Abstract
Ongoing concerns exist regarding the dangers inherent when handling cytotoxics, particularly drugs which are in parenteral formulations. On occasions, nurses and medical doctors have been preparing and administrating these drugs in the open spaces of wards in the absence of suitable personal protective equipment (PPE) and safety cabinets. To explore further into the severity of occupational hazards, we conducted our research in order to evaluate the healthcare’s understanding of occupational exposure to cytotoxics and occurrence of any side effects. A cross-sectional study using a self-administered questionnaire was distributed amongst oncology nurses in nine specialized cancer centers in Tehran. The questionnaire was based on most reputable international guidelines, aiming to evaluate the attitude, knowledge and safe practices of nurses' handling cytotoxic drugs. The gathered data and reported side effects were compared between “oncology/hematology” and “non-oncology” participants. The majority of nurses from oncology wards were aware of the potential hazards associated with handling of chemotherapy and reported high levels of compliance with the use of PPE during reconstitution of antineoplastic agents. Almost all nurses reported the use of a safety cabinet during preparation, however only 55 % reported that they have annual medical checkups and 45 % reported having received specialized training. This work was also to evaluate the experimental procedures as well as cleaning solutions used to reduce the level exposure. While the level of knowledge about antineoplastic agents is high among nurses, along with the level of PPE use, medical surveillance and employee training seems to be lagging behind.
Keywords: cytotoxic drugs, surface sampling, cleaning solutions, contamination, occupational exposure, questionnaire
Introduction
Hazardous drugs, defined by the American Society of Health System Pharmacists (ASHP), are drugs manifesting genotoxicity, carcinogenicity, teratogenicity, fertility impairment, serious organ or any other toxic manifestation at low doses in animal or human experiments (ASHP, 1985[5]). Cytotoxic drugs, sometimes known as antineoplastic, anticancer or cancer chemotherapy drugs are defined as hazardous drugs (Ansari Lari et al., 2001[4]). Their pharmacological property to kill tumor cells is by interfering with cell division. However, their action is not specific to cancerous cells, and non-cancerous cells may also get damaged (Barton Burke and Wilkes, 2006[7]). As a result, they can induce significant side effects in patients or any other person exposed to them in the treatment chain. The medical, clinical, nursing team and workers are exposed to hazardous drugs throughout their career cycle (Gambrell and Moore, 2006[13]). They are involved in manufacturing transport, distribution, use in health care setting and waste disposal (Connor and McDiarmid, 2006[11]; Zeedijk et al., 2005[39]). Acute exposure generally causes transient symptoms such as headache, nausea, malaise, dizziness, rash, dermatitis, skin and mucous membrane irritation or ulceration and eye or throat irritation. The side effects of repeated exposure are significant and dangerous (Valanis et al., 1993[38]). This together with the increasing complexity of chemotherapy, have raised the concerns about the risks to health care workers who are involved in this cycle.
Falck et al. (1979[12]) first reported the evidence of mutagenicity in urine samples of the nurses handling cytotoxic drugs. Other studies have found increased chromosomal aberrations and evidences of mutagenic/carcinogenic risks in exposed nurses’ urine samples (Terui et al., 2011[33]; Moretti et al., 2011[26]; Suspiro and Prista, 2011[32]; Ursini et al., 2006[35]; Kopjar et al., 2010[19]). Fetal loss induced by chronic exposure to cyclophosphamide, doxorubicin and vincristine, in nursing personnel was also observed (Selevan et al., 1985[29]). The report of learning disabilities in children of nurses who had handled chemotherapy drugs was provided by a study of Martin (2005[23]). Numerous studies have been published that have demonstrated contamination of workplace with antineoplastic drugs (Hedmer et al., 2005[15], 2008; Zeedijk et al., 2005[39]; Mason et al., 2003[25]; Maeda et al., 2010[22]; Acampora et al., 2005[1]), use of analytical methods to determine contamination concentration level in surface (Turci et al., 2002[34]) and evaluation of pattern of use of personal protective equipment and compared with current guidelines (Martin and Larson, 2003[24]; Polovich and Martin, 2011[28]; Krstev et al., 2003[20]). Hepatic injuries can also develop, but some other studies have failed to support a relation between exposure and this particular problem. During the past 30 years, professional organizations and government agencies have developed guidelines to protect health care workers from adverse effects of occupational exposure to antineoplastic drugs. The occupational exposure to antineoplastic drugs is directly linked to the poor effectiveness of cleaning procedures or indeed the solutions used for cleaning the work surface (Lé et al., 2013[21]). Various agencies and organizations include the US Department of Labor’s Occupational Safety and Health Administration (US Department of Labor, 1995[36]), the National Institute for Occupational Safety and Health (NIOSH, 1988[27]), the Oncology Nursing Society (Brown et al., 2001[8]), the American Society of Health System Pharmacists (ASHP[5]), and the American Medical Association’s Council on Scientific Affairs (AMA, 1985[3]) do jointly express concerns about failure in cleaning procedures and recommend a need for validated cleaning protocol standards.
