Abstract
Syringocystadenocarcinoma papilliferum (SCACP) is a very rare cutaneous adnexal neoplasm. SCACP presents histologic variability, and it is difficult to establish the diagnosis from a punch biopsy. SCACP has an overall configuration similar to that of syringocystadenoma papilliferum (SCAP). When we diagnose SCACP, the histologic features of SCAP can be contributing and immunohistochemical staining is useful. Our case shows the histologic variability of SCACP and the pitfalls of a punch biopsy for the diagnosis of SCACP.
Key Words: Syringocystadenocarcinoma papilliferum, Perianal syringocystadenocarcinoma papilliferum, Pagetoid spread, Cutaneous adnexal neoplasm, Perianal area, Lymph node metastasis, Adenocarcinoma
Introduction
Syringocystadenocarcinoma papilliferum (SCACP) is a very rare cutaneous adnexal neoplasm and presents histologic variability. Only 28 cases have been reported in the medical literature [1]. Most cases of SCACP seem to have arisen from syringocystadenoma papilliferum (SCAP) [1, 2, 3]. Herein, we report a case of SCACP in the perianal area with histologic and immunohistochemical features which was difficult to diagnose by biopsy only.
Case Report
A 76-year-old man with no prior history of malignancy was referred to our department with an ulcerated enlarging mass in the perianal area measuring 16 cm in diameter that had been first noticed half a year earlier. An exophytic and papillary mass was observed within the erythematous-erosive plaque (fig. 1a). A punch biopsy from the erythema revealed atypical cells with hyperchromatic nuclei in situ (fig. 1b). Immunohistochemical staining revealed that the tumor cells were heterogeneously positive for cytokeratin 5/6 (CK5/6) and strongly positive for CK7, and negative for carcinoembryonic antigen (CEA), CK20 and gross cystic disease fluid protein 15 (GCDFP-15) (table 1). The differential diagnosis was primary or secondary extramammary Paget's disease, squamous cell carcinoma and metastatic adenocarcinoma. The tumor cells were positive for CK5/6 and CK7, so the immunohistochemical features excluded primary extramammary Paget's disease [4]. Screening colonoscopy and cystoscopy revealed no malignancy in the lower gastrointestinal and urinary tract, which excluded secondary extramammary Paget's disease. No distant metastasis could be identified by FDG-PET. From the punch biopsy, the diagnosis was consistent with pagetoid squamous cell carcinoma in situ, thus our initial diagnosis was squamous cell carcinoma. Sentinel lymph node biopsy was performed and the bilateral inguinal sentinel lymph nodes were positive. Surgical resection and bilateral inguinal lymph node dissection were performed.
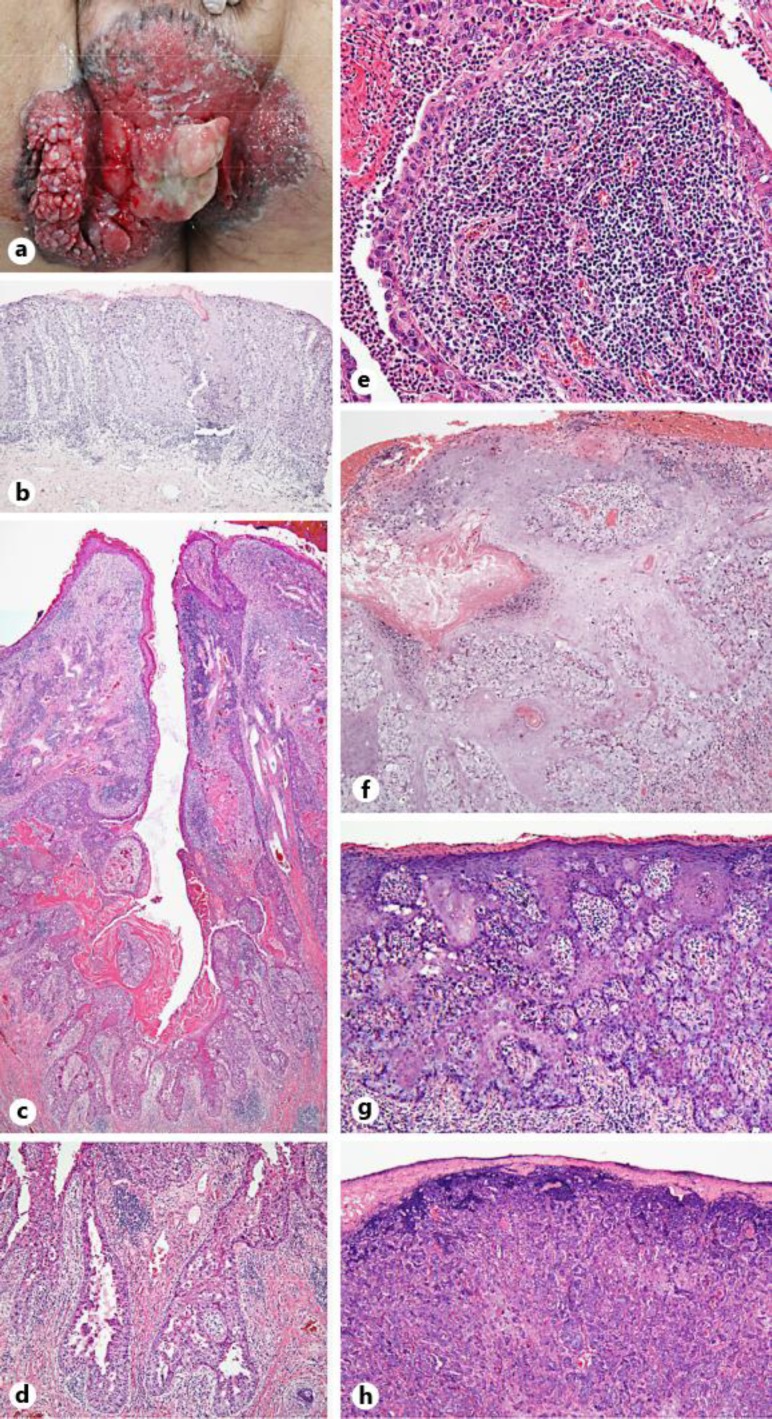
Fig. 1.
