Abstract
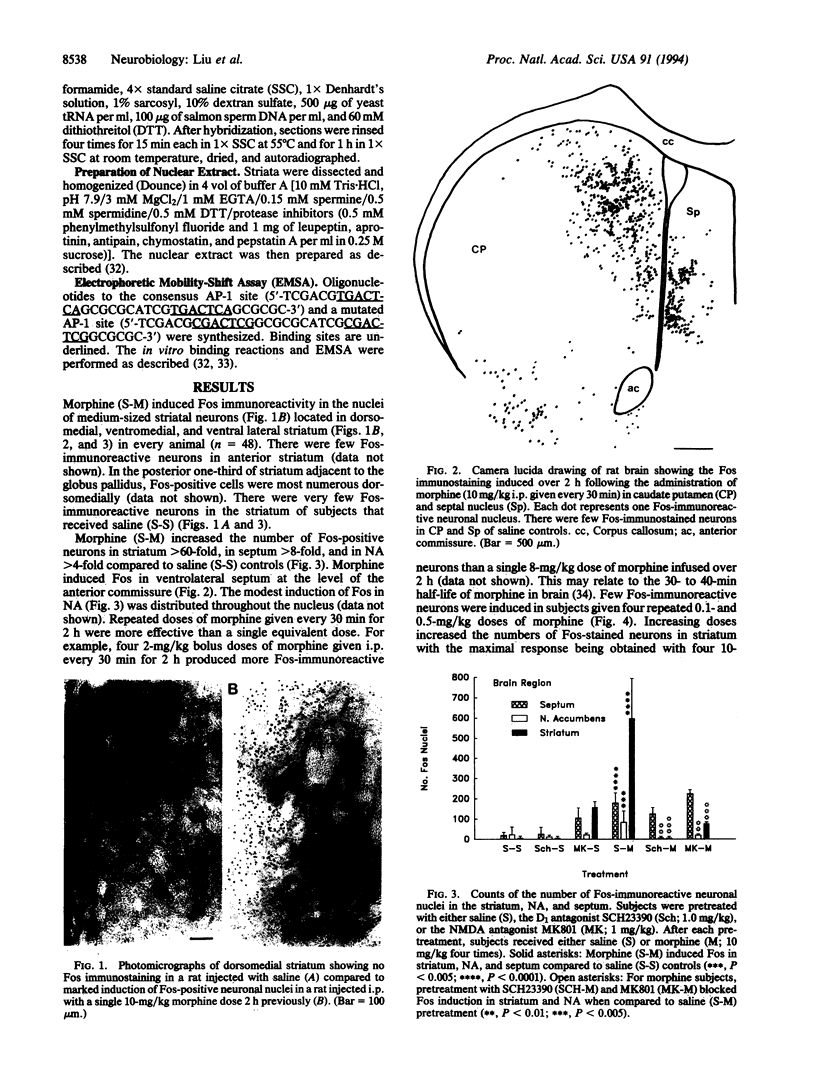
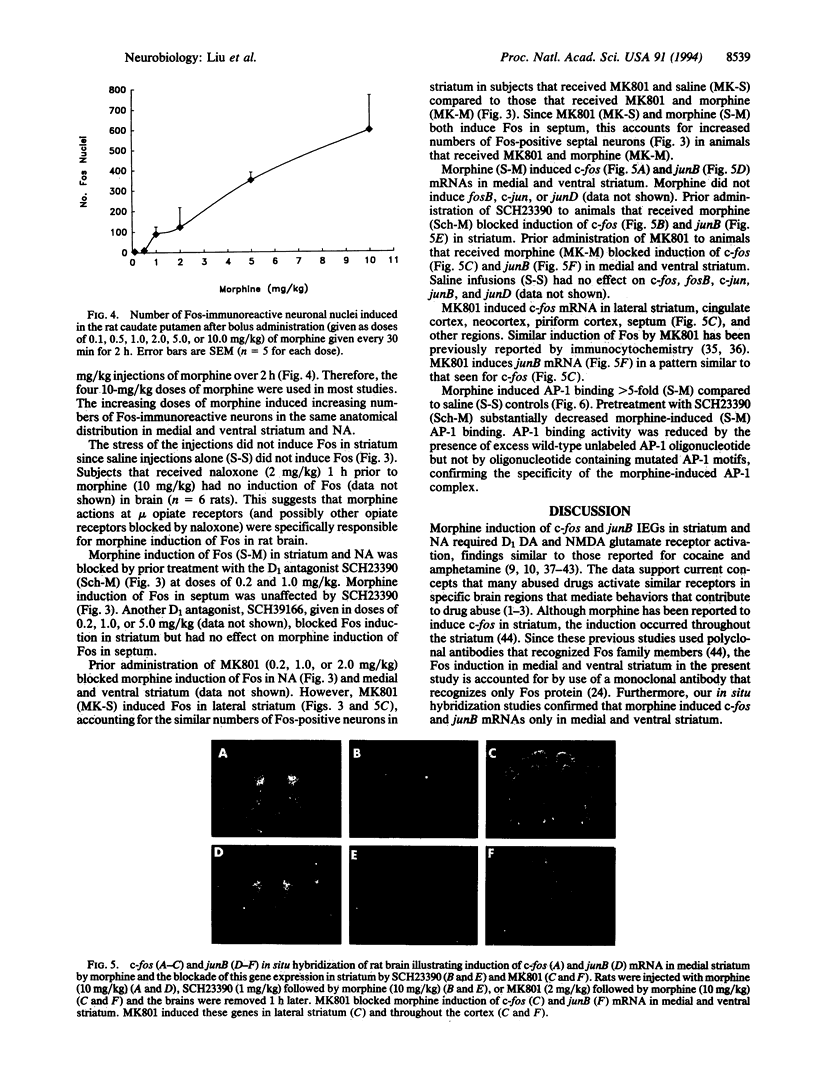
Morphine induced the c-fos and junB immediate early genes in neurons of the medial and ventral striatum and nucleus accumbens. Induction of c-fos and junB mRNA and Fos protein was blocked by naloxone, the D1 dopamine (DA) receptor antagonists SCH23390 and SCH39166, and the N-methyl-D-aspartate (NMDA) glutamate receptor antagonist MK801. SCH23390 attenuated morphine induction of AP-1 binding in striatum, suggesting that c-fos and junB contribute to AP-1 binding. SCH23390 and MK801 did not block morphine induction of c-fos and junB in septum. Since the morphine induction of c-fos and junB in striatum and nucleus accumbens (NA) was similar to that observed with cocaine and amphetamine, these data support current concepts that limbic striatum and NA are among the brain regions that mediate drug abuse. Furthermore, since DA and NMDA receptors may mediate opiate reward and opiate induction of c-fos and junB, the DA/NMDA regulation of c-fos and junB and their target genes may produce long-term changes in the striatal and NA circuits that contribute to opiate drug abuse.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Watson S. J., Young E., Lewis M. E., Khachaturian H., Walker J. M. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Bading H., Ginty D. D., Greenberg M. E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993 Apr 9;260(5105):181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Berretta S., Robertson H. A., Graybiel A. M. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992 Sep;68(3):767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Bhargava H. N., Villar V. M., Rahmani N. H., Larsen A. K. Time course of the distribution of morphine in brain regions and spinal cord after intravenous injection to spontaneously hypertensive and normotensive Wistar-Kyoto rats. J Pharmacol Exp Ther. 1992 Jun;261(3):1008–1014. [PubMed] [Google Scholar]
- Bischoff S., Heinrich M., Sonntag J. M., Krauss J. The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol. 1986 Oct 7;129(3):367–370. doi: 10.1016/0014-2999(86)90449-8. [DOI] [PubMed] [Google Scholar]
- Bouyer J. J., Park D. H., Joh T. H., Pickel V. M. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984 Jun 8;302(2):267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Bozarth M. A., Wise R. A. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984 May 4;224(4648):516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Caboche J., Rogard M., Besson M. J. Comparative development of D1-dopamine and mu opiate receptors in normal and in 6-hydroxydopamine-lesioned neonatal rat striatum: dopaminergic fibers regulate mu but not D1 receptor distribution. Brain Res Dev Brain Res. 1991 Jan 15;58(1):111–122. doi: 10.1016/0165-3806(91)90243-c. [DOI] [PubMed] [Google Scholar]