Previous studies of outpatient and office-based health care workers in Iran show weak compliance of their practice with safe-handling guidelines (Ansari Lari et al., 2001[4]). In addition, since nurses and medical doctors have been preparing these cytotoxic drugs in the open spaces of wards in the absence of appropriate garments and personal protective equipment, it is important to evaluate the current system and the risks associated with the exposure to the cytotoxic drugs in these centers.
Materials and Methods
Site observation
The visiting report was conducted at various specialized cancer centers in Tehran, in order to determine the severity of occupational hazards. The exposure of health care workers to antineoplastic drugs during preparation and administration was the main consideration. This study was one of the largest studies in its own right as nine professional and specialized cancer centers in Tehran (governmental and private centers) were selected. Although, there are in excess of thirty five known cancer treatment centers in Tehran but these selected centers are chosen purely based on the fact that they had the participant’s recruitment criteria for our study and are known as the most reputable cancer centers with good practicing habits in the capital city. These centers were studied through an on-the-spot investigation and data were analyzed in terms of being consisted and close to the standards. Observations were conducted in order to predict how and where (at what stages) nurses would be exposed to antineoplastic drugs.
Population recruitment
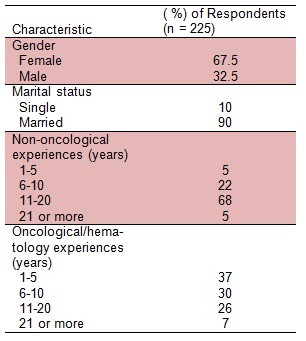
A total number of 225 rotational nursing staffs were provided with the questionnaire, among 152 women and 73 men. All the participants were working at least for 12 months as a fully registered nurse, rotating in “oncology/hematology” for a period of no less than six months compared in parallel with the nurses from “non-oncological” wards with no exposure to the cytotoxic drugs. The participants in both groups were approximately similar in terms of sex and number of years of experience with the median age of 37.3 years old. Exclusion criteria were: active drinkers or smokers, known uncontrolled chronic medical conditions (cardiac problems, diabetes, arthritic related disorders and respiratory diseases), bleeding disorders, any past cancer diagnosis or active complicated medical or surgical therapies in the past 12 months. The aim of this stringent selection in terms of health and age was to avoid any bias or any unrelated symptoms reporting by the nursing staff. Demographic details of all participants in this study are shown in Table 1(Tab. 1).
Table 1. Demographic information of nursing staff participating in the study.
Questionnaire
The questionnaire was in two parts and based on the most reputable international guidelines such as American Society of Health System Pharmacists (ASHP), the Occupational Safety and Health Administration (OSHA) and the Health & Safety Executive (HSE-UK) guidelines for handling cytotoxic drugs in clinical settings. First part was aimed to establish the participant’s understandings of hazards associated with their jobs and existence or lack of resources to help them to minimize such risks. The se¬cond part was aimed at gathering any reported side effects which may be due to acute or chronic exposure to cytotoxic. Acute side effects were classified as irritation of mucous membranes, eyes and skin, dizziness, headache, nausea and vomiting. The questionnaire had to be specific without causing any concerns for participants when answering the questions. The questions had to be translated from English to Persian without any loss of meaning, therefore, expert opinions were considered and once the translation was completed, the questionnaire was validated. The validation process was simply by various pilot studies, cross referencing to clarify the participant’s understandings and ultimately analyzing the findings and comparison against the guidelines recommendations.
Surface sampling and analysis
Surface wipe samples were taken from the surface of the cabinets or isolators (where the drugs were prepared) before and after cleaning, as recommended by the international standards. The method was adopted from previous works (Ziegler et al., 2002[40]). The samples from surfaces were taken by wiping thoroughly the surface with sterile gauze (10 cm×10 cm) which had been wetted with 5 ml of 30 mM sodium hydroxide. All samples were collected into the separate sealed plastic boxes and kept at -18 °C before the analysis. The formic acid with the PH of 3 (5 ml) was added to each sample. The prepared samples were then sonicated and injected in to the HPLC. Analyses were carried out with the HPLC system model SPDM-10ADvp from Shimadzu (Kyoto, Japan) consisting of LC-10Advp binary pumps, a SCL-10Avp controller, a SPD-M10Avp PDA detector and a DGU-14A degasser model and operated with Class-VP software (Shimadzu Scientific instruments, Inc.). Separations were done on a C18-M51002546 250 × 4.6 mm column from Hector (Venture Town, South Korea). The mobile phase used for chromatography was formic acid with the PH of 3, with a wavelength of 200 and flow rate of 1.4. The selectivity of the method and accurate recognition of the substance (5-FU) was verified by the UV spectra.