a Clinical appearance of the perianal tumor: a mass with a papillary surface within a background of erythematous-erosive plaque. b The specimen of the punch biopsy from the erythema: atypical cells with hyperchromatic nuclei were observed in situ. Hematoxylin-eosin staining of the resected tumor specimen showed the following: c Focal connection to the epidermis and papillary projections that protruded into the cystic space. d Apocrine differentiation with decapitation secretion. e The stroma contained infiltrative plasma cells and lymphocytes. f Areas of squamous differentiation in the epidermis. g Pale tumor cells with a pagetoid appearance were observed at all levels of the epidermis. h The invasive adenocarcinoma component.
Table 1.
Immunohistochemical features of our case
| Immunohistochemical staining | Biopsy specimen | Resected specimen |
||
|---|---|---|---|---|
| pagetoid spread | adenocarcinoma component | papillary projections | ||
| CK5/6 | positive | positive | weakly positive | positive (the cells of the outer layer) |
| CK7 | positive | positive | positive | positive |
| CEA | negative | negative | weakly positive | negative |
| CK20 | negative | negative | negative | negative |
| GCDFP-15 | negative | negative | weakly positive | negative |
The overall histologic findings from the resected specimens revealed a focal connection to the epidermis and papillary projections that protruded into the cystic space and were lined with two-layer epithelium (fig. 1c). The luminal layer was composed of columnar cells and the outer layer was composed of small cuboidal cells (fig. 1c). Apocrine differentiation was shown by the presence of decapitation secretion (fig. 1c, d). A focal connection to the epidermis with papillary projections and apocrine differentiation displayed characteristics of a primary cutaneous adnexal origin and showed a silhouette resembling SCAP. The tumor cells in the papillary projections were positive for CK7 and negative for CK20. CK5/6 expression was positive for the cells of the outer layer in the papillary projections (fig. 2a, table 1). The stroma contained dense infiltration of plasma cells and lymphocytes within the fibrovascular core (fig. 1e). There was an area of squamous differentiation in the epidermis (fig. 1f) as well as a prominent pagetoid lateral growth at the periphery of the tumor, and pale tumor cells with a pagetoid appearance were observed at all levels of the epidermis (fig. 1g). The tumor cells with pagetoid spread were heterogeneously positive for CK5/6 (fig. 2b, table 1) and strongly positive for CK7, whereas they were completely negative for CEA, CK20 and GCDFP-15 (table 1). The invasive tumor cells were adenocarcinoma (fig. 1h); they were strongly positive for CK7 and weakly and heterogeneously positive for CK5/6 (fig. 2c), CEA and GCDFP-15, but negative for CK20 (table 1). The tumor cells had large pleomorphic, hyperchromatic nuclei and atypical mitotic figures. From immunohistochemical and histologic findings, the diagnosis of SCACP was established.