- Cenci M. Angela, Campbell Kenneth, Wictorin Klas, Björklund Anders. Striatal c-fos Induction by Cocaine or Apomorphine Occurs Preferentially in Output Neurons Projecting to the Substantia Nigra in the Rat. Eur J Neurosci. 1992;4(4):376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Chang S. L., Squinto S. P., Harlan R. E. Morphine activation of c-fos expression in rat brain. Biochem Biophys Res Commun. 1988 Dec 15;157(2):698–704. doi: 10.1016/s0006-291x(88)80306-1. [DOI] [PubMed] [Google Scholar]
- Chipkin R. E., Iorio L. C., Coffin V. L., McQuade R. D., Berger J. G., Barnett A. Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther. 1988 Dec;247(3):1093–1102. [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Daunais J. B., Roberts D. C., McGinty J. F. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993 May;4(5):543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- De Togni P., Niman H., Raymond V., Sawchenko P., Verma I. M. Detection of fos protein during osteogenesis by monoclonal antibodies. Mol Cell Biol. 1988 May;8(5):2251–2256. doi: 10.1128/mcb.8.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T., Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993 Mar;7(3):479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G., North R. A. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992 May;13(5):185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Dilts R. P., Jr, Helton T. E., McGinty J. F. Selective induction of Fos and FRA immunoreactivity within the mesolimbic and mesostriatal dopamine terminal fields. Synapse. 1993 Mar;13(3):251–263. doi: 10.1002/syn.890130308. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. L. MK-801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett. 1990 Mar 26;111(1-2):39–45. doi: 10.1016/0304-3940(90)90341-6. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr, Sibley D. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990 Dec 7;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., McGinty J. F., Young W. S., 3rd Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991 Apr;11(4):1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992 Apr;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- German D. C., Speciale S. G., Manaye K. F., Sadeq M. Opioid receptors in midbrain dopaminergic regions of the rat. I. Mu receptor autoradiography. J Neural Transm Gen Sect. 1993;91(1):39–52. doi: 10.1007/BF01244917. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Moratalla R., Robertson H. A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990 Jul;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Gysling K., Wang R. Y. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983 Oct 24;277(1):119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. D., Duman R. S., Nestler E. J. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Res. 1990 Aug 20;525(2):256–266. doi: 10.1016/0006-8993(90)90872-9. [DOI] [PubMed] [Google Scholar]