Deactivator or cleaning solutions
We are proposing that one of the reasons for exposure to these drugs is the lack of suitable deactivator or cleaning solution in our investigated centers or indeed the lack of existence of standard cleaning protocols. New combinations of deactivating and cleaning agents were used and comparison between the current brands and recommended cleaning solutions were made. The most used agents are Na Hypochlorite, sterile water, isopropyl alcohol 70 %, hydrogen peroxide and alkaline cleaning agents. These solutions were used alone or in combination with each other. The surface sampling was done before and after cytotoxic drugs preparations using the identical standard operating procedure for cleaning the surface. The results were then compared bearing in mind the variations in cleaning solutions and following a proper cleaning standard operating procedure.
Chemicals and materials
5-Fluorouracil was purchased from Kocak Farma (Tekirdag, Turkey). Sodium hydroxide was from Merck KGaA (Darmstadt, Germany). Formic acid was obtained from Merck Schuchardt OHG (Hohenbrunn, Germany). Na Hypochlorite was purchased from Tizpak (Khorasan, Iran), Isopropyl Alcohol was from Sepidaj (Tehran, Iran), hydrogen peroxide was from Merck KGaA (Darmstadt, Germany) and Triethanolamine was from Sinchem (Dongguo, Tengzhou, Shandong, China).
Results
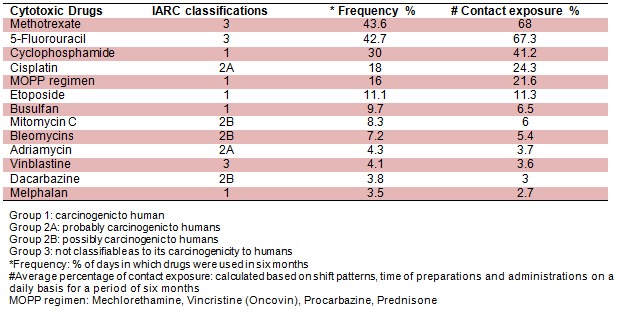
In this cross-sectional study, the site observation was performed on multiple days and on different individuals in order to determine the risk of exposure, and the potential contaminated work surfaces at each side of the hospital. List of all cytotoxic drugs used in these centers were obtained and the most common ones were classified based on International Agency for Research on Cancer (IARC, 1981[17]) (Table 2(Tab. 2)).
Table 2. Classification of common cytotoxic agents based on IARC.
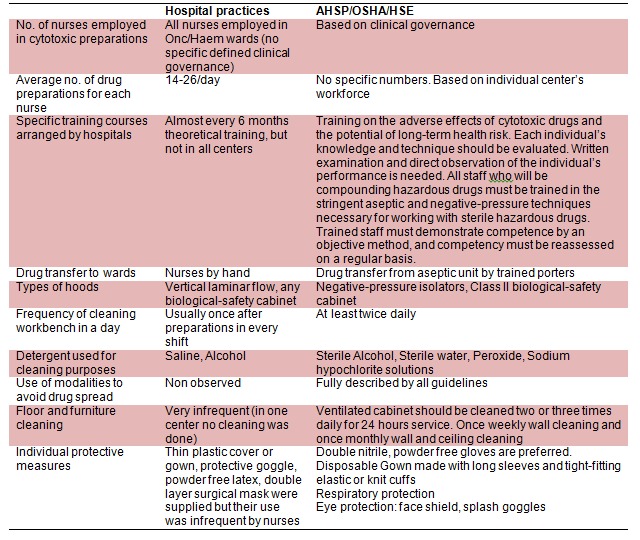
The work force studies were carried out in each center and compared with the recommendations by the guidelines. Recommendations on training, transport, type of isolators and maintenance of the clean room were compared with our centers (Table 3(Tab. 3)).
Table 3. Comparison of recommendations by the guidelines against practices in cancer centers.
