Abstract
Stress-induced cardiomyocyte apoptosis plays an important role in the pathogenesis of a variety of cardiovascular diseases. Our early studies showed that HSP70 effectively inhibited apoptosis, but the underlying mechanism remained unclear. Fas-associated factor 1 (FAF1) is a member of the Fas death-inducing signaling complex (Fas-DISC) that acts upstream of caspase-8. We investigated the interactions among FAF1, HSP70, and FAS in stressed cardiomyocytes to elucidate the protective mechanism of HSP70. FAS and caspase-3/8 activity was higher in cardiomyocytes undergoing stress-induced apoptosis in restraint-stressed rats compared with cardiomyocytes in non-stressed rats, which indicated that the Fas signaling pathway was activated after restraint stress. Geranylgeranylacetone (GGA) induced an increase in HSP70 expression, which reduced stress-induced apoptosis. Additionally, overexpression of HSP70 via transfection with the pEGFP-rHSP70 plasmid attenuated norepinephrine (NE)-induced apoptosis. FAF1 expression increased during stress-induced apoptosis, and overexpression of FAF1 exacerbated NE-induced apoptosis. We also found that HSP70 interacted with FAF1. Overexpression of HSP70 inhibited the binding of FAF1 to FAS in H9C2 cells, which indicated that HSP70 suppressed NE-induced apoptosis by competitively binding to FAF1. An N-terminal deletion mutant of HSP70 (HSP70-△N) was unable to interact with FAF1. After HSP70-△N was transfected into H9C2 cells, the cells were unable to attenuate the NE-induced increases in caspase-8 and apoptosis. These results indicate that the 1–120 sequence of HSP70 binds to FAF1, which alters the interactions between FAS and FAF1 and inhibits the activation of the Fas signaling pathway and apoptosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0589-9) contains supplementary material, which is available to authorized users.
Keywords: Stress, Fas-associated factor 1 (FAF1), HSP70, Apoptosis, Death-inducing signal complex (DISC), Fas signaling pathway
Introduction
Stress overload contributes to the manifestation of many diseases (Hier 2001), such as cardiomyopathy, acute myocardial infarction (Giallauria et al. 2013), atherosclerosis, and other serious cardiovascular diseases (Agid et al. 2000; Manuck et al. 1995). Cardiomyocyte apoptosis, an important mechanism of stress-induced cell death, is also a major factor underlying stress-induced cardiac dysfunction and cardiovascular disease. The abnormal activation of apoptosis is an important risk factor for heart disease (Hier 2001). Excessive apoptotic cell death may cause a loss of contractile cells and weaken a functioning heart. (Abbate et al. 2002; Bardales et al. 1996; Colucci et al. 2000; Feuerstein and Young 2000; Ma et al. 2013; Saraste et al. 1997). Stress alters the levels of catecholamine hormones, which play an important role in hypertension (Louis et al. 2012), cardiac necrosis, arrhythmia, and apoptosis in cardiomyocytes (Kastaun et al. 2014; Schomig et al. 1991; Zaugg et al. 2000; Zhao et al. 2013). Therefore, preventing cardiomyocytes from undergoing apoptosis is an important strategy for the treatment of cardiac diseases. Our early studies indicated that heat shock protein 70 (HSP70) may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis (Zhao et al. 2007); however, the underlying mechanism was unclear. Fas-associated factor 1 (FAF1) is an important component of the Fas signaling pathway and is a member of Fas-DISC, which acts upstream of caspase-8 (Ryu et al. 2003). There is evidence that FAF1 and HSP70 interact (Kim et al. 2005), and there is a region of overlap where both FAS and HSP70 interact with FAF1. Based on these findings, we hypothesized that HSP70 might inhibit norepinephrine (NE)-induced apoptosis in cardiac H9C2 cells by competitively binding to FAF1 and inhibiting the binding of FAF1 to FAS. In this study, we examined the correlation between the anti-apoptotic effect of HSP70 and the activation of the Fas signaling pathway using in vitro experiments in cultured cardiac H9C2 cells. Our results indicate that HSP70 can reduce NE-induced apoptosis in H9C2 cells.
Materials and methods
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Beijing Institute of Basic Medical Sciences (Permit Number: 2012-D-3096).