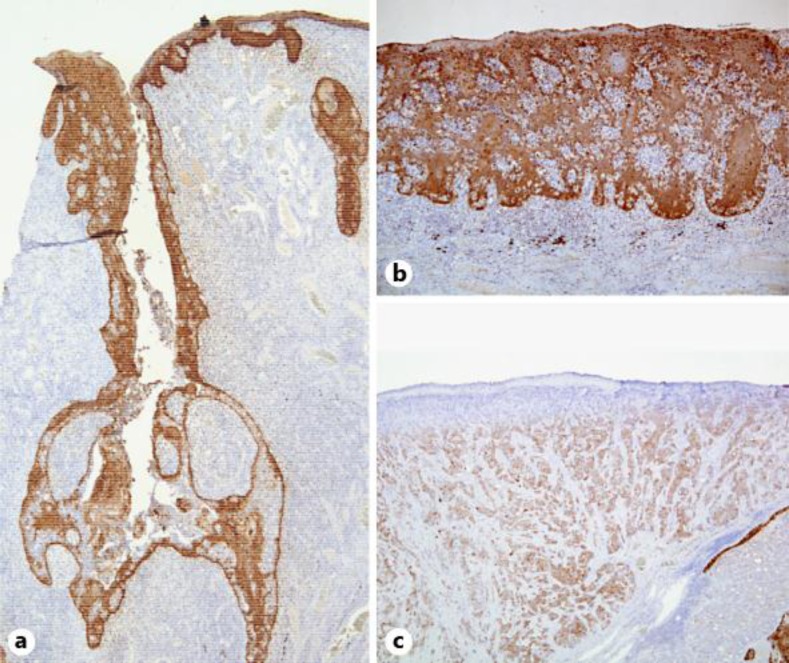
Fig. 2.
Immunohistochemical CK5/6 stainings. a CK5/6 was positive for the cells of the outer layer in the papillary projections. b Tumor cells with pagetoid spread showed heterogeneous staining for CK5/6. c The tumor cells were weakly positive for CK5/6 in the adenocarcinoma component.
Discussion
SCACP presents morphologic diversity as in situ carcinoma, invasive adenocarcinoma, sometimes with squamous differentiation, or pagetoid spread [1, 2, 3]. Diversity of pathological features was observed in our case. When we diagnose SCACP, it is important to evaluate not only a punch biopsy specimen, but also the overall structure from surgical resection, because different histologic features may be observed in each lesion in the resected specimen [1, 2, 3]. Thus, exclusive histologic information from the punch biopsy may mislead the diagnosis. Some papers have suggested that SCACP arises from long-standing SCAP. SCACP has an overall configuration similar to that of SCAP, but shows cytologic atypia [1, 2, 3]. When we diagnose SCACP, the histologic features of SCAP can be contributing and immunohistochemical staining is useful. In particular, CK5/6 positivity supports the primary origin of the cutaneous adnexal tumor and distinguishes it from metastatic adenocarcinoma. In our case, to a varying degree, CK5/6 was positive for the cells of the outer layer in the papillary projections (fig. 2a, table 1), the tumor cells with pagetoid spread (fig. 2b, table 1) and the adenocarcinoma component (fig. 2c, table 1). SCACP has a predilection for the head and neck [1, 3] and to the best of our knowledge, this is the third case of SCACP in the perianal area. The two reported cases of SCACP in the perianal area were both in situ carcinoma. Both of those cases and our case were males [5, 6]. One of the reported cases had existed for over 10 years [6]. SCACP is reportedly a slow-growing tumor, and regional lymph node metastasis is rare [1, 3]. However, our case had bilateral inguinal lymph node metastases. Three cases with lymph node metastases have been previously reported, two of which were located in the scalp (one present since birth [7] and one present for over 20 years [8]), and the third was located in the chest (present for over 20 years [9]). Our case is the first reported case of SCACP with lymph node metastases in the perianal area. It was an invasive carcinoma with regional lymph node metastasis, which suggested that our case had been a long-standing tumor, though the patient noticed it just about half a year prior to his first visit to our department.
