are ascribable to high butyric acid amounts. Rat pheochromocytoma PC12 cells were obtained from RIKEN Cell Bank, Ibaraki, Japan. Mature PC12 cells (1 × 106 cells mL−1) were routinely maintained at 37 °C and 5 % CO2 in Dulbecco’s modified Eagle medium (DMEM) (Sigma) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Thermo Scientific), 50 U mL−1 penicillin (Life Technologies), and 50 μg mL−1 streptomycin (Life Technologies). Two sets were used for downstream biochemical analyses: one set was grown with 100 ng mL−1 NGF [NGF(+)] while the other set has no NGF [NGF(−)]. NGF or the β-nerve growth factor was purchased from R&D Systems. Both sets were treated with 0 (control), 0.5, 1, and 5 mM butyric acid amounts using sodium butyrate (Wako, Japan) for 24 h. N-PERTM Neuronal Protein Extraction Reagent, Pierce Detergent Removal Spin Columns, and Pierce Microplate BCA Protein Assay Kit-Reducing Agent Compatible Kit (all from Thermo Scientific) were used for sample processing and standardization prior to downstream biochemical applications. QuantiChromTM Heme Assay Kit (BioAssay Systems) was used to measure heme levels (free heme and heme-proteins). Red Hydrogen Peroxide Assay Kit (Enzo Life Sciences) was used to measure hydrogen peroxide (H2O2). EnzyChromTM Catalase Assay Kit (BioAssay Systems) was used to measure cytosolic catalase (CAT) activity. Calcium Colorimetric Assay Kit (BioVision) was used to measure calcium amounts. All kits were performed following manufacturers’ recommendations. Assay measurement of a total heme, b hydrogen peroxide, c catalase, and d calcium levels in NGF(+)- and NGF(−)-treated PC12 cells are shown. Varying butyric acid concentrations are indicated. Results presented are mean ± SE utilizing three replicates per independent sample (n = 3). Statistical analyses were performed using Anderson-Darling normality test and, if passed (p > 0.05), Student’s t test († p < 0.05, †† p < 0.01 for NGF(+)-treated; ** p < 0.01 for NGF(−)-treated)
Among NGF(+)-treated cells, increased H2O2 (Fig. 1b), CAT (Fig. 1c), and calcium (Fig. 1d) amounts were only observed at 1 and 5 mM BA concentrations (gray bars). In contrast, among NGF(−)-treated cells, all BA concentrations resulted to an increase in H2O2 (Fig. 1b), CAT (Fig. 1c), and calcium (Fig. 1d) amounts (black bars). We correlated the concurrent increase in H2O2 and CAT levels to oxidative stress (Andersen 2004; Cueno et al. 2013, 2014). Subsequently, we linked BA-induced oxidative stress induction to alterations in calcium homeostasis (Bogeski et al. 2011). Moreover, we observe that BA-linked oxidative stress induction in PC12 cells is influenced by either NGF presence or absence. NGFs are known to exert neuroprotective effects (Yuan and Yankner 2000) and exhibit anti-oxidant properties (Valdovinos-Flores and Gonsebatt 2013). This would suggest that NGF absence made the cells more vulnerable to BA-induced oxidative stress, whereas NGF presence made the cells more resistant to BA-induced oxidative stress, however, only to low BA concentrations. We believe that H2O2 induced in NGF(+)-treated cells exposed to low BA concentration functions more as a signaling molecule rather than oxidative stress induction (Gough and Cotter 2011) which we attributed to the absence of cytosolic CAT activity (Andersen 2004; Balla et al. 2000; Cueno et al. 2013, 2014). This would insinuate that NGF (functioning as an anti-oxidant) is able to maintain non-stress-related H2O2 levels and forgo stimulating cytosolic anti-oxidant activities (like CAT) in NGF(+)-treated cells exposed to low BA concentration. However, at high BA concentration, this is no longer the case and oxidative stress is induced.