Restraint-stress rat model
We randomly divided 30 adult male Wistar rats weighing 180–200 g into five groups: a control group; three restraint-stress groups with restraint times of 1, 2, and 3 weeks; and a geranylgeranylacetone (GGA) group. Each of the restrained rats was placed into a custom-made cabin that restricted its activity. Food and water were not supplied during the restraint times. The rats were restrained from 9:00 AM until 3:00 PM daily. Their plasma and hearts were saved for subsequent experiments at the end of the restraint experiment. The NE levels in their plasma were measured using a competitive ELISA according to the manufacturer’s instructions (KainuoBio, Beijing). GGA (Eisai Co., Ltd. China), a non-toxic inducer of HSP70, was intragastrically administered to the rats in the GGA group at a dosage of 300 mg/kg/day 2 hours prior to initiating the restraint stress. Rats in this group were subjected to a total of 3 weeks of restraint stress.
H9C2 cardiomyocyte cell culture
Cultured H9C2 cardiomyocyte cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Gaithersburg, MD, USA) supplemented with 10 % fetal bovine serum under 95 % air-5 % CO2 at 37 °C. The cells were grown for 2 days in 75-cm2 flasks. The medium was replaced with fresh DMEM before NE treatment and transfection.
Experimental protocol
After 72 h of incubation in six-well plates, the H9C2 cells were moved to serum-free DMEM and incubated for 12 h. Next, the cells were assigned to four groups (3 wells for each group, n = 3) and exposed to NE at 0, 5, 20, or 50 μM for 36 h. An optimal concentration of NE was selected for subsequent experiments. In another experiment, the cells were assigned to three groups (3 wells in each group, n = 3): group 1 was the control group, group 2 was treated with 50 μM NE for 36 h, and group 3 was treated with 50 μM NE for 36 h after transfection with the HSP70 plasmid. Cellular apoptosis was measured by flow cytometry. The activities of caspase-3/8 were measured using the Caspase-3/8 Activity Assay Kit (Beyotime Biotechnology, China). The interactions between FAS and FAF1 were detected by immunoprecipitation.
TdT-mediated dUTP nick-end labeling (TUNEL) assay
Left ventricular tissue was isolated from the rats, fixed with 4 % paraformaldehyde, embedded in paraffin, and cut into 5-mm serial sections. The sections were stained using the In Situ Cell Death Detection Kit (POD Kit; Roche Applied Science, Burgess Hill, UK) according to the manufacturer’s instructions. The apoptotic regions were stained brown with blue nuclei. To determine the apoptotic ratio of the cells, TUNEL-positive cells were counted using Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). Five visual fields in each section (200×) were randomly selected, counted, and averaged.
FCM analysis of apoptosis
The apoptosis of the cardiac H9C2 cells was measured by flow cytometry (FCM) with a 7-AAD and Annexin V-PE Apoptosis Detection Kit (KeyGen Biotechnology, China) according to the manufacturer’s instructions. The apoptosis ratio (%) was the sum ratio of the cells that stained positive for Annexin V-PE plus the cells that were positive for both Annexin V-PE and 7-AAD to the total population of cells.
Western blot assay
The cells were washed twice with phosphate-buffered saline (PBS) solution and then processed with 60 μL of radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors (Beyotime Biotechnology, China). The bicinchoninic acid (BCA) method was used to determine the protein concentration in each well. The proteins were separated by 10 % SDS-polyacrylamide gel electrophoresis (40 μg/lane). A wet-transfer system (Mini Trans-Blot cell, Bio Rad Laboratories, Inc., USA) was used to transfer the protein contents to the membrane via standard procedures. After incubation with 5 % BSA for 2 h at room temperature, the membrane was incubated at 4 °C overnight with one of the following primary antibodies: mouse monoclonal antibodies to HSP70 (Cell Signaling Technology, USA), FAS (Boster Biotechnology Company, China), or GAPDH (Sungene Biotech Company, China) or a goat polyclonal antibody to FAF1 (Santa Cruz Biotechnology, Inc., CA, USA). The membrane was then incubated with an HRP-conjugated secondary antibody at room temperature for 3 h. The ECL chemical luminescence method was employed, and the target protein was detected and analyzed using ImageQuant LAS 4000 software (GE, USA).
Caspase-3/8 activity analysis
Caspases activities were measured using the Caspase Activity Kit (Beyotime Biotechnology, China) according to the manufacturer’s instructions. Briefly, the cells were washed with cold PBS, resuspended in lysis buffer, and placed on ice for 15 min. The lysates were then centrifuged at 16,000g at 4 °C for 15 min. The supernatants were collected, and the protein concentration was determined using the BCA protein assay (Beyotime Biotechnology, China). The activities of caspase-3 and caspase-8 were measured with the substrate peptides Ac-DEVD-pNA and Ac-IETD-pNA, respectively. The release of p-nitroanilide (pNA) was quantified by determining the absorbance at 405 nm with a Varioskan Flash (Thermo).