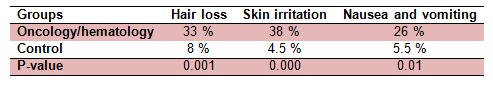
Using the validated questionnaire, we aimed to investigate the prevalence of acute adverse effects. The oncology/hematology rotational nursing staffs (rotation in various medical/surgical as well as oncology/hematology specialties) were compared in parallel to the “non-oncology” nursing staff referred as a control group. An acute adverse effect such as skin irritation is the most reported one (38 %) by the nurses working in the oncology units in comparison to the control. Skin irritation could be any rash and skin redness (dermatitis like reactions) while working with cytotoxic agents. In all cases nurses confirmed that they had no other reasons or life style changes to be the cause for such reactions. Other reported acute adverse effects by this group of nurses in order were hair loss with 33 % of the nurses reporting and nausea and vomiting with 26 %. The questions presented to the nurses were; “In general, have you experienced more episodes of hair loss while working in your current wards?” and “In general, have you experienced more episodes of nausea and vomiting while working in your current ward?” The results of comparing symptoms associated with occupational exposure to chemotherapy agents in both, exposed and control group are indicated in Table 4(Tab. 4).
Table 4. Acute adverse effects reported by nursing staff using questionnaire.
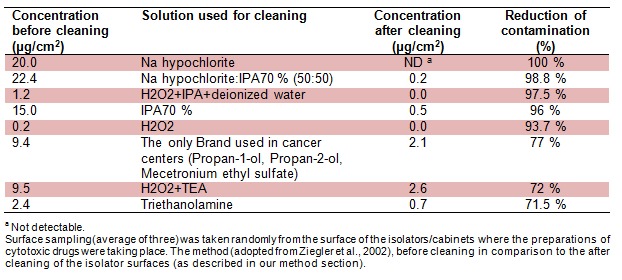
One of the reasons for exposure could be due to use of inappropriate cleaning solutions. Therefore, the common cleaning and deactivating solutions were used to compare the ability for reduction of contamination. Sodium hypochlorite was the only solution with the highest level of reduction and its combination with sterile IPA 70 % in equal parts was the next one in line. The least level of reduction was by using the Triethanolamine with the reduction value of 71.5 %. The only “cleaning solution brand” used in the cancer centers contains Propan-1-ol, Propan-2-ol, Mecetronium ethyl sulfate with the ability of contamination reduction of 77 % (Table 5(Tab. 5)).
Table 5. Comparison of common cleaning solutions using the HPLC method.
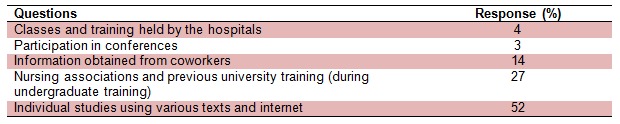
In order to investigate the training arrangements and the sources of such trainings, the questionnaire included both direct and open questions to establish the exact sources. 52 % of nurses received information about cytotoxic drugs from text books and internet, 27 % from nursing associations and previous undergraduate training. Training obtained from nursing co-workers was reported by 14 % of the nurses and participation in conferences was 3 %. Respectively, a minute level, 4 % of the nurses received training program from the clinical settings directly while working as a nursing staff (Table 6(Tab. 6)).
Table 6. Training methods on handling, manufacturing, administrating and disposing cytotoxic agents for nursing staff.
There was very limited training materials offered for nursing staff to read on or indeed any specific continues profession development (CPD). The phrase “CPD” was unfamiliar to 63 % of the registered nursing staff participating in our investigation. However, they all acknowledged that professional development was always strongly recommended by the university lecturers and by many medical professional working alongside of nurses such as pharmacists and medical doctors. The lack of time, encouragement and limitation in number of staffing was a major reason for not being able to take on further CPD shared by all the participants.
Discussion
To the best of our knowledge the presented manuscript is one of the most extensive investigational studies in its own right including the large number of participants and tackling occupational exposure in the healthcare setting. The selected cancer centers in the capital city of Iran were offering the appropriate participants as well as being known as the premium cancer centers. The aim of this investigation was to evaluate the current practices of nursing staff working in the oncology units and the level of exposure to cytotoxic agents. This study was objective in all aspects and was carried out in four different parts;
(i)To investigate the procedure of cytotoxic drug preparation and administration in comparison to the guidelines
(ii)Assess the level of training and knowledge of the nurses on risks associated with cytotoxic exposure and their actual usage of safety measures
(iii) Investigate and compare the level of acute side effects that were associated with occupational exposure to chemotherapy agents in comparison to those working in non-oncological units
(iv) Evaluate the current cleaning procedures and cleaning solutions against the recommended suitable cleaning and deactivator solution.