Interestingly, at 5 mM BA concentration, H2O2, CAT, and calcium assay measurements reversed from those measured at 1 mM BA concentration among NGF(+)-treated cells. Neuronal maturation is accompanied with increased resistance to apoptosis while neurodegeneration returns the neuron to an immature state making them more susceptible to apoptosis (Kole et al. 2013). In this regard, we hypothesize that the reversal of all assay measurements among NGF(+)-treated cells at 5 mM BA concentration may contribute to partial neurodegeneration whereby the neuronal cell reverts back to an immature state ascribable to BA. Admittedly, additional work is needed to further prove this hypothesis.
Neurite retraction is associated to high BA amounts
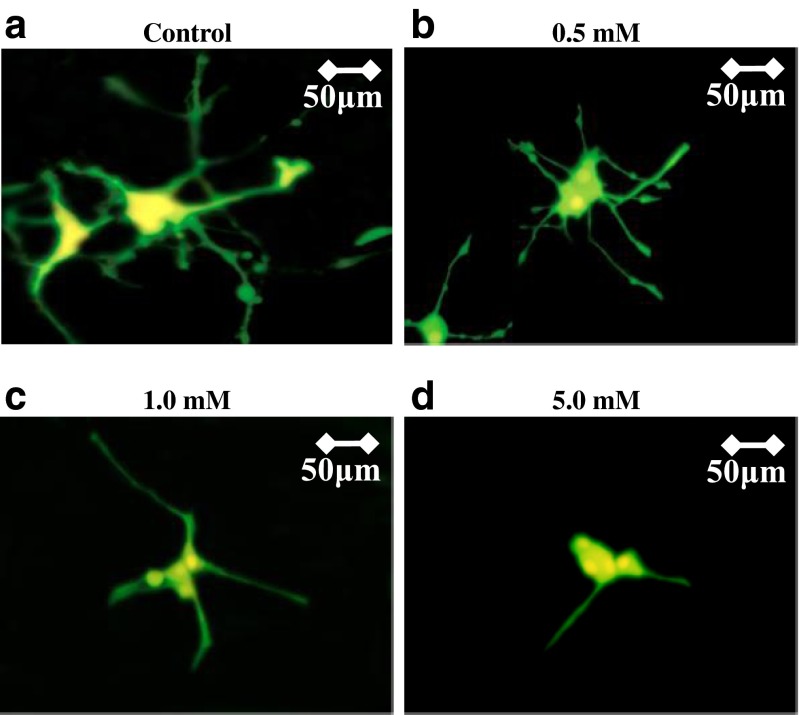
To determine the morphological effects of BA-induced oxidative stress, we visualized the neurite outgrowth of NGF(+)-treated PC12 cells. We found no difference in neurite outgrowth (150–200 μm) between the control (Fig. 2a) and cells treated with low BA concentration (Fig. 2b), whereas neurite retraction (50–75 μm) was observed among cells treated with high BA concentrations (Fig. 2c, d). Neurite retraction has previously been associated to oxidative stress (Garcia-Alloza et al. 2006; Sheehan et al. 2006). This would imply that neurite retraction observed among cells treated with high BA concentrations is linked to BA-induced oxidative stress consistent with our earlier results (Fig. 1).
Fig. 2.