Immunoprecipitation assay
The cells were lysed with RIPA and then centrifuged at 14,000g for 15 min at 4 °C. The protein concentration was determined using a BCA protein assay. After the supernatant was incubated overnight at 4 °C with primary antibody, Protein A+G Agarose (Beyotime Biotechnology, China) was added, and the samples were incubated for an additional 4 h. The immune complexes were washed four times with cold PBS and boiled in SDS sample buffer. The samples were then examined by western blotting according to the method described above.
Construction of pEGFP-HSP70 and pcDNA3.1(+)-rFAF1
The sequences of HSP70 and FAF1 were cloned from a rat myocardium cDNA library. The HSP70 and FAF1 fragments were then obtained, purified, digested with BamHI and EcoRI, and subcloned into pEGFP-N1 and pcDNA3.1(+) vectors with T4 ligase (Promega, Beijing, China).
Construction of the HSP70 deletion mutant
Using the sequence of HSP70 (GenBank ID: L16764.1), a 1–120 N-terminal deletion mutant plasmid (pEGFP-HSP70△N-(121–642)) was constructed with the KOD-Plus-Mutagenesis Kit (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer’s instructions.
Statistical analysis
The data are presented as the mean ± standard deviation. One-way ANOVA and Bonferroni post hoc tests were performed to analyze the differences between two groups. A P value of less than 0.05 was considered to be statistically significant.
Results
Stress-induced activation of the Fas signaling pathway and apoptosis in vivo and in vitro
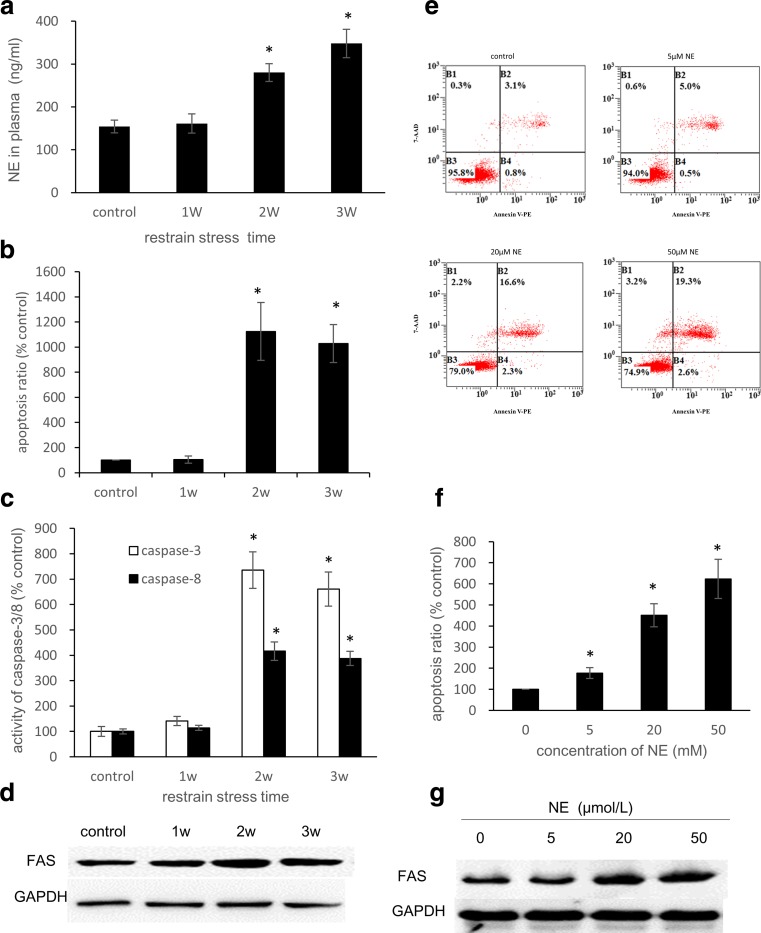
The NE levels in the rat plasma gradually increased with restraint time (Fig. 1a). The plasma NE levels in the 3-week group were 2.26-fold higher than those in the control group (P < 0.05). As evidenced by a higher number of TUNEL-positive cells, there was a significant increase in apoptosis in the 2-week and 3-week groups compared with the control group (P < 0.05) (Fig. 1b). The activities of caspase-3 and caspase-8 were also examined and showed a similar increasing trend. These activities reached maximum levels in the 2-week group, in which caspase-3 was approximately 7.4-fold higher and caspase-8 was approximately 4.2-fold higher than the control group (Fig. 1c). These results indicate that the Fas signaling pathway was activated after restraint stress. In the H9C2 cells treated with NE, an FCM analysis of apoptosis indicated that there was a dose-effect relationship between the concentration of NE and the apoptosis ratio (Figs. 1e–f). Accordingly, we chose 50 μM as the optimal concentration of NE that would induce apoptosis in H9C2 cells. The expression of FAS in the rat myocardia increased in the first 2 weeks and decreased in the third week of experimentation (Fig. 1d). In addition, the FAS levels in the H9C2 cells increased after treatment with NE at various concentrations (Fig. 1g).