- Hisanaga K., Sagar S. M., Sharp F. R. N-methyl-D-aspartate antagonists block fos-like protein expression induced via multiple signaling pathways in cultured cortical neurons. J Neurochem. 1992 May;58(5):1836–1844. doi: 10.1111/j.1471-4159.1992.tb10060.x. [DOI] [PubMed] [Google Scholar]
- Hope B., Kosofsky B., Hyman S. E., Nestler E. J. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd Y. L., Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993 Apr;13(4):357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Bloom F. E. Cellular and molecular mechanisms of drug dependence. Science. 1988 Nov 4;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kornhauser J. M., Nelson D. E., Mayo K. E., Takahashi J. S. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992 Mar 20;255(5051):1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- Krebs M. O., Trovero F., Desban M., Gauchy C., Glowinski J., Kemel M. L. Distinct presynaptic regulation of dopamine release through NMDA receptors in striosome- and matrix-enriched areas of the rat striatum. J Neurosci. 1991 May;11(5):1256–1262. doi: 10.1523/JNEUROSCI.11-05-01256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaud P., Maggio R., Gale K., Grayson D. R. Temporal and spatial patterns of expression of c-fos, zif/268, c-jun and jun-B mRNAs in rat brain following seizures evoked focally from the deep prepiriform cortex. Exp Neurol. 1993 Jan;119(1):20–31. doi: 10.1006/exnr.1993.1003. [DOI] [PubMed] [Google Scholar]
- Laugwitz K. L., Offermanns S., Spicher K., Schultz G. mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993 Feb;10(2):233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- Leviel V., Gobert A., Guibert B. The glutamate-mediated release of dopamine in the rat striatum: further characterization of the dual excitatory-inhibitory function. Neuroscience. 1990;39(2):305–312. doi: 10.1016/0306-4522(90)90269-a. [DOI] [PubMed] [Google Scholar]
- Liu J. L., Chiles T. C., Sen R. J., Rothstein T. L. Inducible nuclear expression of NF-kappa B in primary B cells stimulated through the surface Ig receptor. J Immunol. 1991 Mar 1;146(5):1685–1691. [PubMed] [Google Scholar]
- Loh H. H., Smith A. P. Molecular characterization of opioid receptors. Annu Rev Pharmacol Toxicol. 1990;30:123–147. doi: 10.1146/annurev.pa.30.040190.001011. [DOI] [PubMed] [Google Scholar]
- Maldonado R., Robledo P., Chover A. J., Caine S. B., Koob G. F. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav. 1993 May;45(1):239–242. doi: 10.1016/0091-3057(93)90112-7. [DOI] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987 Aug;7(8):2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Marek P., Ben-Eliyahu S., Gold M., Liebeskind J. C. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res. 1991 Apr 26;547(1):77–81. doi: 10.1016/0006-8993(91)90576-h. [DOI] [PubMed] [Google Scholar]