Neurite retraction in NGF-treated PC12 cells is associated to high butyric acid amounts. NGF-treated PC12 cells exposed to varying butyric acid concentrations were used for neurite outgrowth visualization. Cells were transfected with EGFP using LipofectAMINE TM 2000 (Invitrogen) following manufacturer’s recommendations. PC12-GFP cells were grown for 6 days prior to further downstream applications. Transfected cells were incubated for 24 h in a 0 (control), b 0.5, c 1.0, and d 5.0 mM butyric acid amounts using sodium butyrate (Wako, Japan). Subsequently, cell fixation in 2 % paraformaldehyde solution was performed using Flow Fix, 2 % Paraformaldehyde Fixative Kit (Polysciences, Inc, Germany). Neurite outgrowth was monitored and measured using fluorescent (BHT-RFC; Olympus, Japan) and confocal (LSM 510; Carl Zeiss Japan) microscopy. Briefly, the neurite length of PC12 cells were measured under ×630 oil lens from 9–12 confocal microscopic fields using the LSM 510 image analysis browser (Carl Ziess). For each independent experimental condition, the average neurite outgrowth length of 50 neurites was calculated. Measurement scale (μM) is indicated on the upper right corner
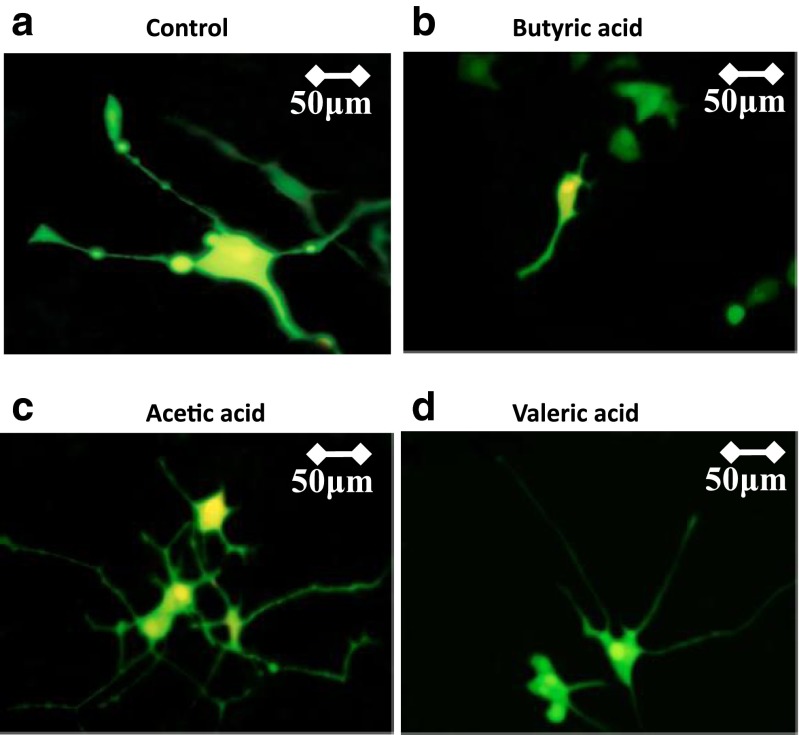
To compare the effects of representative periodontal SCFAs on neurite outgrowth, we exposed PC12 cells in 5 mM acetic, butyric, and valeric acid concentrations, independently. Cells not exposed to any SCFA concentration were used as control. We chose to expose PC12 cells in 5 mM SCFA concentrations since this is the PDL-BA concentration (Kurita-Ochiai et al. 2008). As seen in Fig. 3, we observe that neurite retraction (50–75 μm) is consistent and only evident in BA exposed cells (Fig. 3b) while both acetic (Fig. 3c) and valeric (Fig. 3d) acids have minimal effect on the neurite outgrowth (150–200 μm). SCFAs favor neurite outgrowth only in the presence of NGF, otherwise, SCFAs are minimally effective or not effective (Kamata et al. 2007). This may explain why the neurite outgrowths in acetic and valeric acid-exposed NGF-treated cells were similar to the control. In contrast, we found BA to induce the opposite and instead favor neurite retraction. In this regard, we suspect that BA (possibly through heme-related oxidative stress induction) may uniquely have a detrimental effect on neurite outgrowth of PC12 cells.
Fig. 3.