Fig. 1.
Restraint stress induces apoptosis in rat myocardia, and NE treatment induces apoptosis in H9C2 cells. a Plasma NE levels of the rats after restraint stress. The rat plasma NE levels were measured by ELISA (n = 3). b In the TUNEL assay, the apoptosis ratio was defined as the ratio of positive cells to the total number of cells in each visual field. In each section, five visual fields were counted and averaged (n = 5). c Caspase-3 and Caspase-8 Activity Assay Kits were used to detect caspase-3/8 activity. e, f After 36 h of treatment with NE at concentrations of 0, 5, 20, and 50 μM, H9C2 cells were stained with 7-AAD and Annexin V-PE and analyzed by FCM. d, g Whole-cell lysates were subjected to western blot analysis using the anti-FAS antibody. GAPDH was used as a loading control. The bars represent the mean ± SD of three experiments performed in triplicate. *P < 0.05 vs the control group
Overexpression of HSP70 attenuated stress-induced apoptosis
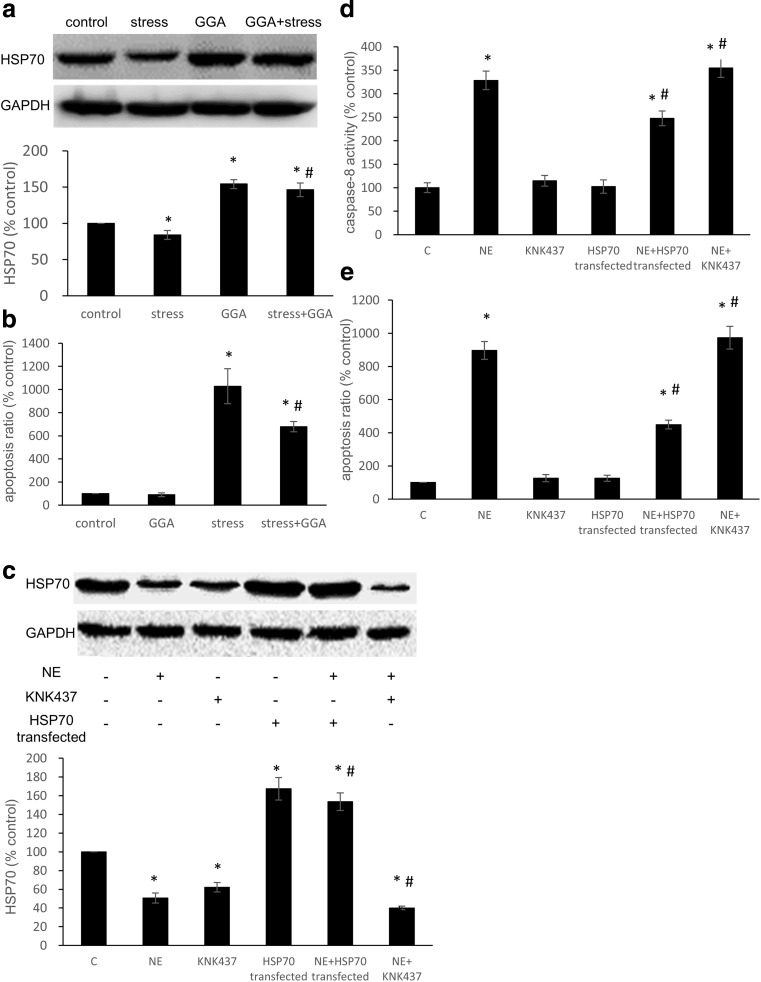
To examine whether HSP70 is capable of inhibiting stress-induced apoptosis, GGA, a non-toxic inducer of HSP70, was intragastrically administered to the rats. The pEGFP-rHSP70 plasmid was constructed and transfected into H9C2 cells using Lipofectamine 2000. After a 3-week restraint period, the expression of HSP70 in rat myocardia was decreased. However, pretreating the rats with GGA enhanced the expression of HSP70 compared with the control group (Fig. 2a). The TUNEL results demonstrated that apoptosis increased after the restraint period, but pretreatment with GGA suppressed stress-induced apoptosis of cardiomyocytes via the induction of HSP70 overexpression (Fig. 2b). In H9C2 cells, the overexpression of HSP70 was enhanced after plasmid transfection, and KNK437 decreased the expression of HSP70 (Fig. 2c). Also, NE had a stimulatory effect on caspase-8 activity and apoptosis, which decreased following the transfection of pEGFP-rHSP70 plasmid. In contrast, the inhibition of HSP70 aggravated NE-induced increase in caspase-8 activity and apoptosis (Fig. 2d–e). These results indicated that HSP70 effectively inhibited apoptosis by Fas signaling pathway.
