Abstract
Tuberculosis (TB) can present with various forms and can occasionally be mistaken for malignancy. Hereby, we report a 53-year-old man diagnosed and treated for Burkitt's lymphoma in 2009 who achieved a complete remission confirmed by a computed tomography (CT) scan. During the follow-up 2 years later, he complained of left hip pain that warranted investigation with magnetic resonance imaging and whole-body 18F-fludeoxyglucose-positron emission tomography (FDG-PET)/CT which showed a benign lesion in the left hip associated with multiple lymph nodes in the chest and abdomen not amenable for biopsy. A follow-up PET/CT scan a few months later showed intense tracer uptake in the lymph nodes with size progression and appearance of new lymph nodes suspicious of lymphoma relapse. The patient was asymptomatic, and all investigations including viral and connective tissue disease studies were negative. Also the tuberculin skin test and QuantiFERON were negative. Lymph node biopsy was planned; however, the patient presented a few days earlier with fever, headache and photophobia. Cerebrospinal fluid (CSF) examination confirmed meningitis with lymphocytic pleocytosis and elevated protein. The CSF Gram stain, culture, viral and acid-fast bacilli were negative. CSF flow cytometry and cytopathology confirmed polyclonal lymphocytosis and suggested reactive causes. CSF TB culture grew Mycobacterium tuberculosis. Mediastinal lymph node biopsy also confirmed TB lymphadenitis. Four antituberculosis drugs were started. One year later, a PET/CT scan showed regression of all the involved lymph nodes. This case highlights the importance of excluding TB in patients with suspected malignancy, especially if they belong to endemic regions, and the increasing role of 18F-FDG-PET/CT in the early detection of extrapulmonary TB.
Key Words: Tuberculosis meningitis, Lymphadenitis, Burkitt's lymphoma, Positron-emission tomography/computed tomography
Introduction
Tuberculosis (TB) remains a worldwide health problem causing morbidity and mortality particularly in the developing and low-income countries. There are several reports of TB occurring during or following the treatment of malignant hemopathy and stem cell transplantation [1, 2, 3]. TB, especially the extrapulmonary subtype, is reputed as one of the great mimickers of malignancy. We report a case of TB meningitis and lymphadenitis mimicking relapse of Burkitt's lymphoma.
Case Presentation
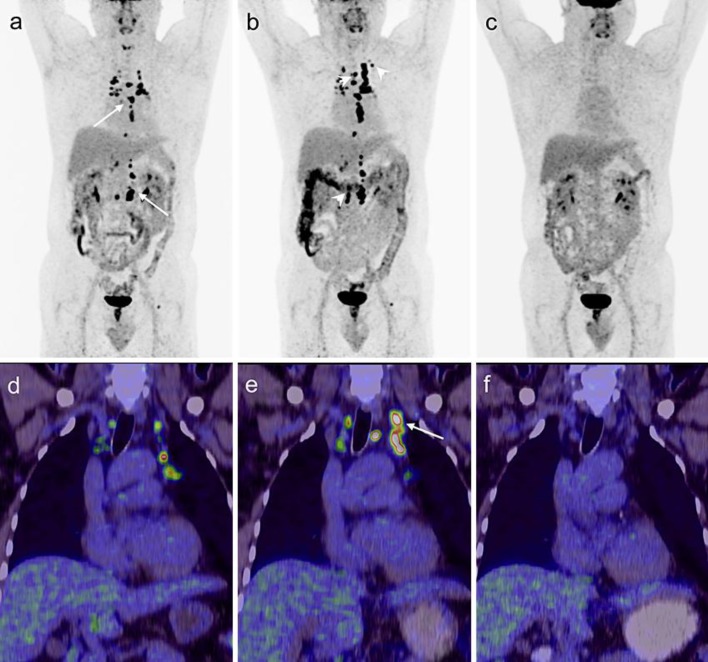
A 53-year-old Indian male known to have diabetes mellitus type 2 and hypertension was diagnosed at the end of 2009 with Epstein-Barr virus (EBV)-negative, stage IVB Burkitt's lymphoma with liver involvement. He was treated with a rituximab plus CODOX-M/IVAC regimen (CODOX-M: cyclophosphamide 800 mg/m2 on days 1 + 2, vincristine 1.4 mg/m2 on days 1 + 8, doxorubicin 50 mg/m2 on day 1, methotrexate 3,000 mg/m2 on day 10 and rituximab 375 mg/m2 on day 8; IVAC: ifosfamide 1,500 mg/m2 on days 1–5, etoposide 60 mg/m2 on days 1–5, cytarabin 2,000 mg/m2 on days 1 + 2 and rituximab 375 mg/m2 on day 4). He received four cycles and 6 treatments of intrathecal methotrexate 12 mg. The therapy was completed in March 2010, and he was in complete remission as evaluated using a computed tomography (CT) scan. During the routine follow-up at 15 months posttreatment, he was in a good general condition but complained of left hip pain. The CT scan of the whole body (July 19, 2011) revealed multiple subcentimetric lymph nodes in the mediastinal, retroperitoneal and para-aortic regions, and also showed the presence of an osteolytic lesion with a sclerotic margin at