Increasing utilization of chemotherapeutic agents in treating patients with malignancy has led to the potential for widespread exposure of healthcare workers who come into contact with patients or these agents in the work place. Unfortunately, these drugs are toxic to both the abnormal and normal somatic cells. This occurs in the patient, and is also likely to affect any individual who is exposed. There appear to be widely divergent opinions concerning the extent of hazard of coming into contact with these agents, in spite of which caution and minimizing exposure risk seems only prudent and appropriate. The known cytotoxic agents are classified by the International Agency for Research on Cancer based on the carcinogenicity to the human due to exposure (Table 2(Tab. 2)). Consumption or administration, direct contact and inhalation are referred as the main exposure routes (IARC, 1981[17]). Our results show for instance, nursing staff (working 40 hours a week for a period of six months) are exposed to group 1 classified agents (carcinogenic to human) in the oncology units with contact exposure between 2.7 % for preparing Melphalan to 41.2 % for preparing Cyclophosphamide. Therefore, initially it is suggested that factors in preparation area like hood type, coverage plan, inlet and outlet filters, and filters replacement are important to reduce the exposure (Kopjar et al., 2009[18]). All employees exposed to chemotherapy agents require wearing the job specific re¬commended level of PPE as the guidelines. Furthermore, the cleaning procedures condition in the preparation and administration areas like appropriate space, exclusive use, access restrictions, presence of warning signs, dressing room, sinks, eyewash, shower, adequate lightening and ventilation, microclimate conditions, intact floor and walls are also important to be considered (Sessink et al., 1994[30]).
Our results indicate that the level of knowledge of the nurses on the risks of exposure is satisfactory. However, the usages of safety measures are not as recommended by the mentioned guidelines (Table 3(Tab. 3)). Similarly, other studies have reported that nurses handling the cytotoxic drugs don’t have satisfactory level of knowledge about the risks (Habib et al., 1992[14]). The lack of knowledge on preventive measures is very much the point of concern as it increases the health workers’ health and safety. Usually by participating in training programs, the level of knowledge will significantly improve and the safe practice is more likely to happen (Sessink et al., 1997[31]). Furthermore, occupational safety and health administration guidelines state that training of all staff involved in any aspect of the handling of hazardous drugs is essential (IARC, 1981[17]).
The nurses’ safe practice and the usage of recommended health safety measures are directly associated with the individual beliefs rather than the rules and regulations pertaining to the cytotoxic drugs. Other studies had similar findings where beliefs about “what protection was required” had stronger correlation with actual use than policy content in relation to the cytotoxic protection (Martin and Larson, 2003[24]). However, eating or drinking was strictly forbidden in the preparation room or indeed anywhere cytotoxic drugs were handled or prepared. In comparison, other studies did disclose that eating or even smoking was observed in the similar clinical settings (Aydemir et al., 2003[6]). The findings about the use of personal protective equipment showed that all of the nurses were using some protective equipment necessary during the handling of cytotoxic drugs. For instance, all nurses were using gloves, masks and gown when preparing the chemotherapeutic agents albeit not the exact recommended types by the guidelines (Al-Ghamdi and Al-Mustafa, 1997[2]).
Questionnaire data on work practices, potential exposure, extent of side effects, use of protective personal equipment and relevant training were collected from all grades of nursing staff and evaluated. For occupational health services it is important to have sensitive and specific methods for monitoring exposure to cytotoxic drugs (Kopjar et al., 2009[18]). The medical surveillance program should include general health questionnaires that should be completed upon hire, periodically (such as yearly), and at job termination. In our centers and in many developing countries no such questionnaires were offered, however, when such questionnaires were offered, no objections were made by the clinical setting’s director (Chaudhary and Karn, 2012[10]). Therefore, this could be argued that this level of ignorance is not intentional and is due to lack of knowledge or resources to deal with this type of issues. In majority of developed countries, clinical or hospital pharmacists are heavily and actively involved in staff training and aseptic manufacturing activities and management (Castiglia et al., 2008[9]). The questionnaires were used to identify the level of exposures and reported acute side effects. In our recruited population, one of the most common acute side effects such as skin irritations were reported in 38 % of all oncology/hematology group in comparison to only 4.5 % of the reported cases in control group. Likewise, other acute side effects such as hair loss as well as nausea and vomiting were significantly higher (Valanis et al., 1993[37]). This extraordinary level of acute adverse reporting was due to the fact that participating oncology/hematology nursing staff were also rotating in various specialties and each were comparing their own adverse effect experiences between oncology and non-oncology rotations (Table 4(Tab. 4)). In similar pattern with other studies, the most prominent reported side effect was skin irritation when the same number of female participants were considered (Valanis et al., 1993[37]). In male participants, the hair loss was the most reported side effects with the average number of 8 % of the male nurse participants. Overall, the number of oncology/hematology nurses complaining of acute side effects due to occupational exposure to drugs in male and female participants was significantly higher when compared against those in control group (Valanis et al., 1993[38], [37]). Due to its importance, in many developed countries following the strict in house guidelines a complete blood count with differential and additional tests such as liver function test are required for those at risk of cytotoxic exposure (Sessink et al., 1994[30]).