Fig. 2.
The role of HSP70 during stress-induced cardiomyocyte apoptosis. a The expression of HSP70 in rat myocardia after stress and GGA administration. b Apoptosis was detected by the TUNEL assay. In each section, five visual fields were counted and averaged (n = 5). c H9C2 cells were transfected with the pEGFP-rHSP70 plasmid or treated with KNK437; whole-cell lysates were then subjected to western blot analysis using the anti-HSP70 antibody. GAPDH was used as a loading control. d Caspase-8 activity was detected with a Caspase-8 Activity Assay Kit. e H9C2 cells were stained with 7-AAD and Annexin V-PE and analyzed by FCM. The data shown represent the mean ± SD from three independent experiments. *P < 0.05 vs the control group. #P < 0.05 vs the NE group
Stress induced an increase in FAF1, and overexpression of FAF1 exacerbated stress-induced apoptosis
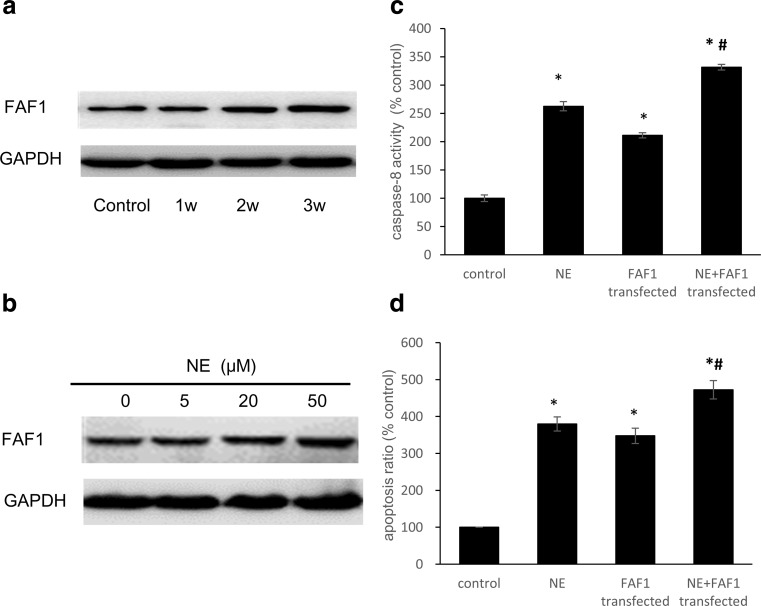
For the rats in the restraint-stress groups, the expression of FAF1 increased with longer restraint times (Fig. 3a). After treatment with NE for 36 h, the FAF1 levels increased as the NE concentration increased, following a dose-response relationship (Fig. 3b). The activity of caspase-8 in the cell lysates was significantly increased after the H9C2 cells were treated with 50 μM NE for 36 h, and this increase was augmented by the overexpression of FAF1 (Fig. 3c). H9C2 cell apoptosis increased significantly after NE treatment. Transfection with the pcDNA3.1(+)-rFAF1 plasmid enhanced this NE-induced increase in apoptosis (Fig. 3d).
Fig. 3.