the left femoral head. The magnetic resonance imaging scan of the femoral head showed a geographical benign looking and slowly growing focal osseous lesion at the proximal left femoral metaphysis. The differential diagnosis included chondroma, fibrous dysplasia and chondromyxoid fibroma. The patient missed the 10-month follow-up. Subsequently, a follow-up 18F-fludeoxyglucose-positron emission tomography (FDG-PET)/CT scan in June 2012 (figure 1a, d) showed intense tracer uptake [standardized uptake value (SUV) 32.4] in several 8- to 18-mm-sized lymph nodes at the thoracic inlet, mediastinum (paravascular, paratracheal, subcarinal and retrocrural regions) and in the abdomen (para-aortic and paracaval regions). The known sclerotic lesion in the left femoral head showed no increased FDG uptake favoring benign origin. Clinically, he looked well and stable. Fever, night sweats, weight loss, palpable peripheral lymph node, hepatosplenomegaly and skin lesions were absent. The case was presented to the multidisciplinary team for suspicion of relapse. The decision was to rule out infection/connective tissue disease and to repeat imaging after 3–4 months because the lymph nodes were small and not accessible for CT-guided biopsy or surgery. His laboratory investigations including viral serology study for herpes simplex type 1, type 2, EBV, cytomegalovirus (CMV), human immunodeficiency virus, hepatitis B, hepatitis C and toxoplasmosis were negative. Serum polymerase chain reaction (PCR) for CMV and EBV was negative. TB workup including tuberculin skin test and QuantiFERON were negative. Autoimmune antinuclear antibody was negative. Complement (C3 and C4) tests and immunoglobulin levels (IgG, IgA and IgM) were normal. A repeat 18F-FDG-PET/CT scan in October 2012 (figure 1b, e) revealed an increase in the size of the previously reported lymph nodes and newly developed active lymph nodes on both sides of the diaphragm. The patient was booked for biopsy through mediastinoscopy. One week prior to the biopsy appointment, he presented to the emergency department with headache, vomiting and photophobia. On physical examination, the patient looked sick. He had a low-grade fever (37.8°C) and stiffness of the neck, but he was conscious, oriented and had no neurological deficit or cranial nerve involvement. The CT scan of the head was normal. The complete blood count revealed the following: white blood cell (WBC) 8.4 × 103/μl, neutrophil count 70%, lymphocyte count 30%, hemoglobin 14 g/dl, platelets 170 × 103/μl. Glucose (9.8 mmol/l), electrolytes, renal function, liver function, lactate dehydrogenase and coagulation workup were normal. Lumbar puncture (LP) showed clear cerebrospinal fluid (CSF). The CSF test revealed the following: WBC 622 × 103/μl, with 84% lymphocytes, 6% monocytes and 10% neutrophils, glucose 2 mmol/l and protein 2.5 g/l. Gram stains and CSF culture were negative. He was started on intravenous ceftriaxone and the next day was transferred to the hematology floor for suspicion of relapse of Burkitt's lymphoma of the central nervous system. Further investigations included magnetic resonance imaging of the brain, which showed mild diffuse meningeal enhancement. LP was repeated, and intrathecal treatment with 15 mg methotrexate and 50 mg hydrocortisone was given. LP showed clear CSF. The WBC count was 908 × 103/μl, with 96% lymphocytes and 4% neutrophils, glucose 2.5 mmol/l and protein 3.5 g/l. Gram stains and culture were negative; acid-fast bacilli smear and TB PCR were negative. The CSF PCR for CMV, EBV, adenovirus, herpes simplex type 1 and 2, varicella virus, enterovirus and mumps virus was negative.