The contamination rate of 5FU as the most commonly used drug in these centers was measured. The sample locations included places in the cytotoxic production area such as working surfaces, in the storage and checking room, refrigerator and storage shelves. The traces of 5FU were commonly detected in our samples. Most of the contamination was found inside the laminar flow or safety cabinets (Ziegler et al., 2002[40]). This finding could be expounded by the facts that nurses are not trained and monitored for their expertise on reconstitutions of cytotoxic drugs and not using the appropriate cleaning solutions (Table 5(Tab. 5)). In similar studies (Acampora et al., 2005[1]; Castiglia et al., 2008[9]), it was proposed that the nursing staff were exposed to the cytotoxic surface contamination due to the haphazard working practices and cleaning procedures.
The health care setting managers do not seem to be acting proactively when it comes to staff training and in some studies it has been suggested that the hospitals do the minimum requirement to get away with the regulations (Sessink et al., 1994[30]; Krstev et al., 2003[20]). The main source of information for more than half of participants (52 %) remains to be the individual readings either from the text books or searching through the internet (Table 6(Tab. 6)). This level of reliant can be very risky as the extent of searches can vary between the individual nursing staff and majority of available information are in English language where many nurses are unfamiliar with it. The staff training should be conducted before beginning duties, at least yearly or more often if deficiencies are observed. The level of detail and complexity of training should be tailored to the specific job area.
This study suggests that although the exact existing guidelines are not compatible to be followed in our centers, but with some modifications, major improvements will be possible. Data from the validated questionnaires are strongly linking the risk of occupational hazard and current practices. The reported adverse effects as a result of current exposure are apparent. In order to reduce the contamination in the working areas, immediate actions should be taken. More efficient cleaning procedure with the use of proper cleaning solvents and enhanced cleaning frequencies should be offered. Ultimately, practical suggestions and training from the clinical and aseptic pharmacists will significantly improve the current system.
Acknowledgements
We would like to thank all medical, clinical and nursing teams who have supported this research throughout.
Declaration
Dr. Mehdi Rajabi is a visiting lecturer, a member of General Pharmaceutical Council of Great Britain and also a freelance clinical pharmacist in various teaching hospitals in UK.
Conflict of interest
None.
References
- 1.Acampora A, Castiglia L, Miraglia N, Pieri M, Soave C, Liotti F, et al. A case study: surface contamination of cyclophosphamide due to working practices and cleaning procedures in two Italian hospitals. Ann Occup Hyg. 2005;49:611–618. doi: 10.1093/annhyg/mei029. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghamdi MS, Al-Mustafa ZH. The handling of anticancer drugs in Riyadh and the Eastern Province. Ann Saudi Med. 1997;17:257–259. doi: 10.5144/0256-4947.1997.257. [DOI] [PubMed] [Google Scholar]
- 3.AMA, American Medical Association. Guidelines for handling parenteral antineoplastic. Council on Scientific Affairs. American Medical Association. JAMA. 1985;253:1590–1592. [PubMed] [Google Scholar]
- 4.Ansari Lari M, Saadat M, Shahryari M, Farhud DD. Sister chromatid exchanges and micronuclei in lymphocyte of nurses handling antineoplastic drugs. Iranian J Publ Health. 2001;30:37–40. [Google Scholar]
- 5.ASHP, American Society of Hospital Pharmacists. ASHP technical assistance bulletin on handling cytotoxic drugs in hospitals. Am J Hosp Pharm. 1985;42:131–137. [Google Scholar]