- Martínez-Fong D., Rosales M. G., Góngora-Alfaro J. L., Hernández S., Aceves J. NMDA receptor mediates dopamine release in the striatum of unanesthetized rats as measured by brain microdialysis. Brain Res. 1992 Nov 13;595(2):309–315. doi: 10.1016/0006-8993(92)91065-m. [DOI] [PubMed] [Google Scholar]
- Matthews R. T., German D. C. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984 Mar;11(3):617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- McLean S., Rothman R. B., Herkenham M. Autoradiographic localization of mu- and delta-opiate receptors in the forebrain of the rat. Brain Res. 1986 Jul 16;378(1):49–60. doi: 10.1016/0006-8993(86)90285-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Portoghese P. S., Takemori A. E. Involvement of delta 2 opioid receptors in acute dependence on morphine in mice. J Pharmacol Exp Ther. 1993 Jun;265(3):1325–1327. [PubMed] [Google Scholar]
- Monstein H. J. Identification of an AP-1 transcription factor binding site within the human cholecystokinin (CCK) promoter. Neuroreport. 1993 Feb;4(2):195–197. doi: 10.1097/00001756-199302000-00020. [DOI] [PubMed] [Google Scholar]
- Moratalla R., Vickers E. A., Robertson H. A., Cochran B. H., Graybiel A. M. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. J Neurosci. 1993 Feb;13(2):423–433. doi: 10.1523/JNEUROSCI.13-02-00423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nakajima S., McKenzie G. M. Reduction of the rewarding effect of brain stimulation by a blockade of dopamine D1 receptor with SCH 23390. Pharmacol Biochem Behav. 1986 Apr;24(4):919–923. doi: 10.1016/0091-3057(86)90437-5. [DOI] [PubMed] [Google Scholar]
- Naranjo J. R., Mellström B., Achaval M., Lucas J. J., Del Rio J., Sassone-Corsi P. Co-induction of jun B and c-fos in a subset of neurons in the spinal cord. Oncogene. 1991 Feb;6(2):223–227. [PubMed] [Google Scholar]
- Naranjo J. R., Mellström B., Achaval M., Sassone-Corsi P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron. 1991 Apr;6(4):607–617. doi: 10.1016/0896-6273(91)90063-6. [DOI] [PubMed] [Google Scholar]
- Nestler E. J. Molecular mechanisms of drug addiction. J Neurosci. 1992 Jul;12(7):2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. S., Vincent S. R., Fibiger H. C. Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res. 1990 Jul 23;523(2):288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- Robertson H. A., Paul M. L., Moratalla R., Graybiel A. M. Expression of the immediate early gene c-fos in basal ganglia: induction by dopaminergic drugs. Can J Neurol Sci. 1991 Aug;18(3 Suppl):380–383. doi: 10.1017/s0317167100032480. [DOI] [PubMed] [Google Scholar]