Fig. 1.
a Restaging 18F-FDG-PET/CT maximum intensity projection image demonstrated multiple intensely hypermetabolic (SUVmax 40) mediastinal and retroperitoneal (arrows) small and moderately enlarged lymph nodes suggestive of lymphoma relapse. b Subsequent study revealed moderate progression with new FDG-avid lymph nodes (arrowheads) on both sides of the diaphragm but no pleuropulmonary involvement. c Posttherapy 18F-FDG-PET/CT performed after antitubercular treatment completion showed complete metabolic remission of the lymphadenitis. d–f Fused 18F-FDG-PET/CT of coronal sections performed at three time points presenting progression of the left upper mediastinal tuberculous nodal involvement (e, arrow) in comparison to the initial picture (d) and scan at the end of anti-TB treatment without any pathological FDG uptake in the mediastinum (f).
Flow cytometry showed polyclonal lymphocytosis, and CSF cytology showed reactive lymphocytosis. CSF findings of low glucose concentration, elevated protein and polyclonal lymphocytosis were more suggestive of TB meningitis than lymphoma; hence, he was started empirically on antituberculosis (a combination of four drugs: isoniazid, rifampicin, pyrazinamide and ethambutol) and steroid treatment. A third LP after 48 h of anti-TB showed a decrease in WBC count (182 × 103/μl, with 88% lymphocytes, 11% neutrophils and 1% monocytes). Bacterial Gram stain and culture were negative; acid-fast bacilli smear and PCR were negative. A CT scan on December 5, 2012, showed absence of pleuropulmonary lesions and the same findings as in the previous imaging. Mediastinal lymph node biopsy through mediastinoscopy confirmed the diagnosis of TB lymphadenitis. Bone marrow biopsy was normal and showed absence of granuloma, and TB culture on bone marrow was negative. Repeat tuberculin skin test, QuantiFERON and TB culture in the sputum were negative. CSF TB culture and TB PCR (GeneXpert) were positive, confirming the diagnosis of Mycobacterium tuberculosis, which is sensitive to conventional anti-TB. The diagnosis of TB meningitis and lymphadenitis was established, and the patient continued taking the antituberculosis drugs for 2 months followed by isoniazid and rifampicin for 10 more months. Repeat 18F-FDG-PET/CT at the end of treatment revealed a total regression of lymph nodes (figure 1c, f).
Discussion
TB infection is a serious and life-threatening complication in patients with malignant hemopathy and in recipients of bone marrow transplant, especially in endemic areas.
The increased risk of reactivation of latent TB could be explained by severely impaired cell-mediated immunity as a result of the underlining disease and its immunosuppressive treatment [1, 2, 3]. The real incidence of TB in hematological malignancies and stem cell transplantation is not well identified; radiological and epidemiological data are based on case reports and retrospective analyses with small cohorts of patients. In Taiwan, TB occurs in approximately 120 per 100,000 adult patients with hematological malignancy [3]. Extrapulmonary disease is common in patients with hematological disorders, ranging from 16 to 70% for all kinds of TB disease [3, 4].
TB can precede or be concomitant with the diagnosis of the malignancy or occur following treatment of malignant disorders [1, 3, 4]. The average time between the completion of cytotoxic chemotherapy and the development of TB is 18–20 months [4]. The timing between TB reactivation and development in patients with hematological malignancy is unknown.
In addition, the diagnosis of TB is often difficult to determine due to its atypical clinical presentation and radiological findings. TB meningitis accounts for 1% of all TB cases and for 6% of all extra pulmonary infections in immunocompetent patients [5]. It is a life-threatening complication; the morality rate remains high at 15–40% despite effective treatment [6].
In immunocompromised patients with malignant hemopathy, only few sporadic cases have been reported [7, 8]. In the present case, the occurrence of mediastinal and retroperitoneal hypermetabolic lymph nodes which gradually increased in size over 1 year followed by lymphocytic meningitis were highly suggestive and mistaken for a recurrence of Burkitt's lymphoma; however, the slow progression of the disorder and the small size of the lymph node was inconsistent with relapse of Burkitt's lymphoma, which is a highly proliferative disease.