FAF1 levels increased with stress intensity, and overexpression of FAF1 exacerbated stress-induced apoptosis. a After restraint stress, whole-cell lysates of rat myocardia were subjected to western blot analysis using an anti-FAF1 antibody. GAPDH was used as a loading control. b Levels of FAF1 in H9C2 cells treated with different concentrations of NE for 36 h. c After transfection with the pcDNA3.1(+)-rFAF1 plasmid, the activity of caspase-8 in whole-cell lysates was detected using a Caspase-8 Activity Assay Kit. d Flow cytometric analysis of apoptosis. The data shown represent the mean ± SD from three independent experiments. *P < 0.05 vs the control group. #P < 0.05 vs the NE group
HSP70 inhibited the activation of the Fas signaling pathway by binding to FAF1 through its N-terminal domain
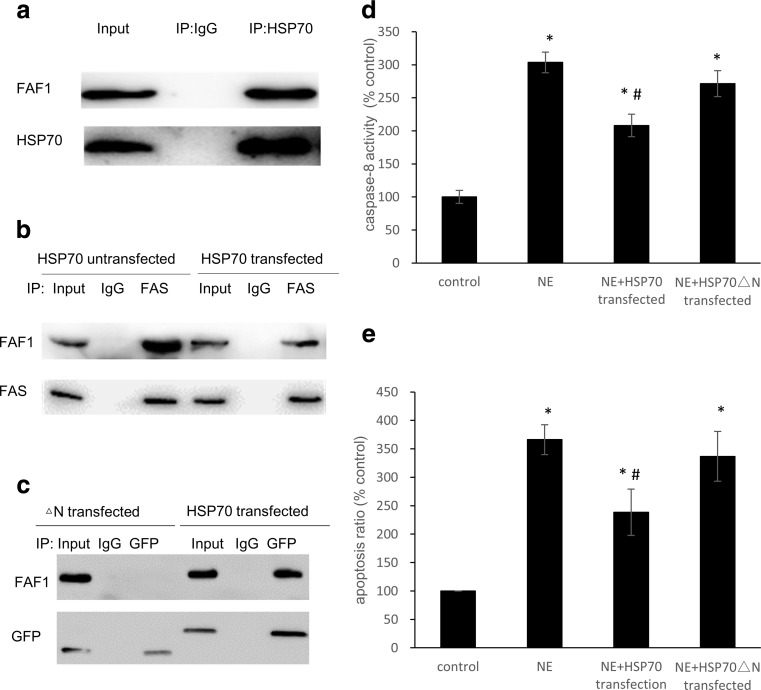
We performed an immunoprecipitation assay to detect the combination of FAF1 and HSP70 in H9C2 cells. Western blot analysis revealed that FAF1 interacts with HSP70 (Fig. 4a). The binding of FAF1 to FAS was analyzed by western blot using an anti-FAF1 antibody after immunoprecipitation with an anti-FAS antibody. The levels of FAF1-FAS binding were lower in the HSP70-transfected H9C2 cells than in the untreated group (Fig. 4b). The pEGFP-HSP70△N-(121–642) and pEGFP-HSP70-(1–642) plasmids were transfected into H9C2 cells, and the cells were lysed and immunoprecipitated with an anti-GFP antibody. The precipitates were analyzed by SDS-PAGE and western blot assays using an anti-FAF1 antibody. After deletion of the N-terminus (amino acids 1–120), HSP70 was no longer bound to FAF1. These data reveal that the N-terminus of HSP70 is necessary for binding with FAF1 (Fig. 4c). As described in Fig. 2d, the NE-induced increase in caspase-8 in the H9C2 cell lysates was attenuated by the overexpression of HSP70. However, transfection with the deletion mutant △N-(121–642) did not attenuate the increase in caspase-8 (P > 0.05) (Fig. 4d) and did not inhibit NE-induced apoptosis (Fig. 4e).
Fig. 4.
HSP70 competitively inhibited the binding of FAF1 to FAS in cardiac H9C2 cells. a H9C2 cells were lysed and immunoprecipitated (IP) with anti-hFAF1 polyclonal antibody and mouse IgG as a control. The precipitates were analyzed by SDS-PAGE and western blot analysis using anti-FAF1 polyclonal antibody (upper panel) and anti-HSP70 monoclonal antibody (lower panel). b FAF1-FAS binding was analyzed by western blot using an anti-FAF1 monoclonal antibody (upper panel) after immunoprecipitation with an anti-FAS polyclonal antibody (lower panel). c After the pEGFP-HSP70△N-(121–642) and pEGFP-HSP70-(1–642) plasmids were transfected into H9C2 cells, cell lysates were immunoprecipitated with anti-FAF1 polyclonal antibody (upper panel) and anti-GFP monoclonal antibody (lower panel). d The activity of caspase-8 was measured with a Caspase-8 Activity Assay Kit. e The apoptosis of H9C2 cells transfected with HSP70 and the HSP70 mutant was measured by staining with 7-AAD and Annexin V-PE followed by FCM analysis. The data shown represent the mean ± SD from three independent experiments. *P < 0.05 vs the control group. #P < 0.05 vs the NE group
Discussion
Stress can be defined as a state of mental or emotional strain that results from exposure to adverse or demanding circumstances. An individual’s perception of a particular situation as “stressful” is a pivotal component of the process whereby the stressor affects the individual’s health (Peters et al. 2007; Steptoe and Kivimaki 2013). The cardiovascular system is the primary target of stress. Because restraint is considered to be a non-specific stressor, the animal model of restraint stress is often used to study the influence of stress on physiological functions and pathological processes. In a previous study, we observed cardiovascular function disruption and pathological alterations in the electrocardiograms of chronically restraint-stressed rats (Wang et al. 2009). Cardiomyocyte damage is considered to be an important cellular basis for stress-induced cardiovascular injury and disease (Liu et al. 2004). However, the mechanism of stress-induced cardiomyocyte injury remains unclear.