- 6.Aydemir G, Sogukpinar N, Türkistanli EC. Prevention and health education: how recent advances in the science and art of health education have been applied in practical ways within medical and other settings for prevention and public health. Asian Pac J Cancer Prev. 2003;4:71–74. [PubMed] [Google Scholar]
- 7.Barton-Burke M, Wilkes G. Cancer therapies. Sudbury, MA: Jones and Bartlett Publ; 2006. [Google Scholar]
- 8.Brown KA, Esper P, Kelleher LO, Brace O’Neill JE, Polovich M, et al. Chemotherapy and biotherapy: guidelines and recommendations for practice. Pittsburgh, PA: Oncology Nursing Press; 2001. [Google Scholar]
- 9.Castiglia L, Miraglia N, Pieri M, Simonelli A, Basilicata P, Genovese G, et al. Evaluation of occupational exposure to antiblastic drugs in an Italian hospital oncological department. J Occup Health. 2008;50:48–56. doi: 10.1539/joh.50.48. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary R, Karn B. Chemotherapy-knowledge and handling practice of nurses working in a medical university of Nepal. J Cancer Ther. 2012;3:110–114. [Google Scholar]
- 11.Connor TH, McDiarmid MA. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J Clin. 2006;56:354–65. doi: 10.3322/canjclin.56.6.354. [DOI] [PubMed] [Google Scholar]
- 12.Falck K, Grohn P, Sorsa M, Vainio H, Heinonen E, Holsti LR. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet. 1979;1(8128):1250–1251. doi: 10.1016/s0140-6736(79)91939-1. [DOI] [PubMed] [Google Scholar]
- 13.Gambrell J, Moore S. Assessing workplace compliance with handling of antineoplastic agents. Clin J Oncol Nurs. 2006;10:473–477. doi: 10.1188/06.CJON.473-477. [DOI] [PubMed] [Google Scholar]
- 14.Habib C, Karam S, Khaled H, Rustom R, Gueutcherian Y, Akatcherian R, et al. Handling of antineoplastic products and nurses' knowledge. J Med Liban. 1992;40:182–186. [PubMed] [Google Scholar]
- 15.Hedmer M, Georgiadi A, Bremberg ER, Jonsson BA, Eksborg S. Surface contamination of cyclophosphamide packaging and surface contamination with antineoplastic drugs in a hospital pharmacy in Sweden. Ann Occup Hyg. 2005;49:629–637. doi: 10.1093/annhyg/mei042. [DOI] [PubMed] [Google Scholar]
- 16.Hedmer M, Tinnerberg H, Axmon A, Jonsson BA. Environmental and biological monitoring of antineoplastic drugs in four workplaces in a Swedish hospital. Int Arch Occup Environ Health. 2008;81:899–911. doi: 10.1007/s00420-007-0284-y. [DOI] [PubMed] [Google Scholar]
- 17.IARC, International Agency for Research on Cancer, editor. Some antineoplastic and immunosuppressive agents. Lyon: IARC; 1981. pp. 370–384. (IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans, Vol. 26). [PubMed] [Google Scholar]
- 18.Kopjar N, Kasuba V, Rozgaj R, Zeljezic D, Milic M, Ramic S, et al. The genotoxic risk in health care workers occupationally exposed to cytotoxic drugs - a comprehensive evaluation by the SCE assay. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:462–479. doi: 10.1080/10934520902719845. [DOI] [PubMed] [Google Scholar]
- 19.Kopjar N, Zeljezić D, Kasuba V, Rozgaj R. Antineoplastic drugs as a potential risk factor in occupational settings: mechanisms of action at the cell level, genotoxic effects, and their detection using different biomarkers. Arh Hig Rada Toksikol. 2010;61:121–146. doi: 10.2478/10004-1254-61-2010-2025. [DOI] [PubMed] [Google Scholar]
- 20.Krstev S, Perunicic B, Vidakovic A. Work practice and some adverse health effects in nurses handling antineoplastic drugs. Med Lav. 2003;94:432–439. [PubMed] [Google Scholar]
- 21.Lé LMM, Jolivot PA, Yaye HS, Rieutord A, Bellanger A, Pradeau D, et al. Effectiveness of cleaning of workplace cytotoxic surface. Int Arch Occup Environ Health. 2013;86:333–41. doi: 10.1007/s00420-012-0769-1. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Miyawaki K, Matsumoto S, Oishi M, Miwa Y, Kurokawa N. Evaluation of environmental contaminations and occupational exposures involved in preparation of chemotherapeutic drugs. Yakugaku Zasshi. 2010;130:903–910. doi: 10.1248/yakushi.130.903. [DOI] [PubMed] [Google Scholar]
- 23.Martin S. The Oncology Nursing Society 30th Annual Congress, April 28–May 1, 2005. 2005. Chemotherapy handling and effects among nurses and their offspring; p. abstr. 13. [Google Scholar]