Neurite outgrowth of NGF-treated PC12 cells exposed to acetic and valeric acids have no effect. NGF-treated PC12 cells exposed to representative periodontal short-chain fatty acids (SCFAs) were used for neurite outgrowth visualization. Cells were transfected with EGFP using LipofectAMINE TM 2000 (Invitrogen) following manufacturer’s recommendations. PC12-GFP cells were grown for 6 days prior to further downstream applications. Transfected cells were incubated for 24 h in a no SCFA (control), b 5.0 mM butyric acid using sodium butyrate, c 5.0 mM acetic acid, and d 5.0 mM valeric acid. All SCFAs used were from Wako, Japan. Subsequently, cell fixation in 2 % paraformaldehyde solution was performed using Flow Fix, 2 % Paraformaldehyde Fixative Kit (Polysciences, Inc, Germany). Neurite outgrowth was monitored and measured using fluorescent (BHT-RFC; Olympus, Japan) and confocal (LSM 510; Carl Zeiss Japan) microscopy. Briefly, the neurite length of PC12 cells were measured under ×630 oil lens from 9–12 confocal microscopic fields using the LSM 510 image analysis browser (Carl Ziess). For each independent experimental condition, the average neurite outgrowth length of 50 neurites was calculated. Measurement scale (μM) is indicated on the upper right corner
In a periodontal disease scenario, mature neuronal cells—which have elevated NGF levels (Kole et al. 2013)—would be exposed to PDL-BA amounts. Subsequently, a major hallmark in the early stages of periodontal disease is that it is often painless (Coventry et al. 2000). Interestingly, neurite retraction was previously correlated to the absence of neuropathic pain (Hobson et al. 2006; Takatori et al. 2006). In this regard, we propose that BA-related neurite retraction may contribute to the absence of neuropathic pain in the early stages of periodontal disease. In a possible future work, it would be interesting to further elucidate on this proposal.
It is worth mentioning that an earlier work showed that heme deficiency causes neurite retraction in NGF-treated PC12 cells via the MAP kinase signaling pathway (Zhu et al. 2002). Considering oxidative stress is involved in MAP kinase activation (McCubrey et al. 2006), we hypothesize that the following sequence of heme-related events occur in PC12 cells treated with high BA amounts: (1) heme increase causing oxidative stress; (2) either heme overconsumption (attributable to oxidative stress induction) or heme synthesis failure (ascribable to neurodegeneration) result to a decrease in heme levels; and, subsequently, (3) heme deficiency contributing to MAP kinase activation and, consequentially, apoptosis (Cayo et al. 2009; Cueno et al. 2014; McCubrey et al. 2006; Zhu et al. 2002). Admittedly, more work is needed to confirm this hypothesis.
In summary, we showed that BA induces oxidative stress and alters calcium homeostasis in NGF-treated PC12 cells. Similarly, we found that NGF(−)-treated cells are susceptible to oxidative stress regardless of BA concentration, whereas NGF(+)-treated cells are susceptible to oxidative stress only in high BA concentrations. In addition, we observed that BA-induced oxidative stress is associated with neurite retraction. Moreover, we showed that unlike acetic and valeric acids, BA uniquely have a detrimental effect on neurite outgrowth.
Acknowledgments
This work was supported by the Dental Research Center-Nihon University School of Dentistry and funded by the Sato Fund-Nihon University School of Dentistry and both the Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan through the Strategic Research Base Development Program for Private Universities 2010–2014 (S1001024).