Heat shock proteins (HSPs) act as molecular chaperones and are involved in basic cellular functions such as protein folding, protein trafficking, and membrane translocation (Evans et al. 2010; Mayer and Bukau 2005; Wisen et al. 2010). HSPs can be induced by diverse environmental stressors. For many years, heat shock and heat stress proteins have been regarded as intracellular molecules that have a range of housekeeping and cytoprotective functions (Evans et al. 2010). Several studies have reported that the induction of HSP70 in myocardia can attenuate a variety of cardiac injuries caused by ischemia/reperfusion, hypoxia/reoxygenation, and heart failure (Esch et al. 2002; Hampton et al. 2003; Kuboki et al. 2007). This suggests that the induction of HSP70 might be correlated with the acquisition of tolerance to stress. HSP70 may inhibit the activation of the Fas signaling pathway (Zhao et al. 2007). In our restraint-stress rat model, HSP70 expression increased, and Fas signaling was inhibited under mild or moderate stress. When the stress level was further increased, HSP70 levels declined, and Fas signaling pathway activation could not be inhibited. Because the Fas signaling pathway causes cardiomyocyte apoptosis, HSP70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis.
FAF1 is an apoptotic signaling molecule that functions downstream in the Fas signal transduction pathway (Chu et al. 1995). It is conceivable that FAF1 is present in Fas-DISC and serves to promote the aggregation of caspase-8 via a fas-associated protein with death domain (FADD)-like mechanism. FAF1 interacts with the death-effector domains (DED) of FADD and caspase-8 through a potentially helix-rich DED-like domain in FAF1 called DEDID, which is known to have apoptotic potential (Ryu et al. 2003). In the present study, we found that restraint stress caused an increase in the expression of FAF1 accompanied by an increase in the rate of cardiomyocyte apoptosis. Our in vitro experiments showed that FAF1 was overexpressed in our NE-induced cardiomyocyte apoptosis model (Fig. 3). This suggests that the FAF1-FAS signaling pathway plays an important role in stress-induced cardiomyocyte apoptosis. These findings are consistent with those in the previous literature. The 1–201-amino acid region of FAF1 acts as a Fas-binding domain, while the 82–180-amino acid region of FAF1 directly interacts with the N-terminus (amino acids 1–120) of HSP70 (Kim et al. 2005).
In the present study, we found that overexpression of HSP70 (via transfection with pEGFP-rHSP70) protected H9C2 cells from NE-induced apoptosis (Fig. 2). Numerous studies have shown that HSP70 contributes to thermotolerance because elevated HSP70 levels decrease cell sensitivity to heat stress and protect the cell against the toxicity induced by tumor necrosis factor (TNF), ultraviolet (UV) radiation, oxidative stress, and numerous chemicals. This protective ability of HSP70 has been recently shown to be a consequence of apoptosis inhibition (Mailhos et al. 1993; Mosser and Martin 1992). Our previous experiments also revealed that HSP70 is capable of protecting cardiomyocytes from restraint stress-induced injury by inhibiting Fas-mediated apoptosis (Zhao et al. 2007). However, the underlying mechanism was unclear. The present study shows that overexpression of HSP70 decreases the interactions between FAF1 and FAS (Fig. 4). HSP70 binds to the FAF1-FAS interaction site, reducing the binding of FAF1 and FAS and thus reducing the activation of the Fas signaling pathway, which reduces NE-mediated cell apoptosis and has a protective effect on myocardial cells.
Our results indicate that HSP70 has a protective effect in cardiomyocytes, in which FAF1 plays an important role. HSP70 and FAS compete for binding to FAF1 and regulate the Fas signaling pathway indirectly. An N-terminal deletion mutant (121–642) of HSP70 displayed an inability to bind to FAF1; consequently, this HSP70 mutant did not exhibit a protective effect. This result suggests that FAF1 plays the role of a scaffold in the interactions among FAS, FAF1, and HSP70. Further research on the regulatory mechanisms of FAF1 will help clarify the molecular mechanism underlying the protective effect of HSP70 on cardiac function. Related research on FAF1 and apoptosis is likely to aid the development of cardiac protection strategies for health improvement (Reeve et al. 2005).
Electronic supplementary material
(PNG 666 kb)
(PNG 31 kb)
(PNG 31 kb)
Acknowledgments
This study was carried out with the support of the National Natural Science Foundation of China (31071022, 81000584, and 81372124).