- 24.Martin S, Larson E. Chemotherapy-handling practices of outpatient and office-based oncology nurses. Oncol Nurs Forum. 2003;30:575–581. doi: 10.1188/03.ONF.575-581. [DOI] [PubMed] [Google Scholar]
- 25.Mason HJ, Morton J, Garfitt SJ, Iqbal S, Jones K. Cytotoxic drug contamination on the outside of vials delivered to a hospital pharmacy. Ann Occup Hyg. 2003;47:681–685. doi: 10.1093/annhyg/meg078. [DOI] [PubMed] [Google Scholar]
- 26.Moretti M, Bonfiglioli R, Feretti D, Pavanello S, Mussi F, Grollino MG, et al. A study protocol for the evaluation of occupational mutagenic/carcinogenic risks in subjects exposed to antineoplastic drugs: a multicentric project. BMC Public Health. 2011;11:195. doi: 10.1186/1471-2458-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NIOSH, National Institute for Occupational Safety and Health, editor. Guidelines for protecting the safety and health of health care workers. (DHHS (NIOSH) Publication No. 88-119). [Google Scholar]
- 28.Polovich M, Martin S. Nurses' use of hazardous drug-handling precautions and awareness of national safety guidelines. Oncol Nurs Forum. 2011;38:718–726. doi: 10.1188/11.ONF.718-726. [DOI] [PubMed] [Google Scholar]
- 29.Selevan SG, Lindbohm ML, Hornung RW, Hemminki K. A study of occupational exposure to antineoplastic drugs and fetal loss in nurses. N Engl J Med. 1985;313:1173–1178. doi: 10.1056/NEJM198511073131901. [DOI] [PubMed] [Google Scholar]
- 30.Sessink PJ, van de Kerkhof MCA, Anzion RB, Noordhoek J, Bos RP. Environmental contamination and assessment of exposure to anti-neoplastic agents by determination of cyclophosphamide in urine of exposed pharmacy technicians: is skin absorption an important exposure route? Arch Environ Health. 1994;49:165–9. doi: 10.1080/00039896.1994.9940377. [DOI] [PubMed] [Google Scholar]
- 31.Sessink PJ, Wittenhorst BC, Anzion RB, Bos RP. Exposure of pharmacy technicians to antineoplastic agents: reevaluation after additional protective measures. Arch Environ Health. 1997;52:240–244. doi: 10.1080/00039899709602893. [DOI] [PubMed] [Google Scholar]
- 32.Suspiro A, Prista J. Biomarkers of occupational exposure do anticancer agents: a minireview. Toxicol Lett. 2011;207:42–52. doi: 10.1016/j.toxlet.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Terui K, Okajima H, Nakajima Y. Safety evaluation of new anticancer chemotherapy administration system: compared to the results from a former study. Gan To Kagaku Ryoho. 2011;38:1483–1487. [PubMed] [Google Scholar]
- 34.Turci R, Sottani C, Ronchi A, Minoia C. Biological monitoring of hospital personnel occupationally exposed to antineoplastic agents. Toxicol Lett. 2002;134:57–64. doi: 10.1016/s0378-4274(02)00163-7. [DOI] [PubMed] [Google Scholar]
- 35.Ursini CL, Cavallo D, Colombi A, Giglio M, Marinaccio A, Iavicoli S. Evaluation of early DNA damage in healthcare workers handling antineoplastic drugs. Int Arch Occup Environ Health. 2006;80:134–140. doi: 10.1007/s00420-006-0111-x. [DOI] [PubMed] [Google Scholar]
- 36.US Department of Labor; US Department of Labor, editor. OSHA Technical Manual. Washington, DC: Occupational Safety and Health Administration; 1995. Controlling occupational exposure to hazardous drugs; p. Section VI, chapter 2. [Google Scholar]
- 37.Valanis BG, Vollmer WM, Labuhn KT, Glass AG. Acute symptoms associated with antineoplastic drug handling among nurses. Cancer Nurs. 1993;16:288–295. [PubMed] [Google Scholar]
- 38.Valanis BG, Vollmer WM, Labuhn KT, Glass AG. Association of antineoplastic drug handling with acute adverse effects in pharmacy personnel. Am J Hosp Pharm. 1993;50:455–462. [PubMed] [Google Scholar]
- 39.Zeedijk M, Greijdanus B, Steenstra FB, Uges DR. Monitoring exposure of cytotoxics on the hospital ward: measuring surface contamination of four different cytostatic drugs from one wipe sample. Eur J Hosp Pharm Sci. 2005;11:18–22. [Google Scholar]
- 40.Ziegler E, Mason HJ, Baxter PJ. Occupational exposure to cytotoxic drugs in two UK oncology wards. Occup Environ Med. 2002;59:608–12. doi: 10.1136/oem.59.9.608. [DOI] [PMC free article] [PubMed] [Google Scholar]