The delay in the diagnosis of TB lymphadenitis after the first PET/CT scan was due to the small size of the lymph node that did not allow biopsy, the negative tuberculin skin test and QuantiFERON, and absence of pleuropulmonary lesion on the consecutive imaging.
The tuberculin skin test is usually negative in patients with malignant hemopathy because of the defect in the immunity related to the disease itself and to cytotoxic chemotherapy [1, 3, 4]. The QuantiFERON TB Gold In-Tube test reflects the release of interferon-γ and is highly recommended in the screening of latent TB in patients with malignant hemopathy and stem cell transplant; its sensitivity and specificity are around 75 and 81%, respectively [9]. Both tests were negative before and after confirmation of TB in our patient, reflecting the severe defect of his immunity. 18F-FDG-PET imaging has a well-documented lack of specificity in discriminating infection and inflammation from malignancy mainly due to the increased macrophage and neutrophil tracer uptake [10, 11, 12]. Differentiation between FDG-avid malignancies such as Burkitt's lymphoma and active TB based on SUVmax is not possible since they both demonstrate intense hypermetabolism with an overlap in SUV values when quantified [13]. Furthermore, the dual time point imaging could not differentiate malignant lymph node involvement from tuberculous lymphadenitis either [14]. For this reason, the 18F-FDG-PET/CT imaging characteristics of TB can be easily confused with malignancy, and therefore positive 18F-FDG-PET/CT results should be interpreted with caution when differentiating benign from malignant disease. The distribution of the 18F-FDG-avid foci in correlation with the clinical signs could suggest TB infection; however, in the case of a nonsymptomatic lymphadenitis and absence of pleuropulmonary lesions in a cancer patient who underwent immunosuppressive treatment, there are no definite investigational modalities to confirm the diagnosis other than histopathological examination and TB culture. In spite of its low specificity to diagnose TB, 18F-FDG-PET/CT plays an increasing role in this patient population. The high sensitivity to detect active tuberculous lesions allows correct evaluation of the extrapulmonary disease extent, which might have an impact on the treatment plan. The metabolic imaging can also guide the biopsy and lead to earlier diagnosis. Treatment response assessment appears to be the most promising application of 18F-FDG-PET/CT in TB. It allows early evaluation of treatment response and differentiates between responders and nonresponders at 2 months and at the end of treatment [15, 16, 17]. Early evaluation of treatment response is essential in the management of patients with TB, particularly extrapulmonary TB, when multidrug resistance is expected and treatment modification is necessary in nonresponders at an early stage of the disease [18, 19]. Results suggest that 18F-FDG-PET/CT may be useful in previously healed TB cases, as patients with pulmonary sequelae demonstrating higher SUV (≥1.5) on 18F-FDG-PET/CT likely have a higher risk for subsequent TB activation [20].
Conclusion
Because of its characteristics, TB can be easily confused with malignancy. We have highlighted the fact that the increased FDG uptake might not necessarily be an indication of high-grade lymphoma relapse, and TB should be checked for especially in patients from endemic areas. The presented case also exemplifies the potential of 18F-FDG-PET/CT imaging in early detection and assessment of the extent of extrapulmonary TB in immunosuppressed cancer patients.
Statement of Ethics
A waiver of informed consent is available from the HMC research office.
Disclosure Statement
The authors report that they have no conflicts of interest.
Acknowledgment
We wish to acknowledge Ms. Mylene Freires for her contribution to the language revision of the paper.