Abbreviations
- FAF1
Fas-associated factor 1
- HSP70
Heat shock protein 70
- NE
Norepinephrine
- DISC
Death-inducing signal complex
- GFP
Green fluorescent protein
- FCM
Flow cytometry
References
- Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002;193:145–153. doi: 10.1002/jcp.10174. [DOI] [PubMed] [Google Scholar]
- Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- Bardales RH, Hailey LS, Xie SS, Schaefer RF, Hsu SM. In situ apoptosis assay for the detection of early acute myocardial infarction. Am J Pathol. 1996;149:821–829. [PMC free article] [PubMed] [Google Scholar]
- Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci U S A. 1995;92:11894–11898. doi: 10.1073/pnas.92.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci WS, Sawyer DB, Singh K, Communal C. Adrenergic overload and apoptosis in heart failure: implications for therapy. J Card Fail. 2000;6:1–7. doi: 10.1016/S1071-9164(00)80002-0. [DOI] [PubMed] [Google Scholar]
- Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res. 2000;45:560–569. doi: 10.1016/S0008-6363(99)00372-7. [DOI] [PubMed] [Google Scholar]
- Giallauria F, et al. Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function. Eur J Nucl Med Mol Imaging. 2013;40:315–324. doi: 10.1007/s00259-012-2302-x. [DOI] [PubMed] [Google Scholar]
- Hampton CR, et al. HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol. 2003;285:H866–H874. doi: 10.1152/ajpheart.00596.2002. [DOI] [PubMed] [Google Scholar]
- Hier DB. The autonomic nervous system in health and disease. Med Sci Sports Exerc. 2001;33:2157. doi: 10.1097/00005768-200112000-00028. [DOI] [Google Scholar]
- Kastaun S, et al. Cortisol awakening and stress response, personality and psychiatric profiles in patients with takotsubo cardiomyopathy. Heart. 2014;100:1786–1792. doi: 10.1136/heartjnl-2014-305745. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Song EJ, Lee YS, Kim E, Lee KJ. Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J Biol Chem. 2005;280:8125–8133. doi: 10.1074/jbc.M406297200. [DOI] [PubMed] [Google Scholar]
- Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1141–G1149. doi: 10.1152/ajpgi.00491.2006. [DOI] [PubMed] [Google Scholar]
- Liu XH, Qian LJ, Gong JB, Shen J, Zhang XM, Qian XH. Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics. 2004;4:3167–3176. doi: 10.1002/pmic.200300845. [DOI] [PubMed] [Google Scholar]
- Louis XL, Murphy R, Thandapilly SJ, Yu L, Netticadan T. Garlic extracts prevent oxidative stress, hypertrophy and apoptosis in cardiomyocytes: a role for nitric oxide and hydrogen sulfide. BMC Complement Altern Med. 2012;12:140. doi: 10.1186/1472-6882-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YX, Guo Z, Sun T. CGRP inhibits norepinephrine induced apoptosis with restoration of Bcl-2/Bax in cultured cardiomyocytes of rat. Neurosci Lett. 2013;549:130–134. doi: 10.1016/j.neulet.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627. doi: 10.1016/0306-4522(93)90428-I. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: an example from coronary artery disease. Psychosom Med. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Martin LH. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol. 1992;151:561–570. doi: 10.1002/jcp.1041510316. [DOI] [PubMed] [Google Scholar]
- Peters JL, et al. Stress as a potential modifier of the impact of lead levels on blood pressure: the normative aging study. Environ Health Perspect. 2007;115:1154–1159. doi: 10.1289/ehp.10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve JL, Duffy AM, O’Brien T, Samali A. Don’t lose heart—therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med. 2005;9:609–622. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SW, Lee SJ, Park MY, Jun JI, Jung YK, Kim E. Fas-associated factor 1, FAF1, is a member of Fas death-inducing signaling complex. J Biol Chem. 2003;278:24003–24010. doi: 10.1074/jbc.M302200200. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.CIR.95.2.320. [DOI] [PubMed] [Google Scholar]
- Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J. 1991;12(Suppl F):38–47. doi: 10.1093/eurheartj/12.suppl_F.38. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. NGFI-B targets mitochondria and induces cardiomyocyte apoptosis in restraint-stressed rats by mediating energy metabolism disorder. Cell Stress Chaperones. 2009;14:639–648. doi: 10.1007/s12192-009-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisen S, et al. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol. 2010;5:611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.CIR.102.3.344. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang W, Qian L. Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones. 2007;12:83–95. doi: 10.1379/CSC-231R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al. Inhibition of cystathionine beta-synthase is associated with glucocorticoids over-secretion in psychological stress-induced hyperhomocystinemia rat liver. Cell Stress Chaperones. 2013;18:631–641. doi: 10.1007/s12192-013-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 666 kb)
(PNG 31 kb)
(PNG 31 kb)