- Robertson H. A., Peterson M. R., Murphy K., Robertson G. S. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res. 1989 Dec 4;503(2):346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Romualdi P., Lesa G., Ferri S. Chronic opiate agonists down-regulate prodynorphin gene expression in rat brain. Brain Res. 1991 Nov 1;563(1-2):132–136. doi: 10.1016/0006-8993(91)91525-6. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sharp F. R., Jasper P., Hall J., Noble L., Sagar S. M. MK-801 and ketamine induce heat shock protein HSP72 in injured neurons in posterior cingulate and retrosplenial cortex. Ann Neurol. 1991 Dec;30(6):801–809. doi: 10.1002/ana.410300609. [DOI] [PubMed] [Google Scholar]
- Sharp F. R., Sagar S. M., Hicks K., Lowenstein D., Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci. 1991 Aug;11(8):2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shippenberg T. S., Bals-Kubik R., Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993 Apr;265(1):53–59. [PubMed] [Google Scholar]
- Shippenberg T. S., Herz A. Motivational effects of opioids: influence of D-1 versus D-2 receptor antagonists. Eur J Pharmacol. 1988 Jul 7;151(2):233–242. doi: 10.1016/0014-2999(88)90803-5. [DOI] [PubMed] [Google Scholar]
- Sivam S. P. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989 Sep;250(3):818–824. [PubMed] [Google Scholar]
- Snyder-Keller A. M. Striatal c-fos induction by drugs and stress in neonatally dopamine-depleted rats given nigral transplants: importance of NMDA activation and relevance to sensitization phenomena. Exp Neurol. 1991 Aug;113(2):155–165. doi: 10.1016/0014-4886(91)90171-8. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T. S. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990 Nov;55(5):1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Steppuhn K. G., Turski L. Diazepam dependence prevented by glutamate antagonists. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6889–6893. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovero F., Herve D., Desban M., Glowinski J., Tassin J. P. Striatal opiate mu-receptors are not located on dopamine nerve endings in the rat. Neuroscience. 1990;39(2):313–321. doi: 10.1016/0306-4522(90)90270-e. [DOI] [PubMed] [Google Scholar]
- Trujillo K. A., Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991 Jan 4;251(4989):85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Walther D., Nishimori T., Buzzi M. G., Moskowitz M. A. Jun B, c-jun, jun D and c-fos mRNAs in nucleus caudalis neurons: rapid selective enhancement by afferent stimulation. Brain Res Mol Brain Res. 1991 Sep;11(2):133–141. doi: 10.1016/0169-328x(91)90115-e. [DOI] [PubMed] [Google Scholar]
- Wang J. K. Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. J Neurochem. 1991 Sep;57(3):819–822. doi: 10.1111/j.1471-4159.1991.tb08224.x. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Rompre P. P. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Young S. T., Porrino L. J., Iadarola M. J. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]