References
- 1.Al-Anazi K, Aljasser A, Evans DA. Infections caused by Mycobacterium tuberculosis in patients with hematological disorders and in recipients of hematopoietic stem cell transplant, a twelve year retrospective study. Ann Clin Microbiol Antimicrob. 2007;6:16. doi: 10.1186/1476-0711-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos JP, Batista MV, Costa SF. Tuberculosis in hematopoietic stem cell transplant recipients. Mediterr J Hematol Infect Dis. 2013;5:e201306. doi: 10.4084/MJHID.2013.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CY, Sheng WH, Cheng A, Tsay W, Huang SY, Tang JL, Chun Chen Y, Wang JY, Fangtien H, Chang SS. Clinical characteristics and outcomes of Mycobacterium tuberculosis disease in adult patients with hematological malignancies. BMC Infect Dis. 2011;11:324. doi: 10.1186/1471-2334-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libtishitz HI, Pannu HK, Elting LS, Cooksley CD. Tuberculosis in cancer patients: an update. J Thorac Imaging. 1997;12:41–46. doi: 10.1097/00005382-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Reported Tuberculosis in the United States, 2004. Atlanta: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 6.Farer LS, Lowell AM, Meador MP. Extrapulmonary tuberculosis in the United States. Am J Epidemiol. 1979;109:205. doi: 10.1093/oxfordjournals.aje.a112675. [DOI] [PubMed] [Google Scholar]
- 7.Stefan DC, Andronikou S, Freeman N, Schoeman J. Recovery of vision after adjuvant thalidomide in a child with tuberculous meningitis and acute lymphoblastic leukemia. J Child Neurol. 2009;24:166–169. doi: 10.1177/0883073808322329. [DOI] [PubMed] [Google Scholar]
- 8.Weiser MA, Obrien S, Escalande C, Manzullo E. Tuberculosis meningitis in a patient with acute myelogenous leukemia. Leuk Lymphoma. 1999;33:187–192. doi: 10.3109/10428199909093741. [DOI] [PubMed] [Google Scholar]
- 9.Kwon JC, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim YJ, Lee S, Kim HJ, Gho SG, Lee JW, Min WS. Clinical characteristics and the usefulness of the QuantiFERON-TB Gold In-Tube test in hematologic patients with hepatic or splenic lesions. Korean J Intern Med. 2013;28:187–196. doi: 10.3904/kjim.2013.28.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selçuk NA, Fenercioglu A, Selçuk HH, Uuçay C, Yencilek E. Multifoci bone tuberculosis and lymphadenitis in mediastinum Mimics malignancy on FDG-PET/CT: a case report. Mol Imaging Radionucl Ther. 2014;23:39–42. doi: 10.4274/Mirt.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'souza MM, Mondal A, Sharma R, Jaimini A, Khanna U. Tuberculosis the great mimicker: 18F-fludeoxyglucose positron emission tomography/computed tomography in a case of atypical spinal tuberculosis. Indian J Nucl Med. 2014;29:99–101. doi: 10.4103/0972-3919.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee A, Shama P, Pkarunanithi S, Dhull VS, Kumar R. Lymphoma and tuberculosis: temporal evolution of dual pathology on sequential 18 F-FDG PET/CT. Clin Nucl Med. 2014;39:736–737. doi: 10.1097/RLU.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 13.Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, Im JG. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006;7:57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J. 2010;100:598–601. doi: 10.7196/samj.4082. [DOI] [PubMed] [Google Scholar]
- 15.Ozmen O, Gökćek A, Tatci E, Biner I, Akkalyoncu B. Integration of PET/CT in current diagnostic and response evaluation methods in patients with tuberculosis. Nucl Med Mol Imaging. 2014;48:75–78. doi: 10.1007/s13139-013-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shejul Y, Chajed PN, Basu S. 18F-FDG PET and PET/CT in diagnosis and treatment monitoring of pyrexia of unknown origin due to tuberculosis with predominant hepatosplenic Involvement. J Nucl Med Technol. 2014;42:235–237. doi: 10.2967/jnmt.113.132985. [DOI] [PubMed] [Google Scholar]
- 17.Vorster M, Sathekge MM, Bormanji J. Advances in imaging of tuberculosis: the role of 18FDG and PET/CT. Curr Opin Pulm Med. 2014;20:287–293. doi: 10.1097/MCP.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 18.Martinez V, Castilla-Lievre MA, Guillet-Caruba C, Grenier G, Fior R, Desarnaud S, Doucet-Populaire F, Boué F. 18F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis. 2012;16:1180–1185. doi: 10.5588/ijtld.12.0010. [DOI] [PubMed] [Google Scholar]
- 19.Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med. 2011;52:880–885. doi: 10.2967/jnumed.110.083709. [DOI] [PubMed] [Google Scholar]
- 20.Jeong YJ, Paeng JC, Nam HY, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. 18F-FDG position-emission tomography/computed tomography findings of radiographic lesions suggesting old healed tuberculosis. J Korean Med Sci. 2014;29:386–391. doi: 10.3346/jkms.2014.29.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]