Contributor Information
Marni E. Cueno, Phone: +81-03-3219-8125, Email: marni.cueno@nihon-u.ac.jp
Kuniyasu Ochiai, Phone: +81-03-3219-8125, Email: ochiai.kuniyasu@nihon-u.ac.jp.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Amoroso S, Pannaccione A, Cataldi M, Pignataro G, D’Alessio A, Sirabella R, Secondo A, Sibaud L, Di Renzo GF. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–133. doi: 10.1016/S0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–1238. [Google Scholar]
- Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood. 2000;95:3442–3450. [PubMed] [Google Scholar]
- Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium. 2011;50:407–423. doi: 10.1016/j.ceca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Cayo MA, Cayo AK, Jarjour SM, Chen H. Sodium butyrate activates Notch1 signaling, reduces tumor markers, and induces cell cycle arrest and apoptosis in pheochromocytoma. Am J Transl Res. 2009;1:178–183. [PMC free article] [PubMed] [Google Scholar]
- Coventry J, Griffiths G, Scully C, Tonetti M. ABC of oral health: periodontal disease. BMJ. 2000;321:36–39. doi: 10.1136/bmj.321.7252.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueno ME, Imai K, Matsukawa N, Tsukahara T, Kurita-Ochiai T, Ochiai K. Butyric acid retention in gingival tissue induces oxidative stress in jugular blood mitochondria. Cell Stress Chaperones. 2013;18:661–665. doi: 10.1007/s12192-013-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueno ME, Imai K, Tamura M, Ochiai K. Butyric acid-induced rat jugular blood cytosolic oxidative stress is associated with SIRT1 decrease. Cell Stress Chaperones. 2014;19:295–298. doi: 10.1007/s12192-013-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65:1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan RN, Schafer AI. Hemin upregulates Egr-1 expression in vascular smooth muscle cells via reactive oxygen species ERK-1/2-Elk-1 and NF-kappaB. Circ Res. 2008;102:42–50. doi: 10.1161/CIRCRESAHA.107.155143. [DOI] [PubMed] [Google Scholar]
- Hobson SA, Holmes FE, Kerr NC, Pope RJ, Wynick D. Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J Neurochem. 2006;99:1000–1010. doi: 10.1111/j.1471-4159.2006.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Dong TS, Dalal SR, Wu F, Bissonnette M, Kwon JH, Chang EB. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS One. 2011;6:e16221. doi: 10.1371/journal.pone.0016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata Y, Shiraga H, Tai A, Kawamoto Y, Gohda E. Induction of neurite outgrowth in PC12 cells by the medium-chain fatty acid octanoic acid. Neuroscience. 2007;146:1073–1081. doi: 10.1016/j.neuroscience.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kole AJ, Annis RP, Deshmukh M. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689. doi: 10.1038/cddis.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T-cells. J Dent Res. 2010;89:689–694. doi: 10.1177/0022034510365456. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Seto S, Suzuki N, Yamamoto M, Otsuka K, Abe K, Ochiai K. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87:51–55. doi: 10.1177/154405910808700108. [DOI] [PubMed] [Google Scholar]
- Liu H, Nowak R, Chao W, Bloch KD. Nerve growth factor induces anti-apoptotic heme oxygenase-1 in rat pheochromocytoma PC12 cells. J Neurochem. 2003;86:1553–1563. doi: 10.1046/j.1471-4159.2003.01978.x. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Kitidis C, Dararutana A, Lodish HF. Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J Biol Chem. 2004;279:23882–23891. doi: 10.1074/jbc.M313460200. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Scandalios JG. The rise of ROS. Trends Biochem Sci. 2002;27:483–486. doi: 10.1016/S0968-0004(02)02170-9. [DOI] [PubMed] [Google Scholar]
- Sheehan J, Eischeid A, Saunders R, Pouratian N. Potentiation of neurite outgrowth and reduction of apoptosis by immunosuppressive agents: implications for neuronal injury and transplantation. Neurosurg Focus. 2006;20:E9. doi: 10.3171/foc.2006.20.5.10. [DOI] [PubMed] [Google Scholar]
- Takada Y, Hachiya M, Park SH, Osawa Y, Ozawa T, Akashi M. Role of reactive oxygen species in cells overexpressing manganese superoxide dismutase: mechanism for induction of radioresistance. Mol Cancer Res. 2002;1:137–146. [PubMed] [Google Scholar]
- Takatori M, Kuroda Y, Hirose M. Local anesthetics suppress nerve growth factor-mediated neurite outgrowth by inhibition of tyrosine kinase activity of TrkA. Anesth Analg. 2006;102:462–467. doi: 10.1213/01.ane.0000194334.69103.50. [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos-Flores C, Gonsebatt ME. Nerve growth factor exhibits an antioxidant and an autocrine activity in mouse liver that is modulated by buthionine sulfoximine, arsenic, and acetaminophen. Free Radic Res. 2013;47:404–412. doi: 10.3109/10715762.2013.783210. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hon T, Ye W, Zhang L. Heme deficiency interferes with the Ras-mitogen-activated protein kinase signaling pathway and expression of a subset of neuronal genes. Cell Growth Differ. 2002;13:431–439. [PubMed] [Google Scholar]