This case shows the histologic variability of SCACP and the pitfalls of relying solely on a punch biopsy for the diagnosis of SCACP. When we diagnose SCACP, it is important to evaluate the whole structure from the surgical resection, and the histologic features of SCAP can be contributing to the diagnosis. SCACP should be considered by clinicians and pathologists as one of the differential diagnoses of a tumor in the perianal area.
Statement of Ethics
We state that our patient gave informed consent and that the study protocol was approved by the institute's committee on human research.
Disclosure Statement
The authors declare no conflict of interests.
Acknowledgements
The authors thank Dr. Toshiaki Manabe (Shiga Medical Center Research Institute, Shiga, Japan) for his kind support in the diagnosis of SCACP.
References
- 1.Castillo L, Moreno A, Tardio JC. Syringocystadenocarcinoma papilliferum in situ: report of a case with late recurrence. Am J Dermatopathol. 2014;36:348–352. doi: 10.1097/DAD.0b013e3182a38bb9. [DOI] [PubMed] [Google Scholar]
- 2.Kazakov DV, Requena L, Kutzner H, Fernandez-Figueras MT, Kacerovska D, Mentzel T, Schwabbauer P, Michal M. Morphologic diversity of syringocystadenocarcinoma papilliferum based on a clinicopathologic study of 6 cases and review of the literature. Am J Dermatopathol. 2010;32:340–347. doi: 10.1097/DAD.0b013e3181b96c0c. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YH, Wang WL, Rapini RP, Torres-Cabala C, Prieto VG, Curry JL. Syringocystadenocarcinoma papilliferum with transition to areas of squamous differentiation: a case report and review of the literature. Am J Dermatopathol. 2012;34:428–433. doi: 10.1097/DAD.0b013e318235dd34. [DOI] [PubMed] [Google Scholar]
- 4.Liegl B, Leibl S, Gogg-Kamerer M, Tessaro B, Horn LC, Moinfar F. Mammary and extramammary Paget's disease: an immunohistochemical study of 83 cases. Histopathology. 2007;50:439–447. doi: 10.1111/j.1365-2559.2007.02633.x. [DOI] [PubMed] [Google Scholar]
- 5.Langner C, Ott A. Syringocystadenocarcinoma papilliferum in situ originating from the perianal skin. APMIS. 2009;117:148–150. doi: 10.1111/j.1600-0463.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Iizuka H. Syringocystadenocarcinoma papilliferum: case report and immunohistochemical comparison with its benign counterpart. Am J Dermatopathol. 2001;45:755–759. doi: 10.1067/mjd.2001.117723. [DOI] [PubMed] [Google Scholar]
- 7.Seco Navedo MA, Fresno Forcelledo M, Orduña Domingo A, Junco Petrement P, Soler Sanchez T. Syringocystadenoma papilliferum with malignant evolution. Presentation of a case (in French) Ann Dermatol Venereol. 1982;109:685–689. [PubMed] [Google Scholar]
- 8.Arslan H, Diyarbakrl M, Batur S, Demirkesen C. Syringocystadenocarcinoma papilliferum with squamous cell carcinoma differentiation and with locoregional metastasis. J Craniofac Surg. 2013;24:e38–e40. doi: 10.1097/SCS.0b013e31826cffc6. [DOI] [PubMed] [Google Scholar]
- 9.Numata M, Hosoe S, Itoh N, Munakata Y, Hayashi S, Maruyama Y. Syringadenocarcinoma papilliferum. J Cutan Pathol. 1985;12:3–7. doi: 10.1111/j.1600-0560.1985.tb00422.x. [DOI] [PubMed] [Google Scholar]